Abstract
Primary isolates of chondrocytes and mesenchymal stem cells are often insufficient for cell-based autologous grafting procedures, necessitating in vitro expansion of cell populations. However, the potential for expansion is limited by cellular senescence, a form of irreversible cell cycle arrest regulated by intrinsic and extrinsic factors. Intrinsic mechanisms common to most somatic cells enforce senescence at the so-called "Hayflick limit" of 60 population doublings. Termed "replicative senescence", this mechanism prevents cellular immortalization and suppresses oncogenesis. Although it is possible to overcome the Hayflick limit by genetically modifying cells, such manipulations are regarded as prohibitively dangerous in the context of tissue engineering. On the other hand, senescence associated with extrinsic factors, often called "stress-induced" senescence, can be avoided simply by modifying culture conditions. Because stress-induced senescence is "premature" in the sense that it can halt growth well before the Hayflick limit is reached, growth potential can be significantly enhanced by minimizing culture related stress. Standard culture techniques were originally developed to optimize the growth of fibroblasts but these conditions are inherently stressful to many other cell types. In particular, the 21% oxygen levels used in standard incubators, though well tolerated by fibroblasts, appear to induce oxidative stress in other cells. We reasoned that chondrocytes and MSCs, which are adapted to relatively low oxygen levels in vivo, might be sensitive to this form of stress. To test this hypothesis we compared the growth of MSC and chondrocyte strains in 21% and 5% oxygen. We found that incubation in 21% oxygen significantly attenuated growth and was associated with increased oxidant production. These findings indicated that sub-optimal standard culture conditions sharply limited the expansion of MSC and chondrocyte populations and suggest that cultures for grafting purposes should be maintained in a low-oxygen environment.
INTRODUCTION
Cell based tissue-engineered grafts offer promising options for the repair and regeneration of bone and hyaline cartilage at sites of osteochondral injury. Because they are created using a patient's own cells, engineered grafts minimize rejection and infection hazards, making them an attractive alternative to conventional allografts.2,3,4,5 Cell based grafts consisting of chondrocytes seeded on various scaffold materials have been used successfully to repair full-thickness osteochondral lesions.7,8,14 More recently, bone marrow-derived mesenchymal stem cells (MSCs) have been used to promote the repair of bone and cartilage defects.6,15,26 These cells are relatively easy to isolate from iliac crest aspirates and differentiate readily into chondrocyte-like and osteoblast-like cells both in vitro and in vivo.17,18,19,22
Partly because of the potential for damage caused by culture or storage conditions, fresh MSCs and chondrocytes are commonly preferred for engraftment procedures. However, the ability to safely expand cell populations in vitro and to store cells for later use is of great practical advantage to tissue engineers. The in vitro expansion of cells over time is calculated in terms of cumulative population doublings (PD). The population-doubling limit (PDL) marks the cessation of growth brought on by irreversible arrest of the cell cycle (cell senescence). Somatic cells that lack telomerase activity, inevitably senesce due to the normal process of telomere erosion. This process, termed "replicative senescence", is the basis of the Hayflick limit that arrests growth after approximately 60 PD. 1,9,11 Telomerase activation via ectopic expression of the human telomerase reverse transcriptase subunit (hTERT) prevents replicative senescence by continuously maintaining telomere length.25 While hTERT expression is sufficient to immortalize human fibroblasts under standard culture conditions, other "telomerized" cell types, including chondrocytes, undergo growth arrest.10,13,20 Such cells appear to senesce in response to stress, a phenomenon that is not dependent on telomere erosion. Stress-induced senescence is often referred to as "premature" since it can occur long before the Hayflick limit is reached.13,16,24 Human chondrocytes and MSCs grown under standard culture conditions grow to only 10-40 PD13,23, well short of the 60 PD Hayflick limit.9 Taken together these findings suggest that standard culture conditions are suboptimal for expansion of MSC and chondrocyte populations.
The in vitro growth characteristics of cells are subject to many culture-related factors, such as initial cell densities, media formulation, and oxygen tension.16,24 Standard culture conditions use oxygen levels (20%-21%) that are hyper-physiologic for many cell types, including articular chondrocytes and bone-marrow derived MSCs, which are adapted to relatively low oxygen levels (< 10%) in vivo.21 This suggests that excessive oxidative stress may limit the growth potential of cells under standard conditions. Indeed, reducing incubator O2 levels has been shown to have beneficial effects on population growth of oxygen sensitive fetal fibroblasts.16 Based on these findings we hypothesized that the standard incubator atmosphere of 21% O2 causes sufficient oxidative stress to induce premature senescence in chondrocytes and MSCs, limiting the number of cells available for tissue engineering. To test this we compared the growth of human MSC and chondrocyte populations in 21% and 5% O2 and measured oxidant production by cells under the two conditions. hTERT transduced chondrocytes were included in the study to help distinguish between stress-induced growth arrest and growth arrest induced by telomere erosion.
