Abstract
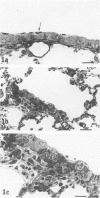
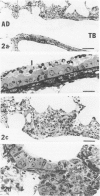
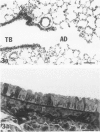
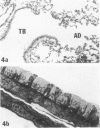
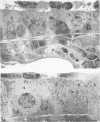
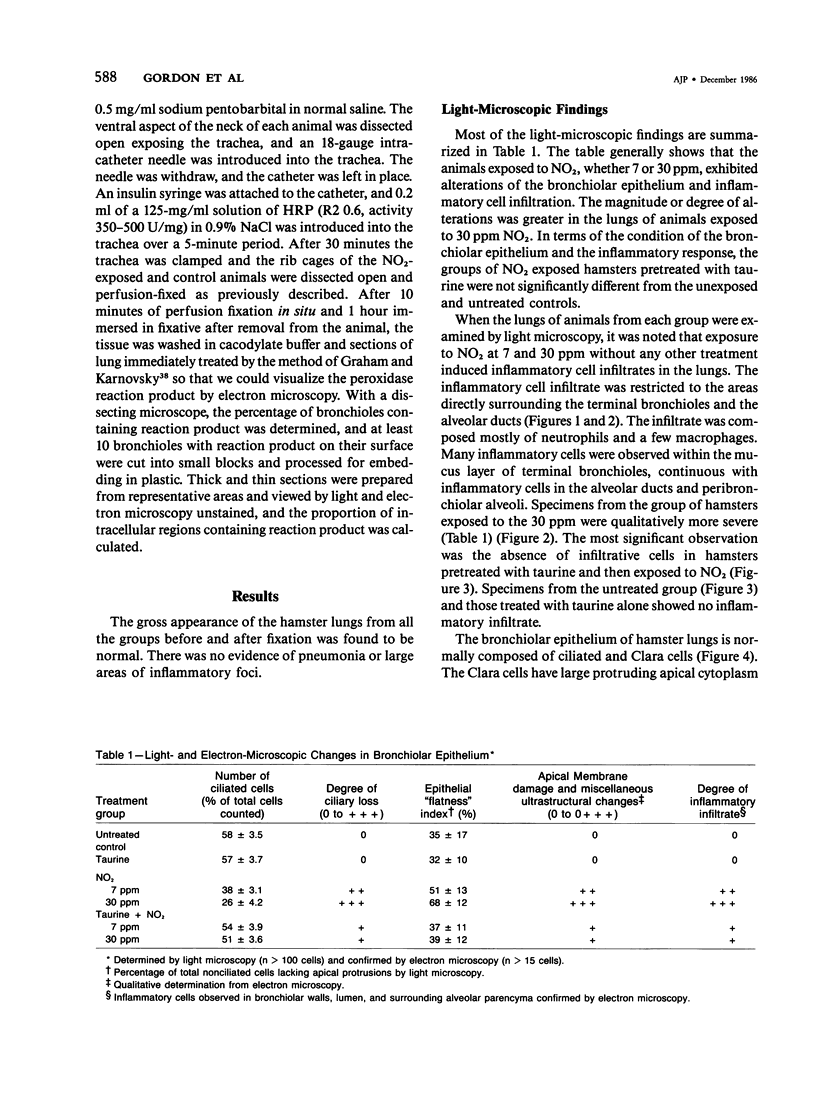
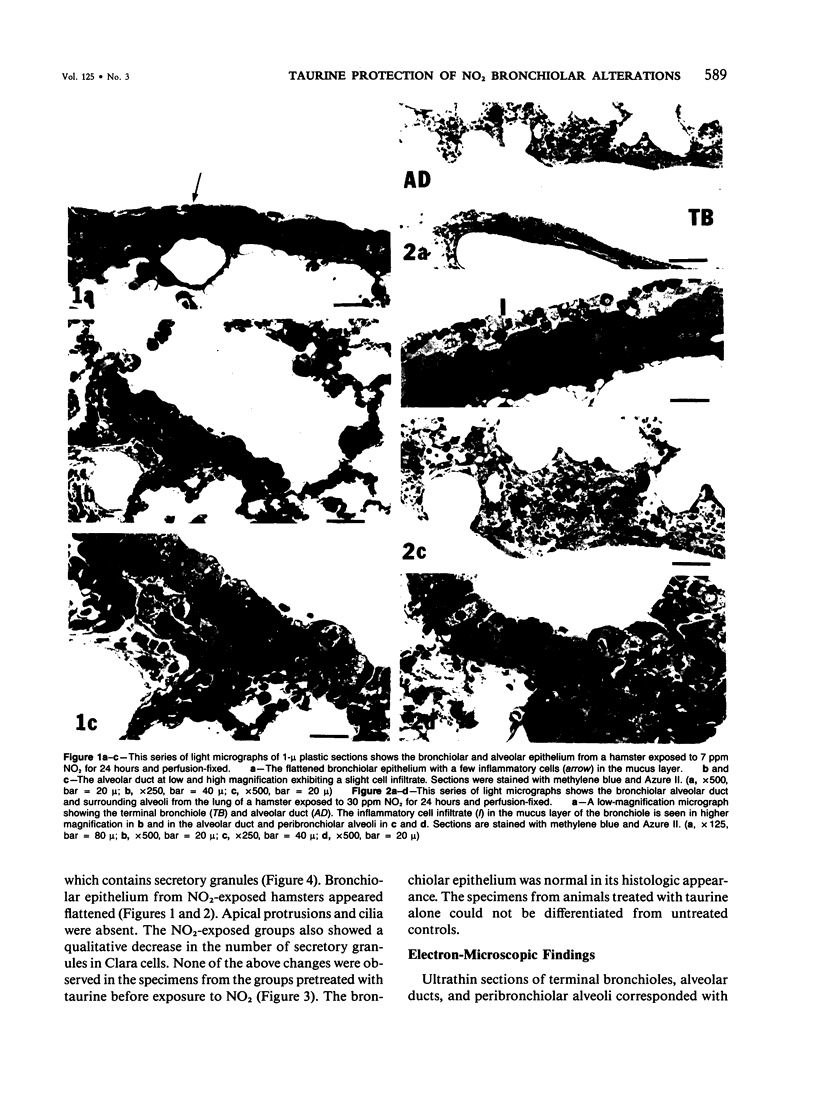
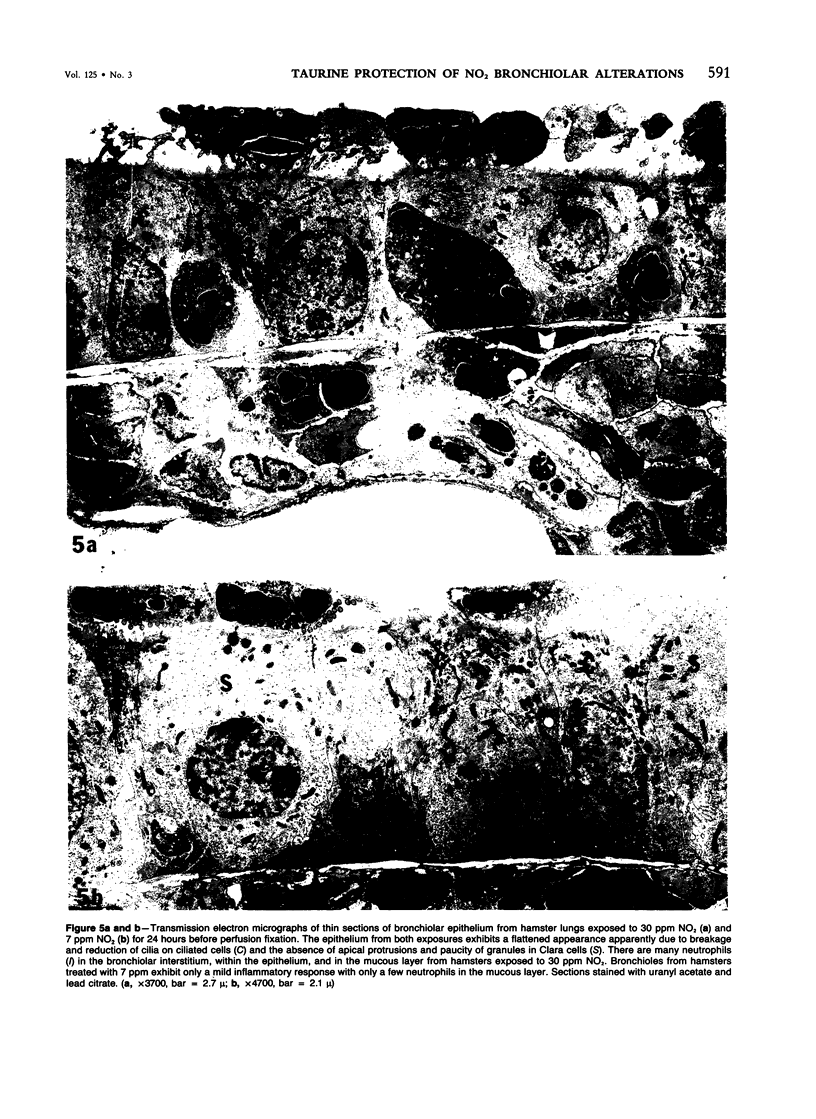
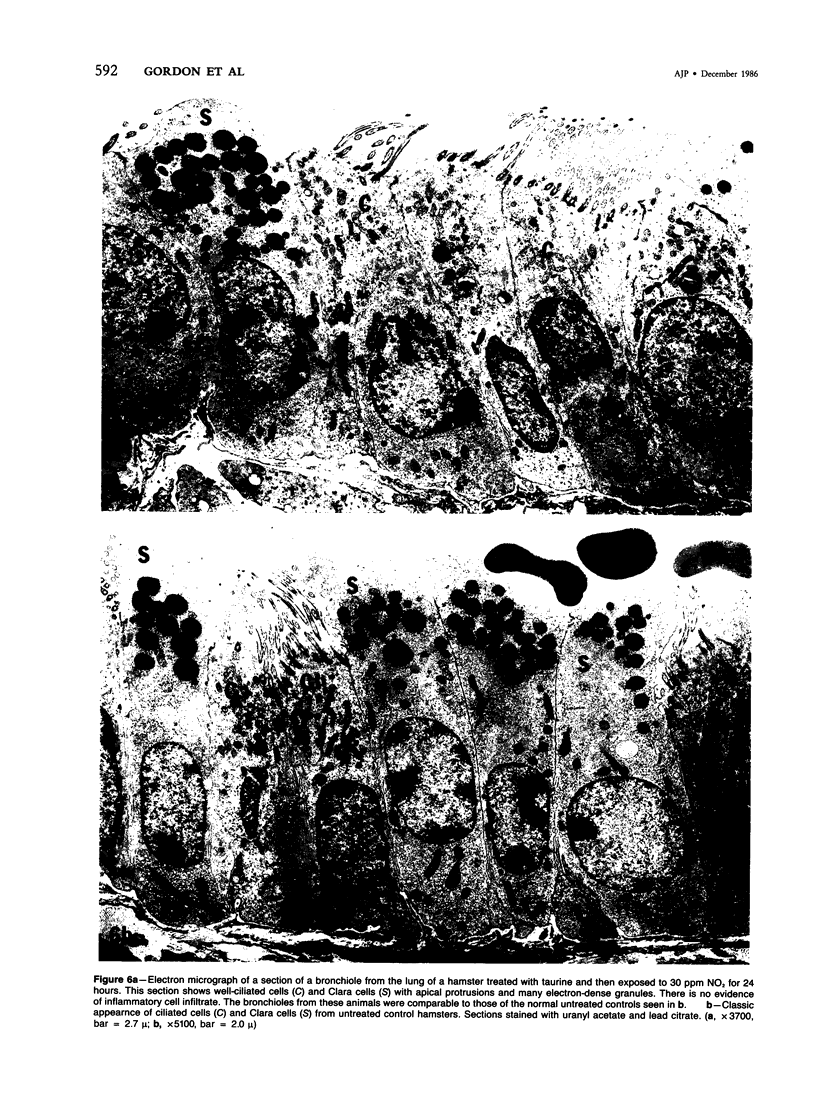
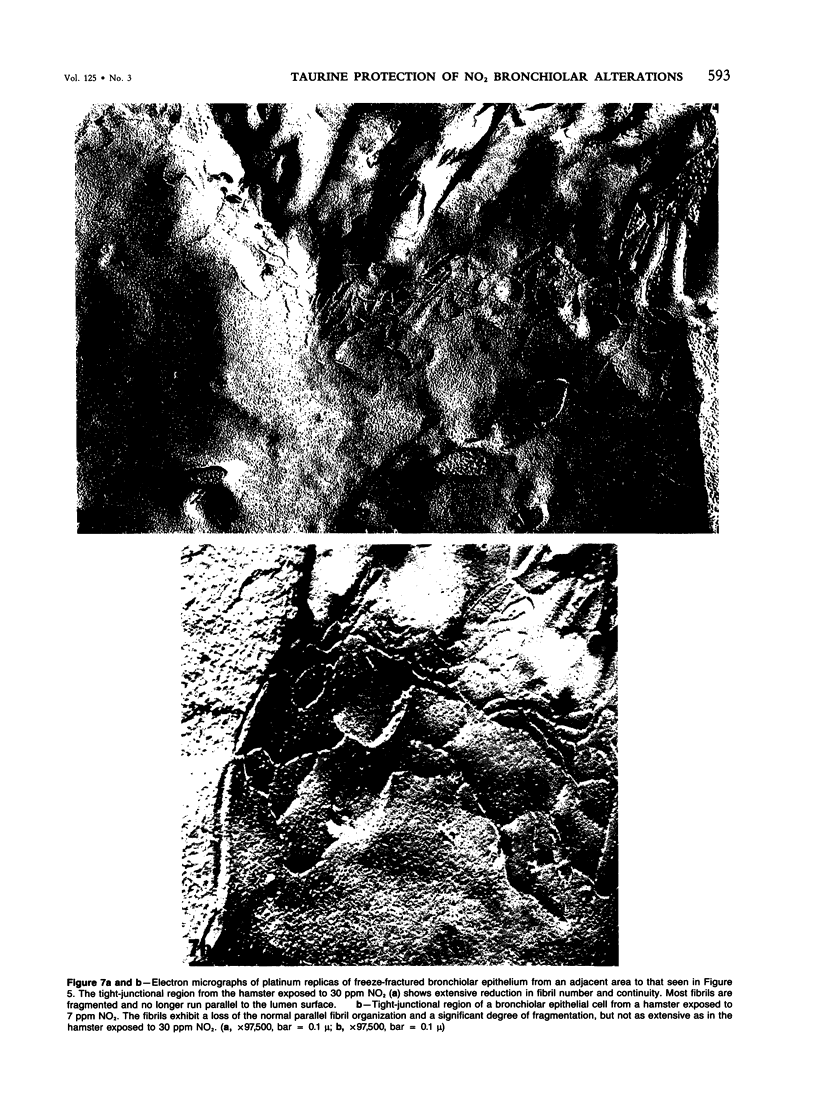
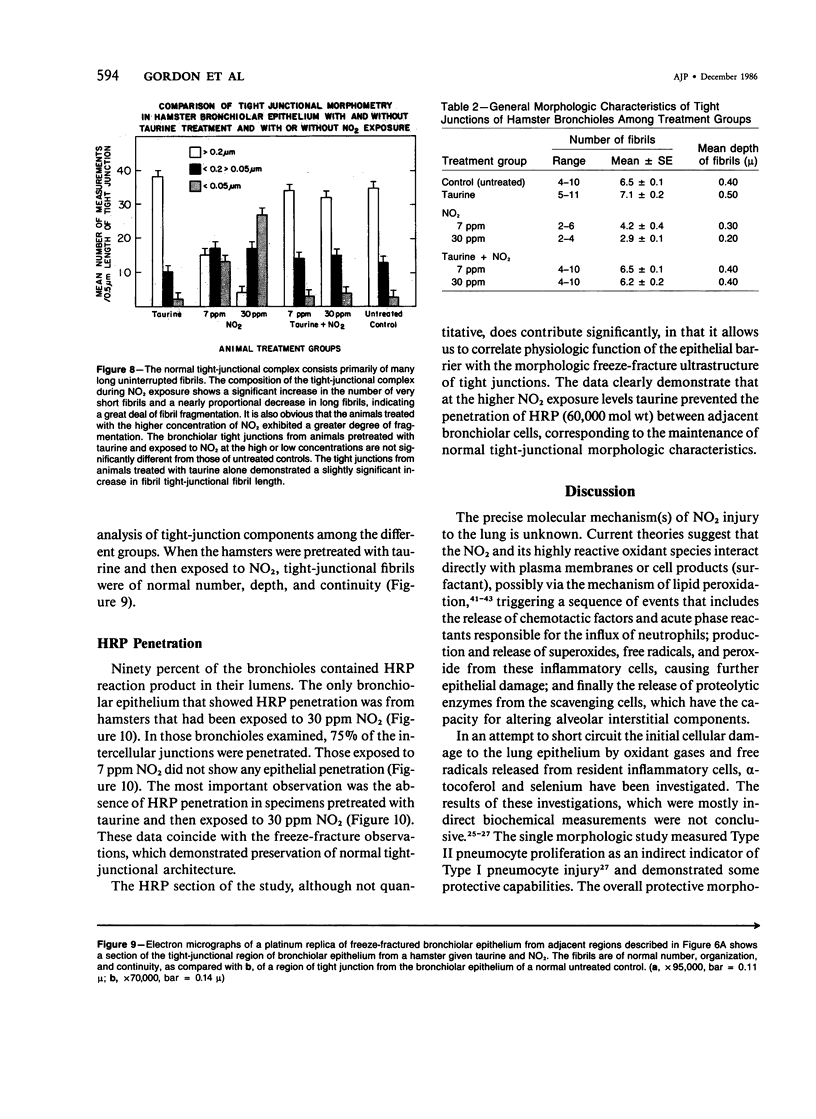
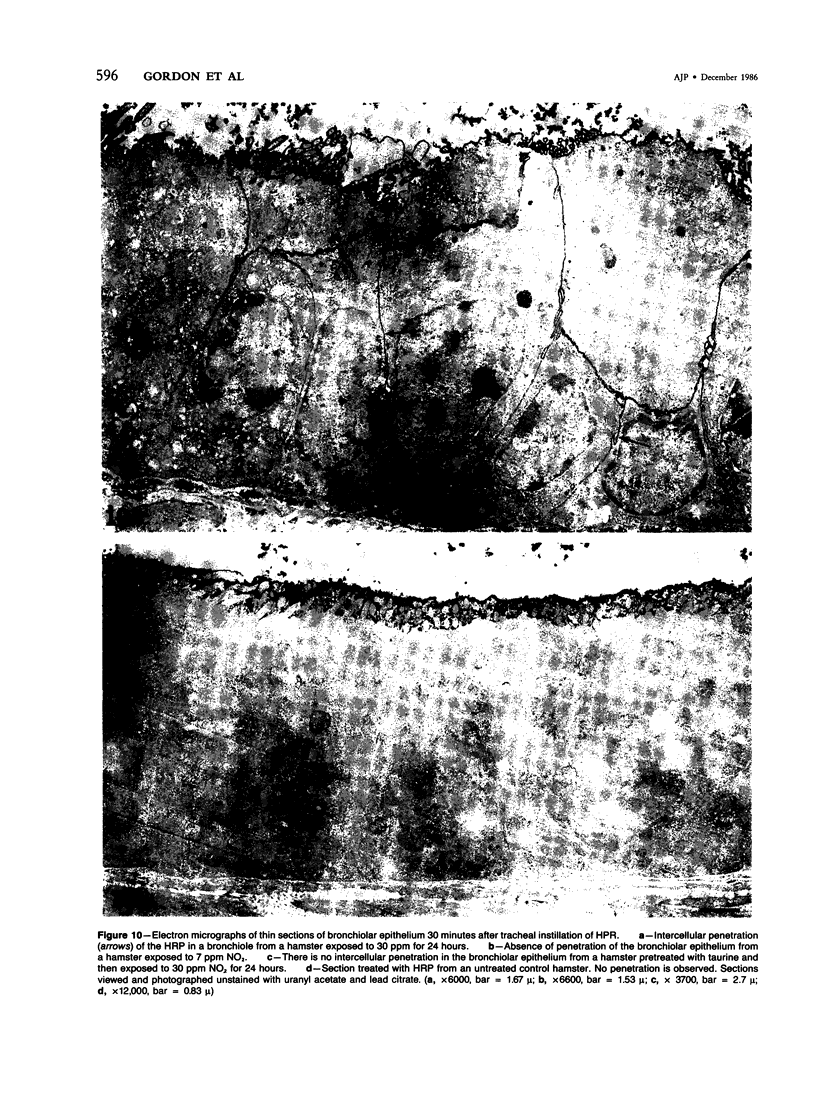
In this study the authors describe the use of dietary taurine to protect hamster lung epithelium from acute nitrogen dioxide (NO2) injury. The conclusions were based on histologic, ultrastructural, and freeze-fracture analyses. Hamsters were pretreated for 14 days with 0.5% taurine in their drinking water. They were then exposed to either 7 or 30 ppm NO2 for 24 hours. The lungs from animals of these experimental groups were compared with those from hamsters treated with only NO2, and those given only taurine and with untreated controls. After treatment, hamsters were anesthetized and perfusion-fixed through the right side of the heart with a solution containing 1% glutaraldehyde, 4% paraformaldehyde, and 0.2 M cacodylate. The trachea and lungs were removed en bloc and stored overnight in cacodylate buffer at 4 C. Terminal and respiratory bronchioles, including alveolar ducts and peribronchiolar alveoli, were dissected from each lobe and processed for embedding in