Abstract
Nerves have been identified in bone. Their function has recently become the focus of intense study. Metabolic control of bone is influenced by the nervous system. Potential transmitters of this influence include glutamate, calcitonin gene-related protein (CGRP), substance P, vasoactive intestinal peptide (VIP), pituitary adenylate cyclase activating polypeptide (PACAP), leptin, and catecholamines. Disorders of nerves - central or peripheral - can have substantial influence on bone health and repair. Specifically considered are the potential neural influences at work in such conditions as osteoporosis, fracture healing, Charcot osteoarthropathy, musculoskeletal pain syndromes, heterotopic ossification, skeletal growth and development, and obesity-related increased bone density. In this article, we review the current state of experimental and clinical evidence implicating the role of nervous tissue in regulating bone biology and discuss the current understanding of molecular signaling between nervous and osseus tissue in the homeostatic maintenance of the skeleton.
INTRODUCTION
From the time of his or her first fracture reduction in the emergency room, no orthopaedic surgeon has ever doubted that bone was innervated. However, the assumption was always that only the periosteum, and not the bone tissue itself, was innervated. Conditions such as Sudeck's atrophy in association with chronic regional pain syndrome, heterotopic ossification in the setting of head injury, and Charcot diabetic neuroarthropathy extend the general consideration that nervous and musculoskeletal systems must interact.
The first documentation of an anatomic relationship between nerves and bone was made via woodcut, by Charles Estienne in Paris in 1545. His diagram demonstrated nerves entering and leaving the bones of a skeleton. More specific study of bone innervation awaited the availability of technology that could microscopically view bone in sufficient detail. Reginald Cooper published his findings from electron microscopy that cortical bone is densely innervated (See Figures 1 and 2.), in his landmark 1966 report in the Journal of Bone and Joint Surgery1 and subsequent report in 1968 in Science.2 The next year Calvo and Fortez-Vila differentiated myelinated and non-myelinated fibers associated with the arterial vessels and venous sinusoids in bone.3 Histofluorescence of noradrenaline identified sympathetic nerve fibers a decade later.4 Finally, in 1986, Hohmann et al. reported on immunohistochemical localization of vasoactive intestinal peptide (VIP) containing sympathetic fibers in bone.5 This began a steady flow of studies of various nerve types in bone by a number of different groups.
Figure 1.
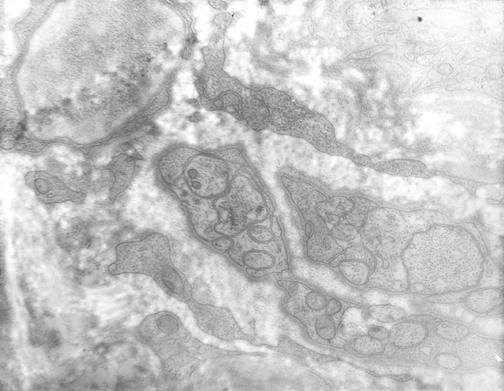
Unmyelinated nerves in bone. Electron microscopy of a human femoral osteon reveals multiple unmyelinated nerves with their neurofilaments within. The nerve cells appear invaginated into the recesses in the Schwann cell plasma membrane. Multiple nerve fibers appear ensheathed in each Schwann cell. Nerve fibers vary from 0.25 to 0.6 micrometers in diameter. They contain mitochondria, and neurofilaments, measuring in diameter about 600 Angstroms and 115 Angstroms, respectively.
Figure 2.
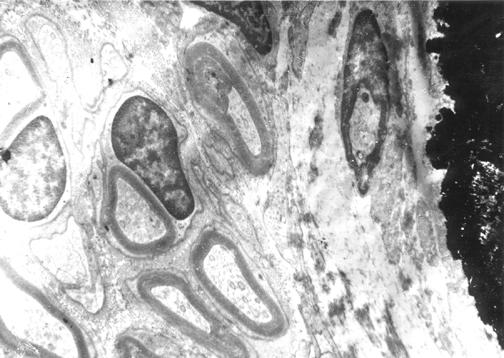
Myelinated nerves in bone. Electron microscopy of a human femoral osteon reveals multiple myelinated nerves adjacent with two nearby osteoblasts. Dark mineralized matrix is visible on one border of the micrograph. The osteoblasts appear to have some electron dense endoplasmic reticulum, more dense than adjacent cytoplasm. One of the osteoblasts has an apparent nucleus with a nucleosome within.
The field has recently been reinvigorated by interest in what was initially thought to be an obesity hormone, leptin. As the story of leptin and its effects on body mass as well as bone mass has slowly unfolded, the implications of neural control of many aspects of bone metabolism that were once thought to be exclusively hormonal, have come more sharply into focus.
NEURO-OSSEUS SIGNAL TRANSMISSION
Nerves are found throughout the periosteum and accompany nutrient vessels in the perivascular spaces of Haversian canals. They have been demonstrated to be especially dense near more metabolically active parts of bones, such as in the epiphysis and metaphysis, especially surrounding the growing physis.
Most nerve-to-nerve signal transmissions along with many nerve-to-end organ communications occur via synapses. While neural synapses represent special cell-to-cell signaling areas isolated from the surrounding interstitial space, they nonetheless utlimately depend on receptor-ligand interactions, just as hormonal endocrine, paracrine, and autocrine functions. Neural transmission ligands include such molecules as catecholamines, glutamate, and a number of small polypeptides formed by alternative RNA splicing from larger genes.
To date, no classical synapses have been found to involve osteoblasts, osteoclasts, or osteocytes. However, nerve fibers with active expression of various neural transmission ligands have been demonstrated to be in close spatial association with bone cells. Further, receptors for these neural ligands are expressed by bone cells, and administration of these neural transmission molecules has potent effects on bone cells.