METHODS
Human articular cartilage was harvested from the distal tibial or talar surfaces of normal ankle joints as previously described.12 Monolayer cultures were grown in medium containing 40% Dulbecco's modified Eagle medium, 40% Modified Eagle Medium-alpha, 10% Ham's F12, 10% fetal calf serum, 0.1 U/ml insulin, 25 µg/ml ascorbate, 5 µM hydrocortisone, and antibiotics (Life Technologies). Growth studies were initiated by trypsinizing the primary cultures, counting the cells manually, and inoculating 100,000 cells in 100 mm tissue culture dishes. These were placed in incubators with either a 5% O2 (5 % O2, 90% N2, 5% CO2) or 21% O2 atmosphere (21% O2, 74% N2, 5% CO2) and incubated with medium changes every 2-3 days until the cells were nearly confluent. At that point the cells were trypsinized, counted, and re-innoculated as before. This procedure was repeated for several passages. Chondrocytes were transduced with FIV-hTERT for telomerase activation or with FIV ß-galactosidase (control) as previously described.13
Human MSCs were obtained from discarded Cellect®, units (DePuy Acromed) immediately after surgery. Filters were washed with MSC medium (70% DMEM, 20% Ham's F12, 10% FBS) and the washings collected in 2-3 150 mm tissue culture dishes. After incubating for 4-5 days at 37°C the cultures were washed extensively with Hanks Balanced Salt Solution (HBSS) to remove red blood cells and other debris, revealing adherent cells. These were re-fed with MSC medium and incubated an additional 14-30 days to expand cell numbers. Growth studies with human MSCs were done in a manner identical to that explained above for chondrocytes.
The production of oxidants in cell cultures was measured using the oxidation-sensitive dye dihydroethidium (DHE) (11). One chondrocyte strain grown in 5% or 21% O2 for approximately 20 PD was stained by incubation for 30 minutes at 37°C with 5 µM DHE (Molecular Probes). After staining, the cultures were washed briefly with PBS, and mounted in Vectashield with DAPI (Vector Labs). The stained cells were imaged using an Olympus BX60 epifluorescence microscope and an Optronics digital camera. All images of the DHE stain were taken using the same exposure time. Image analysis was done using Scion Image software. The threshold and particle size was set to the same level for all images used in the analysis. Total cells (DAPI positive) and number of DHE positive cells were counted in three separate fields (30-40 cells per field) for each of the two conditions, 5% and 21% oxygen. The percent DHE positive cells in each field was calculated by dividing the number of DHE positives in the field by the total number of cells in the field.
Population doubling data and oxidant production data were analyzed using Student's t-test to determine statistical differences between the different culture conditions.
RESULTS
The growth of 3 different chondrocyte populations was enhanced in 5% oxygen (low O2) versus 21% O2 (high O2) (Figure 1A). Control and hTERT-transduced chondrocytes in low O2 reached an average of at least 77 PD, while those in high O2 senesced after only 37.8. This 2-fold increase was highly significant (p <0.001). Without hTERT, mean doubling limits were 64.6 PD in low O2 and 34.5 PD in high O2, a difference that was also highly significant (p < 0.001). Separate analysis of hTERT cells revealed similar significant increases in growth in low O2 (p = .008). The mean doubling limit in low O2 grown cells was 88 PD or more, at least 2-fold greater than the mean for high O2 grown cells (41 PD). The effects of hTERT-transduction depended to some extent on O2 level (Figure 1B). The mean PDL for hTERT cells in high O2 was only slightly greater than for controls (41 PD versus 35 PD, respectively), a difference that was not statistically significant (p = 0.262). However, in low O2 hTERT increased growth from 65 PD to at least 88 PD, a somewhat stronger effect that approached statistical significance (p = 0.052). Two of the three hTERT-transduced strains maintained in low O2 were still growing at the time of this report, suggesting that these cells may be immortalized.
Oxidant generation differed in chondrocytes grown under high and low O2 conditions (Figure 2). Weak DHE staining was observed in cells from a low O2 culture (Figure 2A) but cells from a high O2 culture were intensely stained (Figure 2B). Semi-quantitative analysis revealed that 25% of the cells in the low O2 culture were DHE positive, while 80% of the cells in the high O2 culture were positive. This difference was highly significant (p = 0.004).