Epon and freeze-fracture replication. Light and transmission electron microscopy revealed the typical inflammatory cell infiltrate in the bronchiolar and alveolar duct regions in the lungs of hamsters exposed to NO2. The bronchiolar epithelium appeared flattened because of loss and breakage of cilia on ciliated cells and apical protrusions of Clara cells. Clara-cell secretory granules were reduced or absent. Freeze-fracture replicas of tight junctions of bronchiolar epithelium analyzed by morphometric techniques demonstrated a reduction and fragmentation of fibrils. Only animals exposed to 30 ppm NO2 exhibited physiologic intercellular penetration of horseradish peroxidase. Hamsters pretreated with taurine and then exposed to NO2 showed none of these alterations. They exhibited the same morphologic features as the untreated controls and the hamsters treated only with taurine. On the basis of this evidence, it is suggested that prophylactic dietary taurine can prevent acute NO2-induced morphologic lung injury. Taurine may also be effective in preventing lung injury induced by other oxidant gases.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J. G., Storey B. T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol Reprod. 1983 Oct;29(3):548–555. doi: 10.1095/biolreprod29.3.548. [DOI] [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. II. Hamster trachea, bronchus, and bronchioles. J Natl Cancer Inst. 1978 Aug;61(2):551–561. [PubMed] [Google Scholar]