We will review the roles of the following neural ligands in bone metabolism: glutamate, calcitonin-gene related peptide (CGRP), substance P, vasoactive intestinal peptide (VIP), and catecholamines.
Glutatmate
Glutamatergic synaptic transmission dominates internervous signaling in the central and peripheral nervous systems. There are both ionotropic, or ion channel gating, and metabotropic, or G-protein-coupled, cell protein expression modulating, receptors for glutamate. Among ionotropic glutamate receptors is the NMDA receptor, thought to be responsible for memory and synaptic plasticity in the central nervous system.
Recently, glutamate has been identified in bone both in association with other nerve markers in proximity to bone cells and blood vessels, and as a product released by osteoblasts themselves.6,7 Osteoblasts, osteoclasts, and osteocytes express the NMDA and other glutamate receptors and induce patch-clamp-demonstrable currents (the standard measure of ion channel controlled currents in nerves) in response to glutamate signaling.8–12
Osteoblasts decrease alkaline phosphatase and calcific nodule formation (known in-vitro markers of osteoblast activity) when exposed to NMDA receptor antagonists in culture. Bone marrow stromal cells preferentially differentiate into adipocytes rather than osteoblasts when they are exposed to such antagonists.13 These findings suggest that glutamate, an NMDA agonist, should play a role in encouraging osteoblastic differentiation and activity.
Osteoclasts are halted in bone resorption when exposed to NMDA receptor antagonists.8 This may indicate an expansion of glutamate NMDA-agonist effects to upregulate bone remodeling in general, with increased osteoclastic function as well.
Glutamate function in paracrine signals between bone cells is suspected to be at work when expression of transporters of glutamate is downregulated in response to mechanical loading of osteocytes.14 Glutamate antagonists injected into rats prior to bone loading decrease the responsive bone remodeling that would otherwise result.15
While the signaling is not yet fully understood, glutamate is thought to play a major role in nerve-to-bone signaling, as well as bone-to-bone paracrine signaling to control anabolic and catabolic activities, especially as they relate to responsive remodeling after mechanical loading.16
Calcitonin Gene-Related Protein (CGRP)
CGRP is a 37 amino acid neuropeptide generated by alternative splicing of the calcitonin gene. In the peripheral nervous system, it is expressed in finely myelinated A-delta fibers and unmyelinated C fibers, the major peripheral sensory nerve fibers. In bone, nerve fibers immunostaining for CGRP are found in the periosteum17,18, bone marrow19, and preferentially in the epiphyseal trabecular bone.20 There, the fibers have many varicosities or bulges, which are scarcely covered by Schwann cells and richly loaded with secretory vesicles.21 These varicosities, as well as the free nerve endings, are often closely associated with osteoblasts and osteoclasts. These findings have led to the suspicion that in addition to sensory transmission toward the central nervous system, sensory fibers may also transmit signals to the periphery, specifically to the milieu surrounding bone cells.
Cultured osteoblasts from multiple species demonstrate a characteristic rise in cyclic AMP concentration when exposed to CGRP directly.22,23 CGRP exposure increases insulin-like growth factor expression dramatically24 and interleukin-6 expression weakly.25 It decreases tumor necrosis factor alpha expression.26 These findings suggest that CGRP should increase bone formation and decrease resorption. The bone marrow of mice pretreated with CGRP has increased osteogenic activity in subsequent culture; direct exposure of marrow culture to CGRP also leads to increased bone colony formation. Interestingly, osteoblasts can express CGRP, perhaps as part of an autocrine or paracrine pathway.27 Transgenic mice expressing CGRP driven by the osteocalcin (unique osteoblast gene) promoter have increased bone formation and bone volume;28 this strengthens the supposition that CGRP expressed by osteoblasts themselves can affect osteoblastic activity similar to CGRP signaling from nerves. Interestingly, CGRP staining fibers have been shown to increase during fracture healing29 and bone defect repair.30
Similar to calcitonin itself, CGRP inhibits osteoclast resorption directly in culture31 and decreases serum calcium in vivo.32,33
Substance P
Nerve fibers staining for substance P, a well known nociceptive signaling molecule typically associated with sensory nerves, enter the bone marrow in association with vessels21, but then dissociate and terminate as free nerve endings. Substance P has been shown to increase osteoblast differentiation, bone colony formation, and osteoblast cyclic AMP production. At least one of its receptors, neurokinin-1 is expressed by osteoclasts. Neurokinin-1 drives osteoclast resorption activity in vitro when osteoclasts are exposed to substance P.
Vasoactive Intestinal Peptide (VIP)
VIP is a neuropeptide usually associated with parasympathetic nerve fibers; it is also expressed in post-ganglionic sympathetic nerve fibers. Fibers immunoreactive for VIP were first demonstrated in bone by Hohmann et al. in 1986.5 They were shortly thereafter localized to the periosteum and epiphysis, and only occasionally with blood vessels.34,35 VIP is a ligand for three known receptors, VIP-1R, VIP-2R, and the receptor for pituitary adenylate cyclase activating polypeptide (PACAP). All of these are 7-trans-membrane G-coupled receptors from the VIP/secretin/PTH receptor family. Although results vary as to the timing of specific VIP receptor expression along the differentiation chronology, both VIP receptors have been shown to be expressed on osteoblasts and osteoclasts.36–38
VIP effects on osteoblastic activity have received only limited study. Lundberg et al. in 1999 demonstrated increased osteoblast activity in culture with VIP; however, this was not associated with either osteoblast proliferation or an increase in osteoid production.39 They concluded that VIP induced further differentiation of already committed osteoblasts.