Figure 2. Oxidant Production by Chondrocytes in High and Low Oxygen.
A,B: Fluorescence micrographs of cells stained with DHE (red) and DAPI (blue)
Figure 2A. Cells in low O2 .
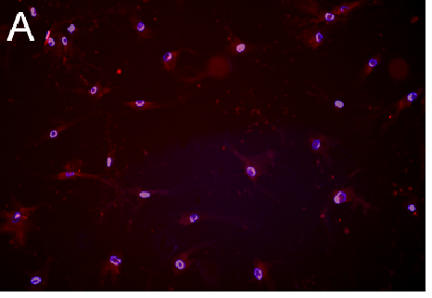
Figure 2B. Cells in high O2 .
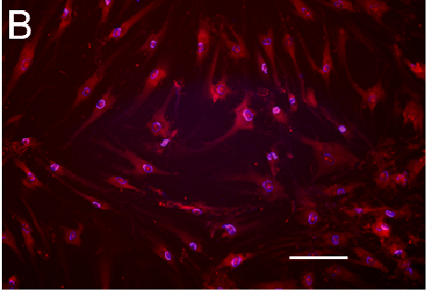
Figure 2C. P=0.004.

The percentage of DHE positive cells (Percent Cells Positive) in high and low O2. Columns show the mean and standard error of the mean (error bars) based on 120 cells. There were significantly more positive cells in high O2 (p = 0.004).
The growth of human bone marrow-derived MSCs was also found to be sensitive to O2 level. Cells from 4 different MSC strains cultured in low O2 outgrew their high O2 counterparts by at least 2-fold (Figure 3A). The mean PDL for all MSC populations in high O2 was 10.5, while the mean for cells cultured in low O2 was 23.6 PD (Figure 3B). This difference was statistically significant (p < 0.05).
DISCUSSION
Our findings support the hypothesis that high oxygen levels used in standard culture conditions are harmful to human chondrocytes and MSCs. Chondrocytes senesced after 25-30 PD when they were cultured under standard conditions, however, the same cells cultured in 5% O2 grew to 60 population doublings, indicating that the earlier growth arrest in standard conditions was premature. Telomerase activity induced by ectopic expression of hTERT allowed chondrocytes grown in 5% O2 to exceed 60 PD, indicating that growth arrest at this stage was due to telomere-dependent replicative senescence. In contrast, hTERT expression had little if any impact on senescence in 21% O2 despite evidence of extensive telomere elongation (data not shown). Thus, premature growth arrest at approximately 30 PD was independent of telomere length, suggesting a stress-induced mechanism of senescence. This conclusion was supported by additional analyses which showed that growth in 21% O2 increased the cellular production of oxidants. High O2 conditions were similarly detrimental to the growth of MSC populations. MSCs cultured in high O2 barely exceeded 10 PD before the cells senesced, but the same populations cultured in low O2 grew to approximately 20 PD.
Two of three hTERT-transduced chondrocyte strains never reached a doubling limit and continue at the present time to proliferate in low O2. This suggests that the combination of telomerase activity and low O2 conditions may be sufficient to immortalize chondrocytes. However, this will be evident only after additional time in culture. In any event, the incomplete growth data reported here probably underestimate the true strength of hTERT effects in chondrocytes cultured in low O2.
MSC growth was clearly affected by oxygen but the absolute PDL values were lower than some published values for human MSCs cultured in high O2.
Much of this apparent discrepancy could have been due to unaccounted-for population doublings that accumulated during expansion of the primary cultures. This will be avoided in future studies by counting the MSCs initially plated so that growth in the primary cultures can be tracked. In some high O2 cultures the number of cells declined following growth arrest (data not shown), suggesting cell death. Thus, our findings to date indicate that high oxygen levels limit are stressful to MSCs and limit their in vitro growth by inducing senescence and apoptosis.
Standard culture conditions were originally developed for growing cell lines and fibroblasts. While fibroblasts perform well under these conditions, reaching their full replicative potential of near 60 PD, other cell types such as keratinocytes, require a more specialized environment to avoid culture-induced premature senescence.20 Our findings indicate that culture induced stress is the primary factor limiting the in vitro growth of chondrocytes and MSCs and that preventing oxidative stress is a key factor in overcoming this barrier. Lowering incubator O2 levels was an effective and simple means to that end, but other modifications of standard conditions, such as the addition of antioxidants to the culture medium, might confer additional protection against oxidative damage. Moreover, even routine cell culture procedures such as initial cell isolation and trypsinization are potential sources of oxidative stress. Such occasional stress exposures might impact growth even in cultures exposed most of the time to low O2 conditions and might explain why one low O2 grown hTERT strain senesced.
