Abstract
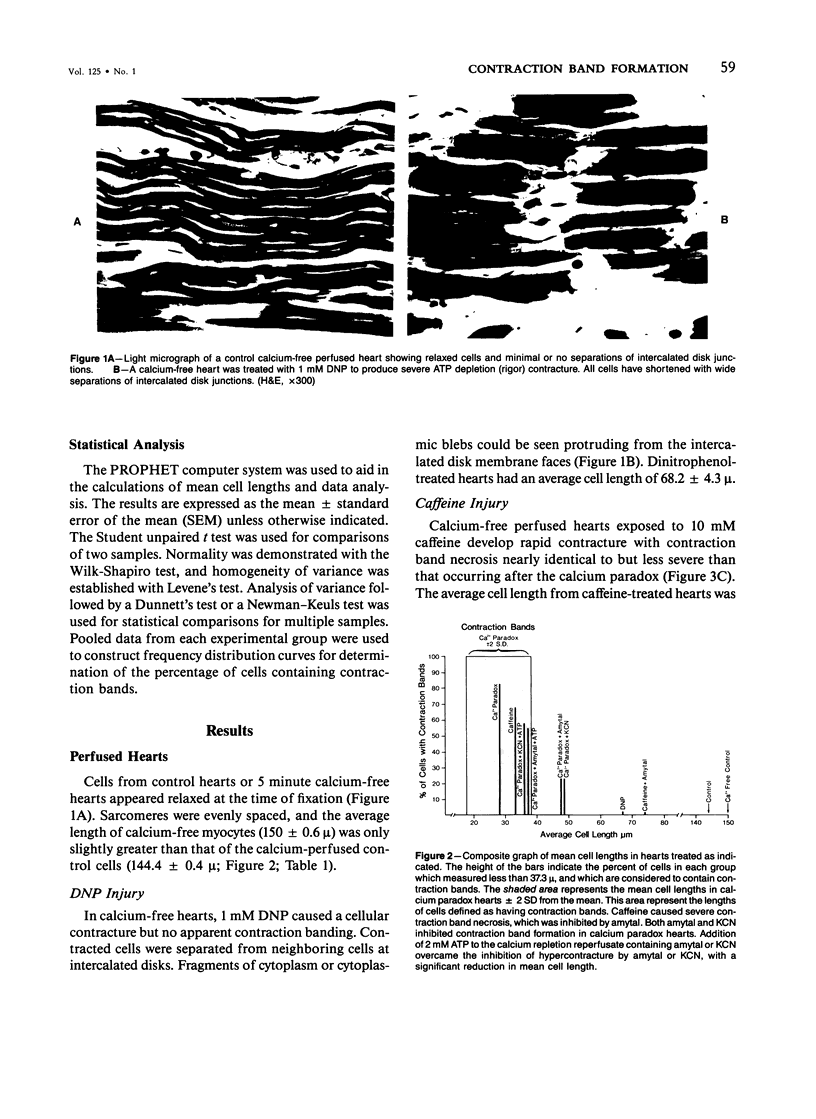
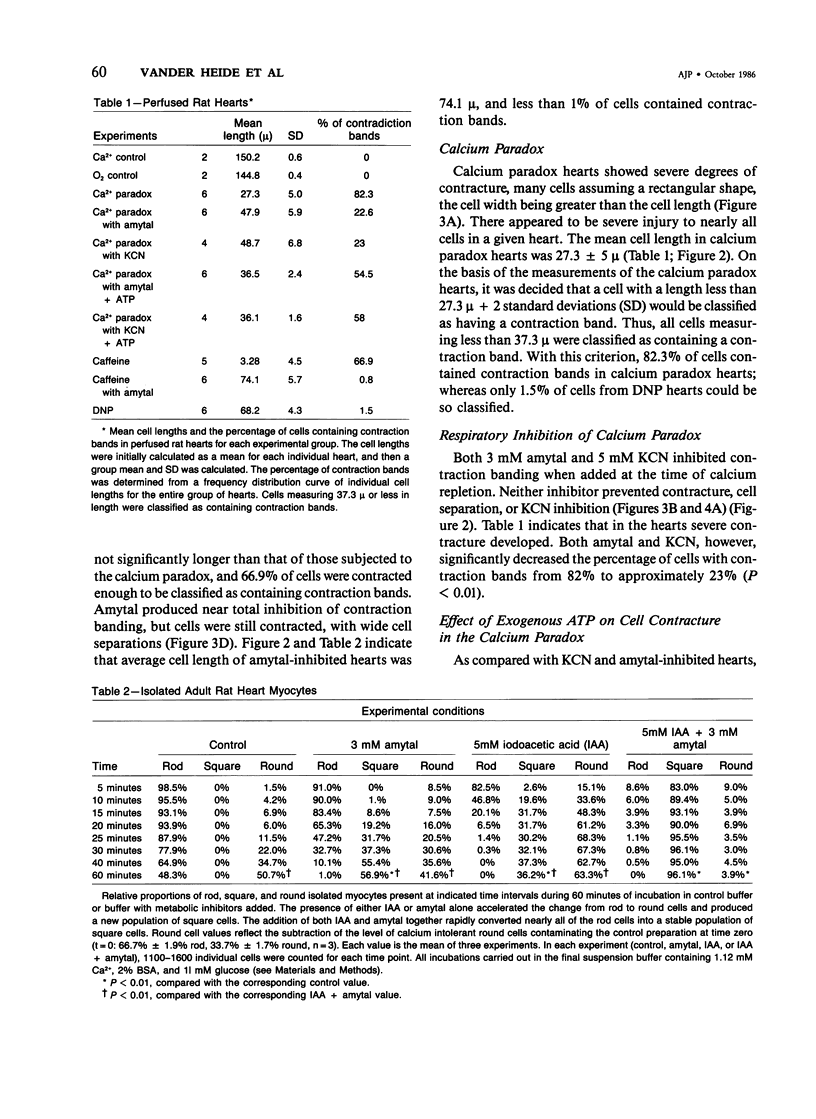
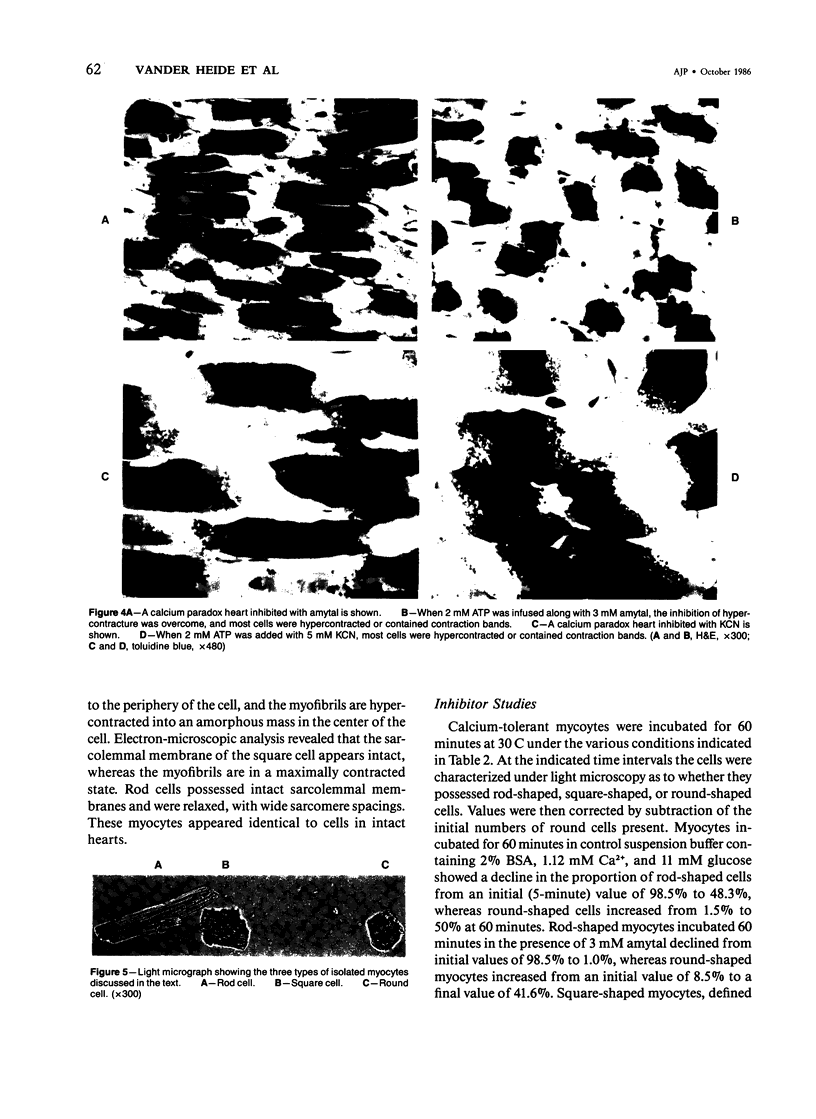
Aggregation of sarcomeres into contraction bands is a prominent feature of the oxygen paradox, the calcium paradox, and caffeine injury to calcium-free perfused hearts. For investigation of the mechanism of contraction banding, it was necessary to devise a method of evaluating the degree of sarcomere contraction and to define objectively a contraction band. Hearts with mechanical detachment of cells caused by hypocalcemic perfusion and isolated myocytes both allow unrestrained contracture of cells and permit direct optical measurements to quantitate the degree of cell contracture. With the use of the calcium paradox as a model of contraction band necrosis, it was found that cells with lengths of less than 37.3 mu could be considered as containing contraction bands. It was found that the mitochondrial inhibitors cyanide and amytal, as well as the uncoupler 2,4-dinitrophenol, allowed cell contracture but inhibited hypercontracture of sarcomeres into contraction bands during both the calcium paradox and caffeine injury to perfused hearts. However, when 2mM adenosine triphosphate (ATP) was included in the perfusion media, contraction band formation occurred despite the continued presence of cyanide or amytal. In isolated myocyte preparations the addition of the glycolytic inhibitor iodoacetate (IAA, 5 mM) and the mitochondrial inhibitor amytal (3 mM) caused relaxed rod-shaped cells (length/width ratio greater than 3:1) to contract into a stable population of square-shaped forms (length/width ratio less than 3:1), indicating an abrupt and severe decline in cellular ATP levels. Removal of amytal from the incubation medium in the presence of IAA produced a significant conversion of square-shaped