Better studied have been the effects of VIP on osteoclasts, some of which are mediated through osteoblast signaling. VIP downregulates osteoclastogenesis by decreasing RANK and RANK ligand expression in osteoclasts and osteoblasts, respectively, and by increasing osteoblast expression of osteoprotegerin, a RANK antagonist.40 Regarding already present osteoclasts, VIP initially decreases their bone resorptive activity with a late reversal of this effect and stimulation of bone resorption. It is thought that this late osteoclast stimulation is mediated by increased expression of IL-6 by either osteoblasts or surrounding stromal cells.41
PACAP is a polypeptide related to VIP, formed by alternative splicing of the calcitonin gene. Its two known forms are PACAP 27 and PACAP 38. Its effects on osteoblasts are thought to be identical to VIP as it acts as a potent ligand to the same receptors.42,43 However, nerve fibers immunostaining for PACAP also stain for substance P and CGRP, suggesting sensory, as opposed to sympathetic origin.44
Catecholamines
Tyrosine hydroxylase (TH) is the rate-limiting enzyme in catecholamine synthesis, which takes place near the nerve terminus of sympathetic nerves. Immunolocalization of TH is the means by which sympathetic adrenergic nerve fibers have been identified in bone.21 Most of these fibers are associated with blood vessels in the bone marrow, but some are found as free nerve endings in the periosteum and bone adherent ligaments.21
Osteoblasts have been demonstrated to express β-2 adrenergic receptors.45 Further, noradrenaline acting at α-1 receptors increases alkaline phosphatase activity in and proliferation of osteoblasts.46 Others have demonstrated increased cyclic AMP and prostaglandin E2 production in response to noradrenaline.22,47,48
The interest in adrenergic innervation of bone has recently been specifically amplified by the same experiments that have truly re-opened the entire field of neuro-osseus transmission in the twenty-first century. Leptin, an obesity and body mass control hormone, has also been shown to induce both osteopenia and cachexia. Central resistance to, or underproduction of leptin results in the clinically frequent combination of obesity and greater than normal bone density. These characteristics, so long thought to be more hormonally related, are now shown to be regulated by the nervous system, as the most powerful effects of leptins are actually in the hypothalamus, and not at peripheral receptors in bone.49 In rather elegant animal experiments, the Karsenty group in Houston demonstrated that adrenergic signaling mediates the bone density reduction resulting from leptin signaling in the hypothalamus.50 While the peripheral pathophysiology at work is not entirely understood, the phenotypic osteopenia of increased central leptin signaling was reversed with systemic administration of beta-blockers. Some groups are now investigating beta-blockers as potential therapeutic options for osteoporosis.
EXPERIMENTAL DENERVATION
Although much of the experimental evidence for varied aspects of the understanding of neuro-osseus interaction comes from in vitro studies, these suggest potential pathways for interaction. The combined effects of multiple nerve-types and their multiplied signals can only begin to be worked out amidst the complex in vivo environment. A number of researchers have attempted to study neuro-osseus control with in vivo models of musculoskeletal denervation.
Neurectomy
The effects of surgically sectioning the sciatic nerve have been studied extensively, but nearly serendipitously. Sciatic neurectomy has been used by many as a standard model of disuse osteopenia, as loss of musculature from denervation effectively stops active motion in the limb. So recent is the prevalence of the thought that nerves may interact with bone metabolism directly, that most of the papers do not even recognize that neurectomy may have direct effects on bone cells from lost bone innervation, above and beyond denervation of muscle and disuse of the limb.51
When measured by DEXA, bone mineral density following rat sciatic neurectomy decreased progressively for 4 weeks post-section, then stabilized, but ceased to recover.52 Interestingly, the contralateral limb with intact innervation also lost bone density - despite lack of apparent disuse.
An ex vivo culture study of bone marrow from neurectomized limbs demonstrated reduced osteoblastic activity markers, increased osteoclast-precursor differentiation after 2 weeks and increased osteoclast number and rate of osteoclastogenesis after 4 weeks.53 The increased marrow osteoclast precursors and osteoclastogenesis may contribute to the contralateral loss in density.
Recently, Ito and colleagues have shown that neurectomy-induced osteopenia has a microstructure distinctly different from ovariectomy-induced osteopenia in the tibia.54 While both show perforation and removal of trabeculae due to accelerated resorption, post-neurectomy trabeculae are flake-like in morphology as opposed to just diffusely absent. In addition, neurectomy more powerfully affects cortical bone density. Similar differences were found in comparison of neurectomy to orchidectomized male rats.55
The effects of neurectomy on osteopenia have been partly prevented experimentally with application of electrical stimulation56 and bisphosphonate therapy.57 In contrast, a substance P receptor antagonist was shown to enhance the bone loss following neurectomy. Substance P was reduced in both tibiae after unilateral sciatic nerve section. This led to the hypothesis that substance P may function systemically to maintain bone mass after denervation.
