Abstract
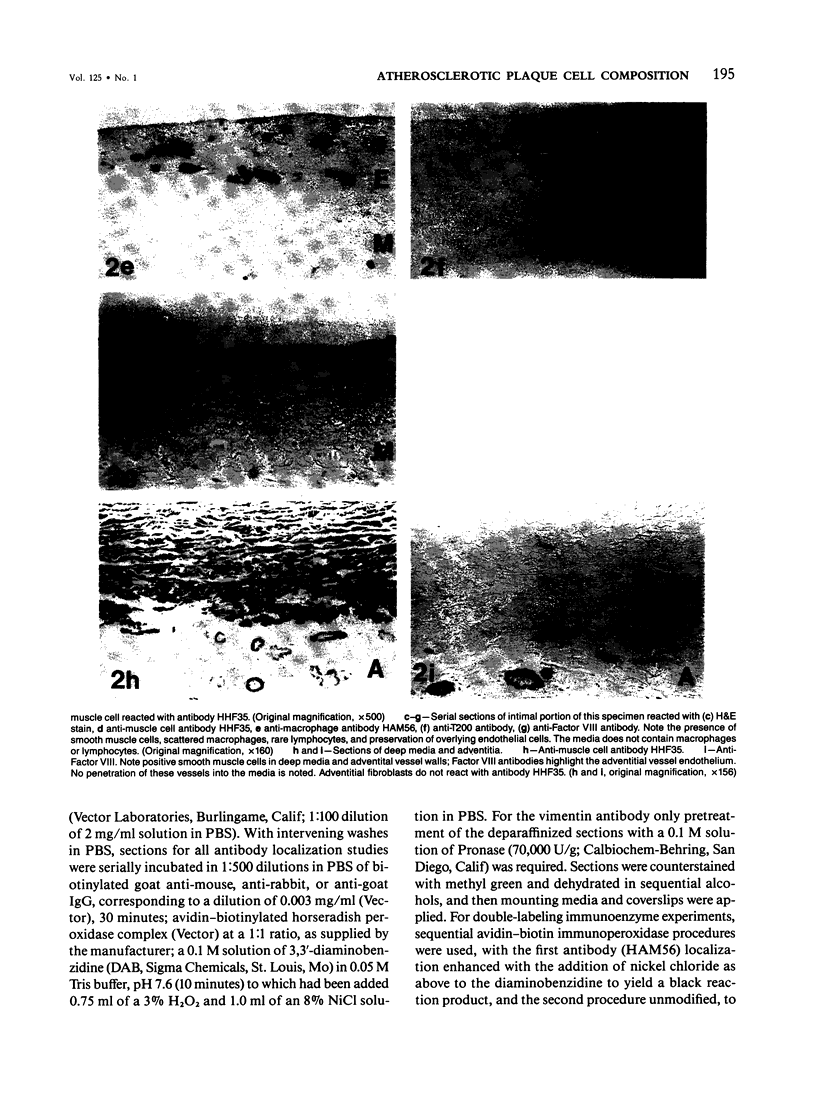
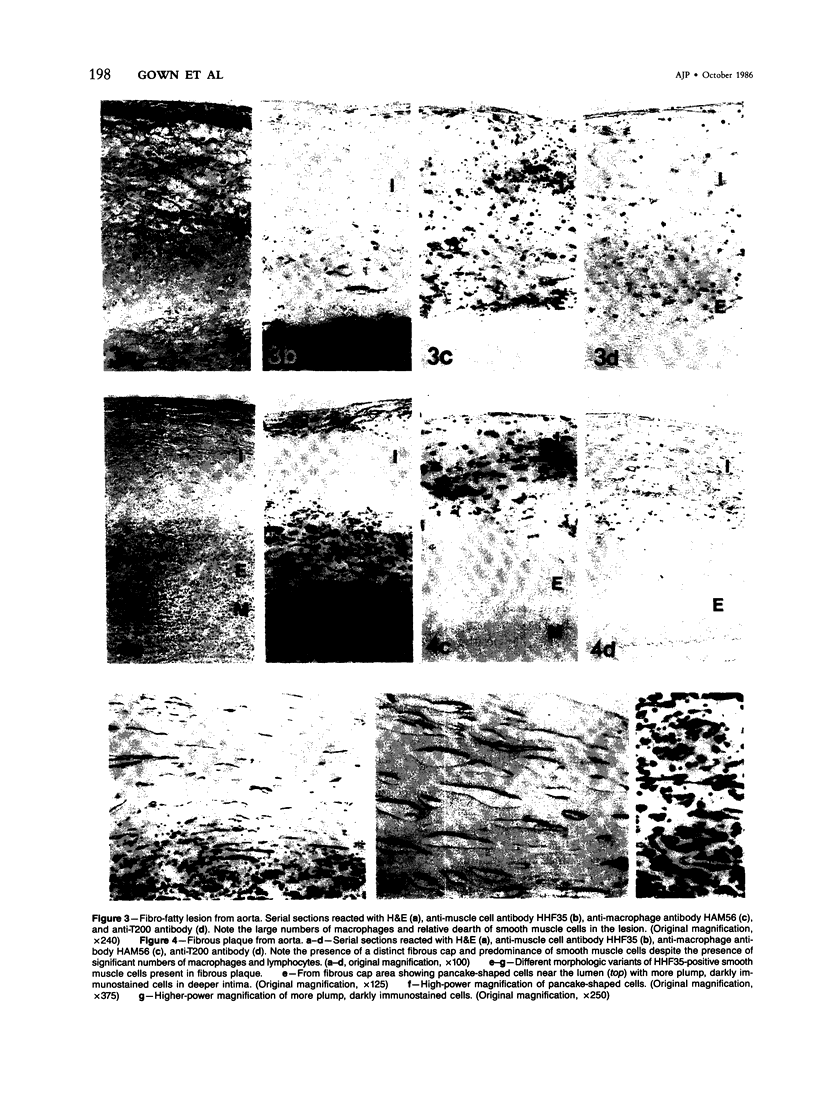
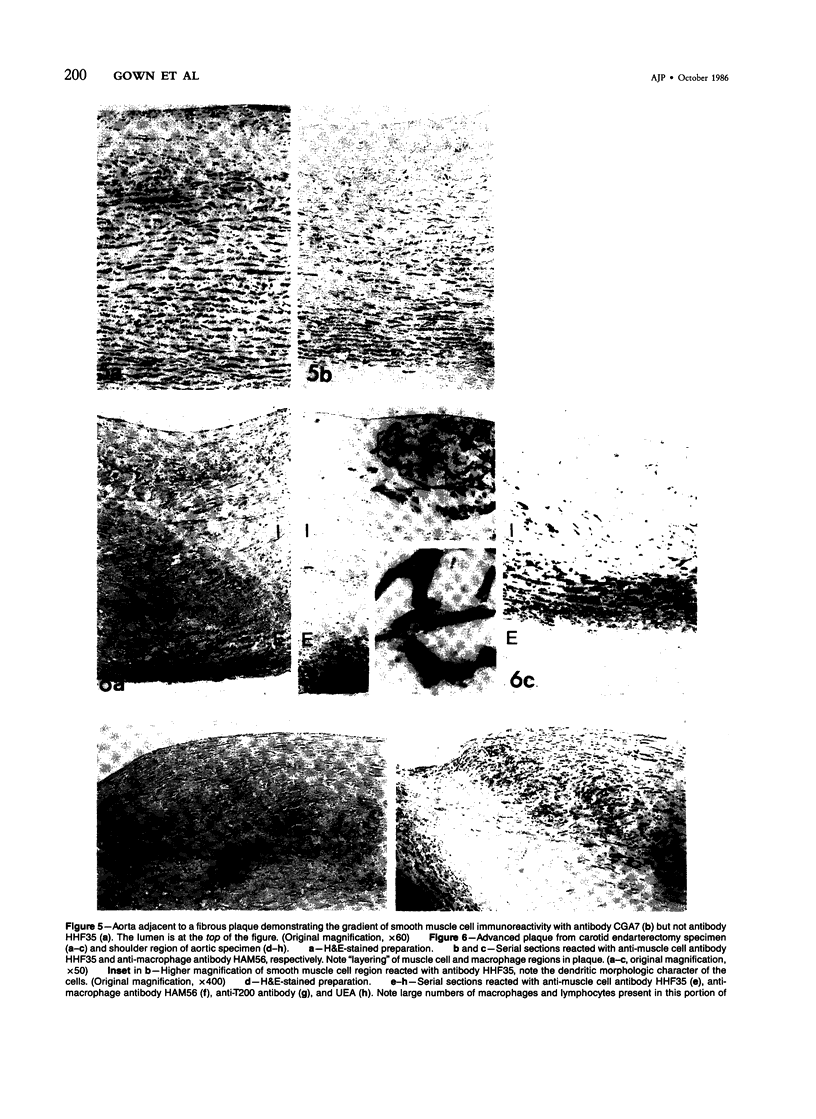
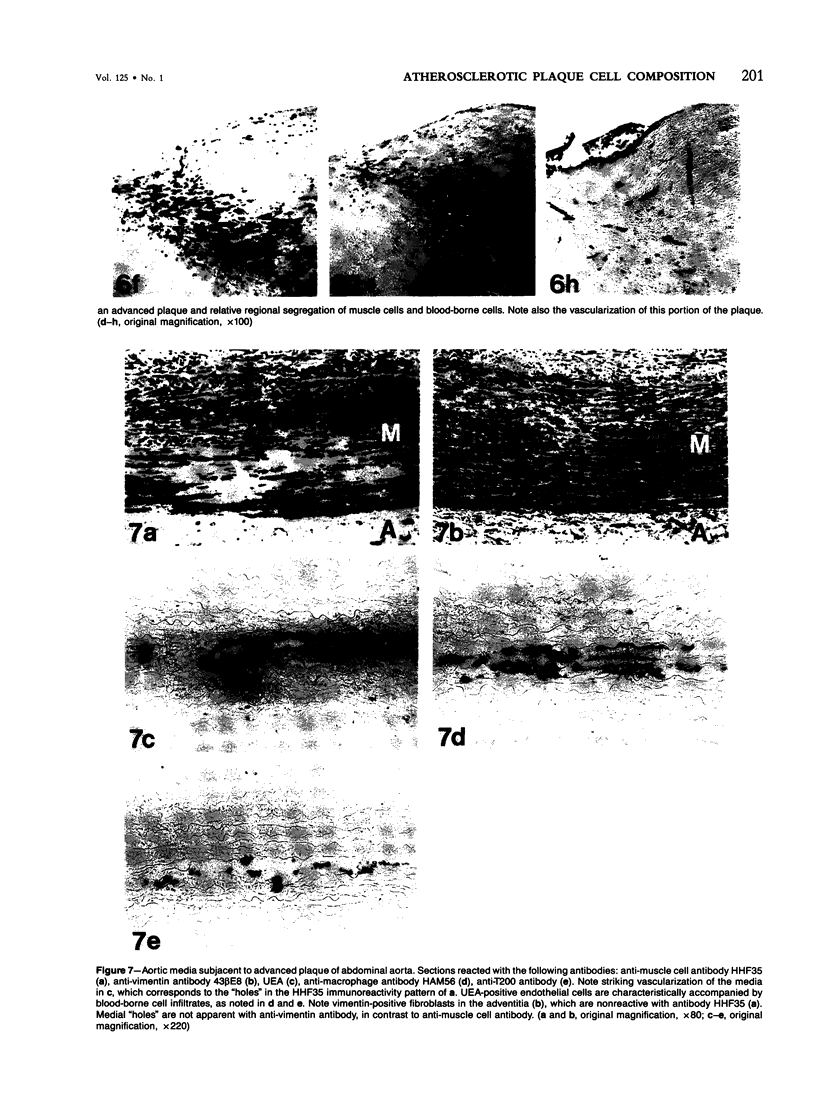
The authors have performed immunocytochemical investigations of the distribution of various cell types in human atherosclerotic plaques using monoclonal antibodies specific to smooth muscle cells (CGA7 [Gown et al, J Cell Biol 1985, 100:807-813] and HHF35 [Tsukada et al, Am J Pathol (In press)] ); lymphocytes (T200 antigen); endothelial cells (Factor VIII and the Ulex europeus agglutinin); and macrophages, the latter with a new macrophage-specific antibody HAM56. All studies were performed on methanol-Carnoy's-fixed, paraffin-embedded tissues. In areas of grossly normal aorta, significant numbers of macrophages were noted within areas of diffuse intimal thickening. The cellular composition of the following three types of raised lesions were analyzed: fibro-fatty lesions, which, despite their gross appearance, consistent with fibrous plaques, were composed almost exclusively of macrophages and lymphocytes and almost devoid of smooth muscle cells; fibrous plaques, which were predominantly composed of smooth muscle cells displaying considerable morphologic heterogeneity and an admixture of blood-borne cells; advanced plaques, which were characterized by complex layers of smooth muscle cells and macrophages with considerable variation from region to region. Also noted were foci of medial and even intimal vascularization subjacent to the more advanced plaques. These studies demonstrate the application of monoclonal antibody technology to the study of the cellular composition of human atherosclerotic lesions.