- Cabral-Anderson L. J., Evans M. J., Freeman G. Effects of NO2 on the lungs of rats. I. Morphology. Exp Mol Pathol. 1977 Dec;27(3):353–365. doi: 10.1016/0014-4800(77)90006-5. [DOI] [PubMed] [Google Scholar]
- Case B. W., Gordon R. E., Kleinerman J. Acute bronchiolar injury following nitrogen dioxide exposure: a freeze fracture study. Environ Res. 1982 Dec;29(2):399–413. doi: 10.1016/0013-9351(82)90041-x. [DOI] [PubMed] [Google Scholar]
- Dawson S. V., Schenker M. B. Health effects of inhalation of ambient concentrations of nitrogen dioxide. Am Rev Respir Dis. 1979 Aug;120(2):281–292. doi: 10.1164/arrd.1979.120.2.281. [DOI] [PubMed] [Google Scholar]
- DeNicola D. B., Rebar A. H., Henderson R. F. Early damage indicators in the lung. V. Biochemical and cytological response to NO2 inhalation. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):301–312. doi: 10.1016/0041-008x(91)90233-5. [DOI] [PubMed] [Google Scholar]
- Dooley M. M., Pryor W. A. Free radical pathology: inactivation of human alpha-1-proteinase inhibitor by products from the reaction of nitrogen dioxide with hydrogen peroxide and the etiology of emphysema. Biochem Biophys Res Commun. 1982 Jun 15;106(3):981–987. doi: 10.1016/0006-291x(82)91807-1. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M., Hacker A. D., Kuehn K., Mustafa M. G., Schrauzer G. N. Dietary antioxidants and the biochemical response to oxidant inhalation. II. Influence of dietary selenium on the biochemical effects of ozone exposure in mouse lung. Toxicol Appl Pharmacol. 1983 Dec;71(3):398–406. doi: 10.1016/0041-008x(83)90027-3. [DOI] [PubMed] [Google Scholar]