cells into round-shaped cells containing contraction bands. Either IAA alone or amytal alone resulted in a mixed population of square and round cells. The results indicate that ATP is required for the formation of contraction bands in intact hearts and for the rounding of isolated myocytes. Formation of contraction bands appears to be an energy-dependent process requiring ATP.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuld R. A., Hostetler J. R., Brierley G. P. Response of isolated rat heart cells to hypoxia, re-oxygenation, and acidosis. Circ Res. 1981 Aug;49(2):307–316. doi: 10.1161/01.res.49.2.307. [DOI] [PubMed] [Google Scholar]
- Altschuld R. A., Wenger W. C., Lamka K. G., Kindig O. R., Capen C. C., Mizuhira V., Vander Heide R. S., Brierley G. P. Structural and functional properties of adult rat heart myocytes lysed with digitonin. J Biol Chem. 1985 Nov 15;260(26):14325–14334. [PubMed] [Google Scholar]
- Altschuld R., Gibb L., Ansel A., Hohl C., Kruger F. A., Brierley G. P. Calcium tolerance of isolated rat heart cells. J Mol Cell Cardiol. 1980 Dec;12(12):1383–1395. doi: 10.1016/0022-2828(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Crozatier B., Ashraf M., Franklin D., Ross J., Jr Sarcomere length in experimental myocardial infarction: evidence for sarcomere overstretch in dyskinetic ventricular regions. J Mol Cell Cardiol. 1977 Oct;9(10):785–797. doi: 10.1016/s0022-2828(77)80056-4. [DOI] [PubMed] [Google Scholar]
- Ganote C. E. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983 Feb;15(2):67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Humphrey S. M. Effects of anoxic or oxygenated reperfusion in globally ischemic, isovolumic, perfused rat hearts. Am J Pathol. 1985 Jul;120(1):129–145. [PMC free article] [PubMed] [Google Scholar]