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Identification of macrophages and smooth muscle cells in human atherosclerosis using monoclonal antibodies. J Pathol. 1985 Jul;146(3):197–204. doi: 10.1002/path.1711460306. [DOI] [PubMed] [Google Scholar]
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 1984 Dec;53(3):265–271. doi: 10.1016/0021-9150(84)90127-8. [DOI] [PubMed] [Google Scholar]
- Barboriak J. J., Yorde D. E., Huang C. J. Apolipoprotein B (Apo B) in vein graft atherosclerosis. An immunoperoxidase study. Atherosclerosis. 1980 Nov;37(3):403–407. doi: 10.1016/0021-9150(80)90145-8. [DOI] [PubMed] [Google Scholar]
- Barger A. C., Beeuwkes R., 3rd, Lainey L. L., Silverman K. J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984 Jan 19;310(3):175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Charbord P., Gown A. M., Keating A., Singer J. W. CGA-7 and HHF, two monoclonal antibodies that recognize muscle actin and react with adherent cells in human long-term bone marrow cultures. Blood. 1985 Nov;66(5):1138–1142. [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Davis H. R., Vesselinovitch D., Wissler R. W. Histochemical detection and quantification of macrophages in rhesus and cynomolgus monkey atherosclerotic lesions. J Histochem Cytochem. 1984 Dec;32(12):1319–1327. doi: 10.1177/32.12.6501864. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Fowler S., Berberian P. A., Shio H., Goldfischer S., Wolinsky H. Characterization of cell populations isolated from aortas of rhesus monkeys with experimental atherosclerosis. Circ Res. 1980 Apr;46(4):520–530. doi: 10.1161/01.res.46.4.520. [DOI] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Fritz K. E., Daoud A. S., Jarmolych J. Non-specific esterase activity during regression of swine aortic atherosclerosis. Artery. 1980;7(5):352–366. [PubMed] [Google Scholar]
- GEIRINGER E. Intimal vascularization and atherosclerosis. J Pathol Bacteriol. 1951 Apr;63(2):201–211. doi: 10.1002/path.1700630204. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Rungger-Brändle E., de Chastonay C., Franke W. W. Vimentin-containing smooth muscle cells in aortic intimal thickening after endothelial injury. Lab Invest. 1982 Sep;47(3):265–269. [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gladstone, Pious D. Stable variants affecting B cell alloantigens in human lymphoid cells. Nature. 1978 Feb 2;271(5644):459–461. doi: 10.1038/271459a0. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Gordon D., Lu P. L. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985 Mar;100(3):807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H. F., Ruggles B. M., Bond M. G. A technique for localizing LDL by immunofluorescence in formalin-fixed and paraffin-embedded atherosclerotic lesions. Artery. 1980;6(4):328–339. [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Kariniemi A. L., Hormia M., Linder E., Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest. 1982 Jul;47(1):60–66. [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A. Endothelial cells and the biology of factor VIII. N Engl J Med. 1977 Feb 17;296(7):377–383. doi: 10.1056/NEJM197702172960707. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Klurfeld D. M. Identification of foam cells in human atherosclerotic lesions as macrophages using monoclonal antibodies. Arch Pathol Lab Med. 1985 May;109(5):445–449. [PubMed] [Google Scholar]
- Knieriem H. J., Kao V. C., Wissler R. W. Actomyosin and myosin and the deposition of lipids and serum lipoproteins. Arch Pathol. 1967 Aug;84(2):118–129. [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- McCullagh K. G., Duance V. C., Bishop K. A. The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol. 1980 Jan;130(1):45–55. doi: 10.1002/path.1711300107. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Karpova I. I., Tertov V. V., Rudchenko S. A., Andreeva E. R., Krushinsky A. V., Smirnov V. N. Cellular composition of atherosclerotic and uninvolved human aortic subendothelial intima. Light-microscopic study of dissociated aortic cells. Am J Pathol. 1984 Apr;115(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Schaefer H. E. The role of macrophages in atherosclerosis. Haematol Blood Transfus. 1981;27:137–142. doi: 10.1007/978-3-642-81696-3_15. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schütte H. E. Changes in the vasa vasorum of the atherosclerotic aortic wall. Angiologica. 1968;5(3):210–222. [PubMed] [Google Scholar]
- Schütte H. E. Plaque localization and distribution of vasa vasorum. A micro-angiological study of the human abdominal aorta. Angiologica. 1966;3(1):21–39. [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatskii S. P., Rudin A. V., Rukosuev V. S. Immunomorfologicheskaia kharakteristika raspredeleniia kollagena I, III, IV, V tipov v normal'noi intime i pri ateroskleroze krupnykh arterii i aorty cheloveka. Arkh Patol. 1984;46(3):18–24. [PubMed] [Google Scholar]
- Vlaicu R., Niculescu F., Rus H. G., Cristea A. Immune deposits in human aortic atherosclerotic wall. Med Interne. 1983 Jan-Mar;21(1):3–8. [PubMed] [Google Scholar]
- Warnke R. A., Gatter K. C., Falini B., Hildreth P., Woolston R. E., Pulford K., Cordell J. L., Cohen B., De Wolf-Peeters C., Mason D. Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N Engl J Med. 1983 Nov 24;309(21):1275–1281. doi: 10.1056/NEJM198311243092102. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- Wolinsky H., Glagov S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circ Res. 1969 Dec;25(6):677–686. doi: 10.1161/01.res.25.6.677. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Turner R. R., Shiurba R. A., Eng L., Warnke R. A. Human dendritic cells and macrophages. In situ immunophenotypic definition of subsets that exhibit specific morphologic and microenvironmental characteristics. Am J Pathol. 1985 Apr;119(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Yomantas S., Elner V. M., Schaffner T., Wissler R. W. Immunohistochemical localization of apolipoprotein B in human atherosclerotic lesions. Arch Pathol Lab Med. 1984 May;108(5):374–378. [PubMed] [Google Scholar]