- Elsayed N. M., Mustafa M. G. Dietary antioxidants and the biochemical response to oxidant inhalation. I. Influence of dietary vitamin E on the biochemical effects of nitrogen dioxide exposure in rat lung. Toxicol Appl Pharmacol. 1982 Dec;66(3):319–328. doi: 10.1016/0041-008x(82)90298-8. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Freeman G., Crane S. C., Stephens R. J., Furiosi N. J. The subacute nitrogen dioxide-induced lesion of the rat lung. Arch Environ Health. 1969 Apr;18(4):609–612. doi: 10.1080/00039896.1969.10665460. [DOI] [PubMed] [Google Scholar]
- Geggel H. S., Ament M. E., Heckenlively J. R., Martin D. A., Kopple J. D. Nutritional requirement for taurine in patients receiving long-term parenteral nutrition. N Engl J Med. 1985 Jan 17;312(3):142–146. doi: 10.1056/NEJM198501173120302. [DOI] [PubMed] [Google Scholar]
- Gordon R. E., Case B. W., Kleinerman J. Acute NO2 effects on penetration and transport of horseradish peroxidase in hamster respiratory epithelium. Am Rev Respir Dis. 1983 Sep;128(3):528–533. doi: 10.1164/arrd.1983.128.3.528. [DOI] [PubMed] [Google Scholar]
- Gordon R. E., Solano D., Kleinerman J. Tight junction alterations of respiratory epithelium following long-term NO2 exposure and recovery. Exp Lung Res. 1986;11(3):179–193. doi: 10.3109/01902148609064295. [DOI] [PubMed] [Google Scholar]