The attenuation of growth imposed by culture-related stress seriously restricts cell yields and may have a negative impact on subsequent differentiation of MSCs and chondrocytes. Additional studies will be needed to determine what are likely to be diverse phenotypic effects of oxidative damage. However, the present study shows that low O2 culture is an effective means to control oxidative stress and to increase the proliferative potential of MSCs and chondrocytes destined for grafting procedures.
Figure 1. Effects of Oxygen and Telomerase Activation on Chondrocyte Population Growth.
Figure 1A.
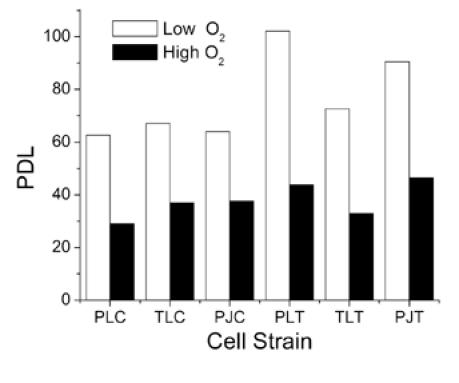
Population doubling limits (PDL) for 3 chondrocyte strains (PL, TL, PJ) cultured in 5% oxygen (Low O2) or 21% oxygen (High O2). Results are shown for control cells (PLC, TLC, PJC) and for cells transduced with hTERT (PLT, TLT, PJT).
Figure 1B.

Columns represent mean PDL and standard error of the mean (error bars) for control and hTERT cells in either low or high O2. PDL values for low O2 grown cells were significantly greater than for high O2 grown cells (p < 0.001). The differences between hTERT and control cells were not significant in high O2 (p = 0.262) but were very nearly significant in low O2 (p = 0.052).
Figure 3. Effects of Oxygen on Mesenchymal Stem Cell Population Growth.
Figure 3A.

Population doubling limits (PDL) for 4 MSC strains (8, 7, K, F) cultured in 5% oxygen (Low O2) or 21% oxygen (High O2)
Figure 3B.

Columns represent mean PDL and standard error of the mean (error bars) for low or high O2 grown cells. PDL values for low O2 grown cells were significantly greater than for high O2 grown cells (p < 0.05).
References
- 1.Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay JW, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 2.Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86–96. [PubMed] [Google Scholar]
- 3.Brittberg M, Lindahl A, Nillson A, Ohlsson C, Isaksson O, Peterson L. Treatment of Deep Cartilage Defects in the Knee With Autologous Chondrocyte Transplantation. New Eng J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Tallheden T, Sjogren-Jansson B, Lindahl A, Peterson L. Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop. 2001;391(Suppl):S337–348. doi: 10.1097/00003086-200110001-00031. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 6.Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–256. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Accio F, Vanlauwe J, Bellemans J, Neys J, De Bari C, Luyten FP. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21:123–131. doi: 10.1016/S0736-0266(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 8.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7:363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 9.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 10.Herbert BS, Wright WE, Shay JW. p16(INK4a) inactivation is not required to immortalize human mammary epithelial cells. Oncogene. 2002;21:7897–7900. doi: 10.1038/sj.onc.1205902. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Miller F, Brown M, Chatterjee P, Aylsworth G, Shao J, Spector A, Oberley L, Weintraub N. Enhanced H2O2-induced cytotoxicity in "epithelioid" smooth muscle cells implications for neointimal regression. Arterioscler Thromb Vasc Biol. 2000;20:1473–1479. doi: 10.1161/01.atv.20.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol Biol Sci. 2001;56A:B172–179. doi: 10.1093/gerona/56.4.b172. [DOI] [PubMed] [Google Scholar]
- 13.Martin JA, Mitchell CJ, Klingelhutz AJ, Buckwalter JA. Effects of telomerase and viral oncogene expression on the in vitro growth of human chondrocytes. J Gerontol: Biol Sci. 2002;57:B48–53. doi: 10.1093/gerona/57.2.b48. [DOI] [PubMed] [Google Scholar]
- 14.Micheli LJ, Browne JE, Erggelet C, Fu F, Mandelbaum B, Moseley JB, Zurakowski D. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11:223–228. doi: 10.1097/00042752-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop. 2002;395:66–80. doi: 10.1097/00003086-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003 Aug;5(8):741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 20.Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O'Toole KM. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott JE. Oxygen and the connective tissues. Trends Biochem Sci. 1992;17:340–343. doi: 10.1016/0968-0004(92)90307-u. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg. (Am) 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Toussaint O, Medrano E, Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (sips) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 25.Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/s0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- 26.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg. (Am) 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]