- Ganote C. E., McGarr J., Liu S. Y., Kaltenbach J. P. Oxygen-induced enzyme release. Assessment of mitochondrial function in anoxic myocardial injury and effects of the mitochondrial uncoupling agent 2,4-dinitrophenol (DNP). J Mol Cell Cardiol. 1980 Apr;12(4):387–408. doi: 10.1016/0022-2828(80)90049-8. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. Contracture in isolated adult rat heart cells. Role of Ca2+, ATP, and compartmentation. Circ Res. 1981 Nov;49(5):1119–1128. doi: 10.1161/01.res.49.5.1119. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Hearse D. J. Reperfusion of the ischemic myocardium. J Mol Cell Cardiol. 1977 Aug;9(8):605–616. doi: 10.1016/s0022-2828(77)80357-x. [DOI] [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Hohl C., Ansel A., Altschuld R., Brierley G. P. Contracture of isolated rat heart cells on anaerobic to aerobic transition. Am J Physiol. 1982 Jun;242(6):H1022–H1030. doi: 10.1152/ajpheart.1982.242.6.H1022. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Lambert M. R., Johnson J. D., Lamka K. G., Brierley G. P., Altschuld R. A. Intracellular free Ca2+ and the hypercontracture of adult rat heart myocytes. Arch Biochem Biophys. 1986 Mar;245(2):426–435. doi: 10.1016/0003-9861(86)90234-1. [DOI] [PubMed] [Google Scholar]
- Nayler W. G. Regulation of myocardial function--a subcellular phenomenon. J Mol Cell Cardiol. 1973 Jun;5(3):213–219. doi: 10.1016/0022-2828(73)90062-x. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Chien A. M., Capogrossi M. C., Pelto D. J., Lakatta E. G. Direct observation of the "oxygen paradox" in single rat ventricular myocytes. Circ Res. 1985 Jun;56(6):899–903. doi: 10.1161/01.res.56.6.899. [DOI] [PubMed] [Google Scholar]
- Vander Heide R. S., Ganote C. E. Caffeine-induced myocardial injury in calcium-free perfused rat hearts. Am J Pathol. 1985 Jan;118(1):55–65. [PMC free article] [PubMed] [Google Scholar]