- Gordon R. E. The effects of NO2 on ionic surface charge on type I pneumocytes of hamster lungs. Am J Pathol. 1985 Nov;121(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Gordon R. E. The effects of NO2 on ionic surface charge on type I pneumocytes of hamster lungs. Am J Pathol. 1985 Nov;121(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gregory K. L., Malinoski V. F., Sharp C. R. Cleveland Clinic Fire Survivorship Study, 1929-1965. Arch Environ Health. 1969 Apr;18(4):508–515. doi: 10.1080/00039896.1969.10665445. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Thomas E. L. Role of monochloramine in the oxidation of erythrocyte hemoglobin by stimulated neutrophils. J Biol Chem. 1984 Jun 10;259(11):6757–6765. [PubMed] [Google Scholar]
- Heller R. F., Gordon R. E. Chronic effects of nitrogen dioxide on cilia in hamster bronchioles. Exp Lung Res. 1986;10(2):137–152. doi: 10.3109/01902148609061489. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. G., Smith L. H. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968 Apr;48(2):424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Jones G. R., Proudfoot A. T., Hall J. I. Pulmonary effects of acute exposure to nitrous fumes. Thorax. 1973 Jan;28(1):61–65. doi: 10.1136/thx.28.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman J., Cowdrey C. R. The effects of continuous high level nitrogen dioxide on hamsters. Yale J Biol Med. 1968 Apr-Jun;40(5-6):579–590. [PMC free article] [PubMed] [Google Scholar]