Sciatic neurectomy has been used to study pathophysiology other than osteopenia. Dietz demonstrated that complete and partial denervation after limb differentiation but before complete ossification and growth in tadpoles yield increased retained cartilaginous anlagen and decreased bone length, cross-sectional area, and foot size.58
Fracture repair has specifically been studied as a setting for sciatic neurectomy in the rat. Tibia fractures with intramedullary fixation showed decreased free and perivascular CGRP containing nerve fibers within a more voluminous callus compared to non-neurectomized, but still immobilized, limbs.59 Another experiment found increased bone mineral content and increased bending moment and energy absorption after sciatic neurectomy concomitant to tibia fracture and intramedullary nailing.60
Deafferentation via dorsal root ganglionectomy of a limb with superimposed ACL transection resulted in knee instability and joint breakdown within weeks. This was interpreted to be a model for Charcot osteoarthropathy.61
Chemical Sympathectomy
Guanethidine is a sympathetic neurotoxin that can be administered intraperitoneally. In rats, TH and VIP staining fibers are dramatically decreased and CGRP and substance P fibers slightly increased in response. Treatment at birth62 has demonstrated increased osteoclast resorption; but administration to adult rats has demonstrated reduced osteoclast numbers, and presence of pre-osteoclasts in the bone.63
Chemical Sensory Denervation
Capsaicin, a sensory nerve specific neurotoxin has also been studied in in vivo bone resorption models. CGRP and substance P containing nerve fibers are reduced significantly after treatment. When rats were treated at birth, resorption induction later in life yielded less resorption than in neurointact, untreated controls.62 Adult rats treated also have decreased osteoclast recruitment and decreased attachment via the ruffled border to the bone surface.64
CLINICAL CORRELATION AND RELATED RESEARCH
While recent neuroscience discoveries have shed light on clinical neurological conditions, the role of neural pathophysiology in musculoskeletal disorders has been largely ignored. The obvious clinical problems with neural-bone pathology include bone pain from nonunion/fracture/joint degeneration, Charcot neuroarthropathy, Sudeck's atrophy from complex regional pain syndrome (reflex sympathetic dystrophy), and heterotopic bone formation (ossification) following severe head injury. Evidence now suggests roles for neural control in fracture healing, bone development, bone mass control, and osteoporosis. These and other clinical scenarios of altered bone growth and metabolism require orthopaedic scientists to re-think underlying basic orthopaedic pathophysiology in light of recent insight of a neuro-osseus axis.
Immobilization
Functional immobilization by varied means (bed rest, cast immobilization, extremity trauma) results in what is termed disuse osteopenia. Although this phenomenon has been appreciated for some time, the mechanisms of remodeling due to load are not entirely understood. With the new appreciation of glutamate signaling changing with variation in load history, it has surfaced as one of many possible mediators of this bone loss due to immobilization.
Central Nerve Lesions
A hemiplegic stroke victim may be osteoporotic in both involved extremities, but interestingly, this osteoporosis is totally independent of pre-existing body muscle composition or weight.65 This implies that effects from more than muscle inactivity alone are at play.
Spinal Cord Injury
Early after spinal cord injury (SCI), a rapid onset of bone loss occurs. This is clearly detectable in the first three months by serum and urinary type I collagen C-telopeptide assays,66 and by six months by standard bone density measurement techniques.67 With quadripalegia, bone mineral loss occurs throughout the entire skeleton, except the skull.68 The time course and location of these changes suggests that more than simple disuse osteoporosis is at play. A bone maintenance role for peripheral nerves may be implicated. Supporting this concept of a neurologically mediated effect rather than disuse osteporosis, is the data that shows that electromyostimulation to promote muscle mass and bone mass has failed.69 More than lack of mechanical bone loading is at work in the osteopenia following SCI.70 We suspect that nerve lesions, central and peripheral, contribute directly to abnormal bone metabolism through direct peripheral nerve signaling in bone.
Heterotopic Bone Formation
Heterotopic ossification (HO) is another sequelum from a range of "neurologic" injuries, including SCI, head injury, and brain tumors. HO is estimated to occur in up to 50% of SCI patients, in whom the hip is most often involved, followed by the knee.71 It affects 20% of traumatic brain-injured patients, with hip, shoulder, and elbow being the most common sites. Beyond its experimental reproduction by bone morphogenic protein administration in muscle, HO etiology is poorly understood. Interestingly, limb spasticity, joint trauma, decubitus ulcers, and complete spinal cord lesions have all been correlated with increased risk of HO in SCI.72,73 Is it possible that some inflammatory signal in and between muscles defaults differentiation of mesenchymal progenitors toward bone formation when not suppressed by some peripheral nerve signal?
Charcot Osteoarthropathy
In the developed countries, the most common cause of neuropathy in adults is diabetes mellitus. A special musculoskeletal complication of diabetic neuropathy in the extremities is Charcot neuroarthropathy, a slowly progressive inflammatory joint destruction. Disastrous complications of infection and frequent amputation make it a major concern for those involved in the care of the over 100 million patients with diabetes in the world.74
The pathophysiology is unclear. Charcot's personal etiological theory has come to be known as the French theory that without any obvious trauma history, some primary underlying neurological problem exists. A counter German theory has advocated that recurrent trauma is the culprit.
Insensate foot trauma coupled to abnormal neuro-osseus signals from neuropathy may together result in Charcot, proving the French and German theories each partially correct. It has been demonstrated that abnormal bone metabolism is at work in at least some patients with Charcot.75–77 Whether Charcot arthropathy causes, is caused by, or results from a common etiologic factor along with altered bone metabolism has yet to be determined.
Growth and Development
Clinical disorders such as idiopathic leg length discrepancy, hemihypertrophy, and clubfoot, all represent focal abnormalities of bone development and morphology, but essentially normal resultant bone quality and properties. This suggests that an influence with local effects, but anatomically separate origin has been brought to bear. The nervous system is an obvious potential source for such an influence given its organization to deliver geographically specific signals from a central origin.
Musculoskeletal Pain
Orthopaedists have always appreciated that bone is richly supplied with pain sensitive fibers, thus explaining the pain associated with fractures, nonunions, inflammatory bone conditions, and neoplastic bone lesions. Perhaps, nerve pathophysiology may cause or worsen these clinical conundrums more than simply relay pain from them. For example, some of the pain in osteoarthritis has been attributed to vascular engorgement response to perivascular autonomic nerve dysfunction. Some neural pain phenoma in extremities have been associated with abnormal regional bone density. Regional osteopenia is one of the hallmarks of chronic regional pain syndrome or reflex sympathetic dystrophy. Transient osteoporosis is another painful bone lesion, associated with MRI evidence of marrow hyperemia. Such poorly understood disease processes, along with osteolytic syndromes like pubic osteolysis and acroosteolysis, may present unique opportunities to understand nerve-bone interaction.