- Kleinerman J., Ip M. P., Sorensen J. Nitrogen dioxide exposure and alveolar macrophage elastase in hamsters. Am Rev Respir Dis. 1982 Feb;125(2):203–207. doi: 10.1164/arrd.1982.125.2.203. [DOI] [PubMed] [Google Scholar]
- Kleinerman J. Some effects of nitrogen dioxide on the lung. Fed Proc. 1977 Apr;36(5):1714–1718. [PubMed] [Google Scholar]
- LOWRY T., SCHUMAN L. M. Silo-filler's disease; a syndrome caused by nitrogen dioxide. J Am Med Assoc. 1956 Sep 15;162(3):153–160. doi: 10.1001/jama.1956.02970200001001. [DOI] [PubMed] [Google Scholar]
- Larcan A., Calamai M., Lambert H., Mentre B. Pneumopathie par inhalation de vapeurs nitreuses (combusion de poupées de cellulöide) Poumon Coeur. 1970;26(8):957–960. [PubMed] [Google Scholar]
- Marin M. L., Gordon R. E., Case B. W., Kleinerman J. Ultrastructure of hamster bronchiolar epithelium in freeze fracture replicas. Lung. 1982;160(5):257–266. doi: 10.1007/BF02719299. [DOI] [PubMed] [Google Scholar]
- Merrill W. W., Naegel G. P., Matthay R. A., Reynolds H. Y. Alveolar macrophage-derived chemotactic factor: kinetics of in vitro production and partial characterization. J Clin Invest. 1980 Feb;65(2):268–276. doi: 10.1172/JCI109668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochitate K., Kaya K., Miura T., Kubota K. In vivo effects of nitrogen dioxide on membrane constituents in lung and liver of rats. Environ Res. 1984 Feb;33(1):17–28. doi: 10.1016/0013-9351(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Norwood