Obesity and Bone Mass
Many theories have been proposed to explain why obese individuals are frequently protected from osteoporosis. Little is actually understood about the complex mechanisms of bone maintenance in obesity. Is it mechanical loading, among a relatively non-active population? Is it peripheral conversion of estrogen due to increased adipocytic metabolic tissue volumes? A large body of research regarding obesity and bone mass now centers around the effects of the adipocyte hormone leptin. But leptin does not seem to affect bone metabolism as an endocrine hormone. Instead via the hypothalamus and the sympathetic nervous system, leptin signals centrally and results in systemic peripheral bone loss. Clinical studies in this area are controversial and active. In a recent national study of over 5,000 U.S. adults, serum leptin concentration did not appear to directly effect bone mineral density. Curiously, in younger men who are at lower risk of osteoporosis, this study showed a consistent inverse association of lower leptin levels and higher bone mineral density.78 What are the implications? Do obese patients with supranormal leptin levels develop a centrally mediated resistance for both anorexic and antiosteogenic pathways? Will orthopaedic surgeons indicating obese patients (with theoretically better bone growth potential) for total joint replacement consider them to be better candidates for bone ingrowth prostheses rather than cemented prostheses, as has been suggested?79 Much clinical investigation is still pending, but it is clear that the past maxims that obesity protects against osteoporosis through increased bone loading is too simple, given the current complex relationship between fat, the brain, and bone.
FUTURE DIRECTIONS
Nerve signals to bone are not necessarily the master controllers, but are now recognized as part of a vastly complex system for metabolic regulation in bone. The evolution of these findings are noteworthy, and at each step it has required scientists to recognize the importance of unexpected biological findings. First, nerves had to be recognized in bones. Then, the concept of neurons as one-way wires had to be challenged. Only now can we permit the thought that efferent and afferent may not be mutually exclusive descriptions.
To autocrine (self-signaling), paracrine (neighbor-cell signaling), and endocrine (systemic signaling via blood supply), perhaps neurocrine or axonocrine should be terms added to the vocabulary of hormone delivery. Nerves, even sensory efferents, clearly deliver signaling molecules in a unique way to the immediate milieu around bone cell surface receptors.
Given this newly conceived notion, the challenge is all before us. Much remains to be done. Basic scientists will remain diligent in the search for signals and their effects on cellular metabolic function, but enough information is now available that clinicians must begin to get involved in directing this science toward important questions of pathophysiology and disease.
TABLE 1.
Summary of neurotransmitters characterized in bone
| Neurotransmitter | Receptor | Putative intermediary mechanism | Putative action |
|---|---|---|---|
| Glutamate | NMDA | ion-gated channels | ▴ bone remodeling |
| Calcitonin gene-related peptide | CGRP-R1, CGRP-R2 |
▴ cAMP | ▴ bone formation ▾ bone resorption |
| Substance P | Neurokinin-1 | ▴ cAMP | ▴ bone formation ▴ bone resorption |
| Vasoactive Intestinal Peptide | VIP-1R, VIP-2R, PACAP-R | ▾ RANK ▾ RANKL ▴ OPG ▴ IL-6 |
▴ bone formation? ▾ osteoclast formation ▴ osteoclast resorption? |
| Catecholamines | β-2, α-1 adrenergic receptors | ▴ cAMP ▴ PGE-2 |
▴ bone formation ▴ bone resorption |
References
- 1.Cooper RR, Milgram JW, Robinson RA. Morphology of the osteon. An electron microscopic study. J Bone Joint Surg. (Am) 1966;48:1239–1271. [PubMed] [Google Scholar]
- 2.Cooper RR. Nerves in cortical bone. Science. 1968;160:327–328. doi: 10.1126/science.160.3825.327. [DOI] [PubMed] [Google Scholar]
- 3.Calvo W, Forteza-Vila J. On the development of bone marrow innervation in new-born rats as studied with silver impregnation and electron microscopy. Am J Anat. 1969;126:355–371. doi: 10.1002/aja.1001260308. [DOI] [PubMed] [Google Scholar]
- 4.Duncan CP, Shim SS. J Edouard Samson Address: the autonomic nerve supply of bone. An experimental study of the intraosseous adrenergic nervi vasorum in the rabbit. J Bone Joint Surg. (Br) 1977;59:323–330. doi: 10.1302/0301-620X.59B3.19482. [DOI] [PubMed] [Google Scholar]
- 5.Hohmann EL, Elde RP, Rysavy JA, et al. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 6.Bhangu PS, Genever PG, Spencer GJ, et al. Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone. 2001;29:16–23. doi: 10.1016/s8756-3282(01)00482-3. [DOI] [PubMed] [Google Scholar]
- 7.Serre CM, Farlay D, Delmas PD, et al. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/s8756-3282(99)00215-x. [DOI] [PubMed] [Google Scholar]
- 8.Chenu C, Serre CM, Raynal C, et al. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–299. doi: 10.1016/s8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- 9.Espinosa L, Itzstein C, Cheynel H, et al. Active NMDA glutamate receptors are expressed by mammalian osteoclasts. J Physiol. 1999;518( Pt 1):47–53. doi: 10.1111/j.1469-7793.1999.0047r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Y, Genever PG, Skerry TM, et al. The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcif Tissue Int. 2002;70:194–203. doi: 10.1007/s00223-001-2004-z. [DOI] [PubMed] [Google Scholar]
- 11.Laketic-Ljubojevic I, Suva LJ, Maathuis FJ, et al. Functional characterization of N-methyl-D-aspartic acid-gated channels in bone cells. Bone. 1999;25:631–637. doi: 10.1016/s8756-3282(99)00224-0. [DOI] [PubMed] [Google Scholar]
- 12.Peet NM, Grabowski PS, Laketic-Ljubojevic I, et al. The glutamate receptor antagonist MK801 modulates bone resorption in vitro by a mechanism predominantly involving osteoclast differentiation. Faseb J. 1999;13:2179–2185. doi: 10.1096/fasebj.13.15.2179. [DOI] [PubMed] [Google Scholar]
- 13.Dobson KR, Skerry TM. The NMDA-type glutamate receptor antagonist MK801 regulates differentiation of rat bone marrow osteoprogenitors and influences adipigenesis. J Bone Miner Res. 2000;15:S272. [Google Scholar]
- 14.Mason DJ, Suva LJ, Genever PG, et al. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20:199–205. doi: 10.1016/s8756-3282(96)00386-9. [DOI] [PubMed] [Google Scholar]
- 15.Taylor AF, Brabbs AC, Peet NM, et al. Bone formation/resorption and osteoblast/adipocyte plasticity mediated by AMPA/Kainate receptors in vitro and in vivo. J Bone Miner Res. 2000;14:S37. [Google Scholar]
- 16.Skerry TM, Taylor AF. Glutamate signalling in bone. Curr Pharm Des. 2001;7:737–750. doi: 10.2174/1381612013397771. [DOI] [PubMed] [Google Scholar]
- 17.Bjurholm A, Kreicbergs A, Brodin E, et al. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–171. doi: 10.1016/0196-9781(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 18.Hill EL, Elde R. Calcitonin gene-related peptide-immunoreactive nerve fibers in mandibular periosteum of rat: evidence for primary afferent origin. Neurosci Lett. 1988;85:172–178. doi: 10.1016/0304-3940(88)90347-3. [DOI] [PubMed] [Google Scholar]
- 19.Hukkanen M, Konttinen YT, Rees RG, et al. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J Rheumatol. 1992;19:1252–1259. [PubMed] [Google Scholar]
- 20.Imai S, Matsusue Y. Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Tech. 2002;58:61–69. doi: 10.1002/jemt.10119. [DOI] [PubMed] [Google Scholar]
- 21.Imai S, Tokunaga Y, Maeda T, et al. Calcitonin gene-related peptide, substance P, and tyrosine hydroxylase-immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res. 1997;15:133–140. doi: 10.1002/jor.1100150120. [DOI] [PubMed] [Google Scholar]
- 22.Bjurholm A, Kreicbergs A, Schultzberg M, et al. Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR-106-01, ROS 17/2.8, MC3T3-E1, and Saos-2) and primary bone cells. J Bone Miner Res. 1992;7:1011–1019. doi: 10.1002/jbmr.5650070903. [DOI] [PubMed] [Google Scholar]
- 23.Michelangeli VP, Fletcher AE, Allan EH, et al. Effects of calcitonin gene-related peptide on cyclic AMP formation in chicken, rat, and mouse bone cells. J Bone Miner Res. 1989;4:269–272. doi: 10.1002/jbmr.5650040220. [DOI] [PubMed] [Google Scholar]
- 24.Vignery A, McCarthy TL. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone. 1996;18:331–335. doi: 10.1016/8756-3282(96)00017-8. [DOI] [PubMed] [Google Scholar]
- 25.Sakagami Y, Girasole G, Yu XP, et al. Stimulation of interleukin-6 production by either calcitonin gene-related peptide or parathyroid hormone in two phenotypically distinct bone marrow-derived murine stromal cell lines. J Bone Miner Res. 1993;8:811–816. doi: 10.1002/jbmr.5650080706. [DOI] [PubMed] [Google Scholar]
- 26.Millet I, Vignery A. The neuropeptide calcitonin gene-related peptide inhibits TNF-alpha but poorly induces IL-6 production by fetal rat osteoblasts. Cytokine. 1997;9:999–1007. doi: 10.1006/cyto.1997.0245. [DOI] [PubMed] [Google Scholar]
- 27.Drissi H, Hott M, Marie PJ, et al. Expression of the CT/CGRP gene and its regulation by dibutyryl cyclic adenosine monophosphate in human osteoblastic cells. J Bone Miner Res. 1997;12:1805–1814. doi: 10.1359/jbmr.1997.12.11.1805. [DOI] [PubMed] [Google Scholar]
- 28.Ballica R, Valentijn K, Khachatryan A, et al. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J Bone Miner Res. 1999;14:1067–1074. doi: 10.1359/jbmr.1999.14.7.1067. [DOI] [PubMed] [Google Scholar]
- 29.Hukkanen M, Konttinen YT, Santavirta S, et al. Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience. 1993;54:969–979. doi: 10.1016/0306-4522(93)90588-7. [DOI] [PubMed] [Google Scholar]
- 30.Aoki M, Tamai K, Saotome K. Substance P- and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. Int Orthop. 1994;18:317–324. doi: 10.1007/BF00180235. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi M, Chambers TJ, Gaines Das RE, et al. A direct action of human calcitonin gene-related peptide on isolated osteoclasts. J Endocrinol. 1987;115:511–518. doi: 10.1677/joe.0.1150511. [DOI] [PubMed] [Google Scholar]