W. D., Wisehart D. E., Earl C. A., Adley F. E., Anderson D. E. Nitrogen dioxide poisoning due to metal-cutting with oxyacetylene torch. J Occup Med. 1966 Jun;8(6):301–306. doi: 10.1097/00043764-196606000-00002. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Cruz C. Protective effect of taurine and zinc on peroxidation-induced damage in photoreceptor outer segments. J Neurosci Res. 1984;11(3):303–311. doi: 10.1002/jnr.490110310. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Cruz C. Protective effect of taurine and zinc on peroxidation-induced damage in photoreceptor outer segments. J Neurosci Res. 1984;11(3):303–311. doi: 10.1002/jnr.490110310. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Wright C. E., Gaull G. E. Protective effect of taurine, zinc and tocopherol on retinol-induced damage in human lymphoblastoid cells. J Nutr. 1984 Dec;114(12):2256–2261. doi: 10.1093/jn/114.12.2256. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Lightsey J. W. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous Acid. Science. 1981 Oct 23;214(4519):435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- Roehm J. N., Hadley J. G., Menzel D. B. Antioxidants vs lung disease. Arch Intern Med. 1971 Jul;128(1):88–93. [PubMed] [Google Scholar]
- Sagai M., Ichinose T., Oda H., Kubota K. Studies on biochemical effects of nitrogen dioxide: I. Lipid peroxidation as measured by ethane exhalation of rats exposed to nitrogen dioxide. Lipids. 1981 Jan;16(1):64–67. doi: 10.1007/BF02534923. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E. Heterogeneity of tight junction morphology in extrapulmonary and intrapulmonary airways of the rat. Anat Rec. 1980 Oct;198(2):193–208. doi: 10.1002/ar.1091980207. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Mead J. F., Stein R. A. Epoxides as products of lipid autoxidation in rat lungs. Lipids. 1979 Jul;14(7):634–643. doi: 10.1007/BF02533449. [DOI] [PubMed] [Google Scholar]
- Sturman J. A., Rassin D. K., Gaull G. E. Taurine in development. Life Sci. 1977 Jul 1;21(1):1–22. doi: 10.1016/0024-3205(77)90420-9. [DOI] [PubMed] [Google Scholar]
- Sugahara K., Cott G. R., Parsons P. E., Mason R. J., Sandhaus R. A., Henson P. M. Epithelial permeability produced by phagocytosing neutrophils in vitro. Am Rev Respir Dis. 1986 May;133(5):875–881. [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Melton D. F., Jefferson M. M. Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J Biol Chem. 1985 Mar 25;260(6):3321–3329. [PubMed] [Google Scholar]
- Tse R. L., Bockman A. A. Nitrogen dioxide toxicity. Report of four cases in firemen. JAMA. 1970 May 25;212(8):1341–1344. [PubMed] [Google Scholar]
- Walker D. C., MacKenzie A., Wiggs B. R., Hulbert W. C., Hogg J. C. The structure of tight junctions in the tracheal epithelium may not correlate with permeability. Cell Tissue Res. 1984;235(3):607–613. doi: 10.1007/BF00226959. [DOI] [PubMed] [Google Scholar]