- 32.Roos BA, Fischer JA, Pignat W, et al. Evaluation of the in vivo and in vitro calcium-regulating actions of noncalcitonin peptides produced via calcitonin gene expression. Endocrinology. 1986;118:46–51. doi: 10.1210/endo-118-1-46. [DOI] [PubMed] [Google Scholar]
- 33.Struthers AD, Brown MJ, Macdonald DW, et al. Human calcitonin gene related peptide: a potent endogenous vasodilator in man. Clin Sci (Lond) 1986;70:389–393. doi: 10.1042/cs0700389. [DOI] [PubMed] [Google Scholar]
- 34.Bjurholm A, Kreicbergs A, Terenius L, et al. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25:119–125. doi: 10.1016/0165-1838(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 35.Hill EL, Elde R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg P, Lundgren I, Mukohyama H, et al. Vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide receptor subtypes in mouse calvarial osteoblasts: presence of VIP-2 receptors and differentiation-induced expression of VIP-1 receptors. Endocrinology. 2001;142:339–347. doi: 10.1210/endo.142.1.7912. [DOI] [PubMed] [Google Scholar]
- 37.Ransjo M, Lie A, Mukohyama H, et al. Microisolated mouse osteoclasts express VIP-1 and PACAP receptors. Biochem Biophys Res Commun. 2000;274:400–404. doi: 10.1006/bbrc.2000.3151. [DOI] [PubMed] [Google Scholar]
- 38.Togari A, Arai M, Mizutani S, et al. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233:125–128. doi: 10.1016/s0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg P, Bostrom I, Mukohyama H, et al. Neuro-hormonal control of bone metabolism: vasoactive intestinal peptide stimulates alkaline phosphatase activity and mRNA expression in mouse calvarial osteoblasts as well as calcium accumulation mineralized bone nodules. Regul Pept. 1999;85:47–58. doi: 10.1016/s0167-0115(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 40.Mukohyama H, Ransjo M, Taniguchi H, et al. The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem Biophys Res Commun. 2000;271:158–163. doi: 10.1006/bbrc.2000.2599. [DOI] [PubMed] [Google Scholar]
- 41.Cai Y, Xin X, Shim GJ, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) stimulate interleukin-6 production through the third subtype of PACAP/VIP receptor in rat bone marrow-derived stromal cells. Endocrinology. 1997;138:2515–2520. doi: 10.1210/endo.138.6.5169. [DOI] [PubMed] [Google Scholar]
- 42.Lerner UH, Lundberg P, Ransjo M, et al. Helodermin, helospectin, and PACAP stimulate cyclic AMP formation in intact bone, isolated osteoblasts, and osteoblastic cell lines. Calcif Tissue Int. 1994;54:284–289. doi: 10.1007/BF00295952. [DOI] [PubMed] [Google Scholar]
- 43.Winding B, Wiltink A, Foged NT. Pituitary adenylyl cyclase-activating polypeptides and vasoactive intestinal peptide inhibit bone resorption by isolated rabbit osteoclasts. Exp Physiol. 1997;82:871–886. doi: 10.1113/expphysiol.1997.sp004070. [DOI] [PubMed] [Google Scholar]
- 44.Strange-Vognsen HH, Arnbjerg J, Hannibal J. Immunocytochemical demonstration of pituitary adenylate cyclase activating polypeptide (PACAP) in the porcine epiphyseal cartilage canals. Neuropeptides. 1997;31:137–141. doi: 10.1016/s0143-4179(97)90082-2. [DOI] [PubMed] [Google Scholar]
- 45.Moore RE, Smith CK, 2nd, Bailey CS, et al. Characterization of beta-adrenergic receptors on rat and human osteoblast-like cells and demonstration that beta-receptor agonists can stimulate bone resorption in organ culture. Bone Miner. 1993;23:301–315. doi: 10.1016/s0169-6009(08)80105-5. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, Palmer G, Bonjour JP, et al. Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone. 1998;23:197–203. doi: 10.1016/s8756-3282(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 47.Kusaka M, Oshima T, Yokota K, et al. Possible induction of fatty acid cyclooxygenase in mouse osteoblastic cells (MC3T3-E1) by cAMP. Biochim Biophys Acta. 1988;972:339–346. doi: 10.1016/0167-4889(88)90210-8. [DOI] [PubMed] [Google Scholar]
- 48.Oshima T, Yoshimoto T, Yamamoto S, et al. cAMP-dependent induction of fatty acid cyclooxygenase mRNA in mouse osteoblastic cells (MC3T3-E1) J Biol Chem. 1991;266:13621–13626. [PubMed] [Google Scholar]
- 49.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 50.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 51.Zeng QQ, Jee WS, Bigornia AE, et al. Time responses of cancellous and cortical bones to sciatic neurectomy in growing female rats. Bone. 1996;19:13–21. doi: 10.1016/8756-3282(96)00112-3. [DOI] [PubMed] [Google Scholar]
- 52.Kingery WS, Offley SC, Guo TZ, et al. A substance P receptor (NK1) antagonist enhances the widespread osteoporotic effects of sciatic nerve section. Bone. 2003;33:927–936. doi: 10.1016/j.bone.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Sakai A, Nakamura T, Tsurukami H, et al. Bone marrow capacity for bone cells and trabecular bone turnover in immobilized tibia after sciatic neurectomy in mice. Bone. 1996;18:479–486. doi: 10.1016/8756-3282(96)00042-7. [DOI] [PubMed] [Google Scholar]
- 54.Ito M, Nishida A, Nakamura T. Differences of three-dimensional trabecular microstructure in osteopenic rat models caused by ovariectomy and neurectomy. Bone. 2002;30:594–598. doi: 10.1016/s8756-3282(02)00684-1. [DOI] [PubMed] [Google Scholar]
- 55.Iwamoto J, Takeda T, Ichimura S, et al. Comparative effects of orchidectomy and sciatic neurectomy on cortical and cancellous bone in young growing rats. J Bone Miner Metab. 2003;21:211–216. doi: 10.1007/s00774-003-0411-0. [DOI] [PubMed] [Google Scholar]
- 56.Brighton CT, Tadduni GT, Pollack SR. Treatment of sciatic denervation disuse osteoporosis in the rat tibia with capacitively coupled electrical stimulation. Dose response and duty cycle. J Bone Joint Surg. (Am) 1985;67:1022–1028. [PubMed] [Google Scholar]
- 57.Iwamoto J, Takeda T, Katsumata T, et al. Effect of etidronate on bone in orchidectomized and sciatic neurectomized adult rats. Bone. 2002;30:360–367. doi: 10.1016/s8756-3282(01)00687-1. [DOI] [PubMed] [Google Scholar]
- 58.Dietz FR. Effect of peripheral nerve on limb development. J Orthop Res. 1987;5:576–585. doi: 10.1002/jor.1100050413. [DOI] [PubMed] [Google Scholar]
- 59.Hukkanen M, Konttinen YT, Santavirta S, et al. Effect of sciatic nerve section on neural ingrowth into the rat tibial fracture callus. Clin Orthop. 1995. pp. 247–257. [PubMed]
- 60.Madsen JE, Aune AK, Falch JA, et al. Neural involvement in post-traumatic osteopenia: an experimental study in the rat. Bone. 1996;18:411–416. doi: 10.1016/8756-3282(96)00027-0. [DOI] [PubMed] [Google Scholar]
- 61.O'Connor BL, Palmoski MJ, Brandt KD. Neurogenic acceleration of degenerative joint lesions. J Bone Joint Surg. (Am) 1985;67:562–572. [PubMed] [Google Scholar]
- 62.Hill EL, Turner R, Elde R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience. 1991;44:747–755. doi: 10.1016/0306-4522(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 63.Cherruau M, Morvan FO, Schirar A, et al. Chemical sympathectomy-induced changes in TH-, VIP-, and CGRP-immunoreactive fibers in the rat mandible periosteum: influence on bone resorption. J Cell Physiol. 2003;194:341–348. doi: 10.1002/jcp.10209. [DOI] [PubMed] [Google Scholar]
- 64.Adam C, Llorens A, Baroukh B, et al. Effects of capsaicin-induced sensory denervation on osteoclastic resorption in adult rats. Exp Physiol. 2000;85:62–66. doi: 10.1017/s0958067000019308. [DOI] [PubMed] [Google Scholar]
- 65.Ramnemark A, Nyberg L, Lorentzon R, et al. Hemiosteoporosis after severe stroke, independent of changes in body composition and weight. Stroke. 1999;30:755–760. doi: 10.1161/01.str.30.4.755. [DOI] [PubMed] [Google Scholar]
- 66.Maimoun L, Couret I, Micallef JP, et al. Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism. 2002;51:958–963. doi: 10.1053/meta.2002.34013. [DOI] [PubMed] [Google Scholar]
- 67.Warden SJ, Bennell KL, Matthews B, et al. Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporos Int. 2002;13:586–592. doi: 10.1007/s001980200077. [DOI] [PubMed] [Google Scholar]
- 68.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 69.Bickel CS, Slade JM, Haddad F, et al. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol. 2003;94:2255–2262. doi: 10.1152/japplphysiol.00014.2003. [DOI] [PubMed] [Google Scholar]
- 70.Frost HM. From Wolff's law to the Utah paradigm: insights about bone physiology and its clinical applications. Anat Rec. 2001;262:398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 71.Singh RS, Craig MC, Katholi CR, et al. The predictive value of creatine phosphokinase and alkaline phosphatase in identification of heterotopic ossification in patients after spinal cord injury. Arch Phys Med Rehabil. 2003;84:1584–1588. doi: 10.1053/s0003-9993(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 72.Bravo-Payno P, Esclarin A, Arzoz T, et al. Incidence and risk factors in the appearance of heterotopic ossification in spinal cord injury. Paraplegia. 1992;30:740–745. doi: 10.1038/sc.1992.142. [DOI] [PubMed] [Google Scholar]
- 73.Lal S, Hamilton BB, Heinemann A, et al. Risk factors for heterotopic ossification in spinal cord injury. Arch Phys Med Rehabil. 1989;70:387–390. [PubMed] [Google Scholar]
- 74.Myerson MS, Henderson MR, Saxby T, et al. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int. 1994;15:233–241. doi: 10.1177/107110079401500502. [DOI] [PubMed] [Google Scholar]
- 75.Childs M, Armstrong DG, Edelson GW. Is Charcot arthropathy a late sequela of osteoporosis in patients with diabetes mellitus? J Foot Ankle Surg. 1998;37:437–449. doi: 10.1016/s1067-2516(98)80054-9. discussion 49. [DOI] [PubMed] [Google Scholar]
- 76.Herbst SA, Jones KB, Saltzman CL. Pattern of Diabetic Neuropathic Osteoarthropathy Associated With Peripheral Skeletal Bone Mineral Density. J Bone Joint Surg. Br. 2004. In Press. [DOI] [PubMed]
- 77.Jirkovska A, Kasalicky P, Boucek P, et al. Calcaneal ultrasonometry in patients with Charcot osteoarthropathy and its relationship with densitometry in the lumbar spine and femoral neck and with markers of bone turnover. Diabet Med. 2001;18:495–500. doi: 10.1046/j.1464-5491.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- 78.Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17:1896–1903. doi: 10.1359/jbmr.2002.17.10.1896. [DOI] [PubMed] [Google Scholar]
- 79.Einhorn TA. Brain, bone, and body mass: fat is beautiful again. J Bone Joint Surg. (Am) 2001;83-A:1782. doi: 10.2106/00004623-200112000-00002. [DOI] [PubMed] [Google Scholar]


