Abstract
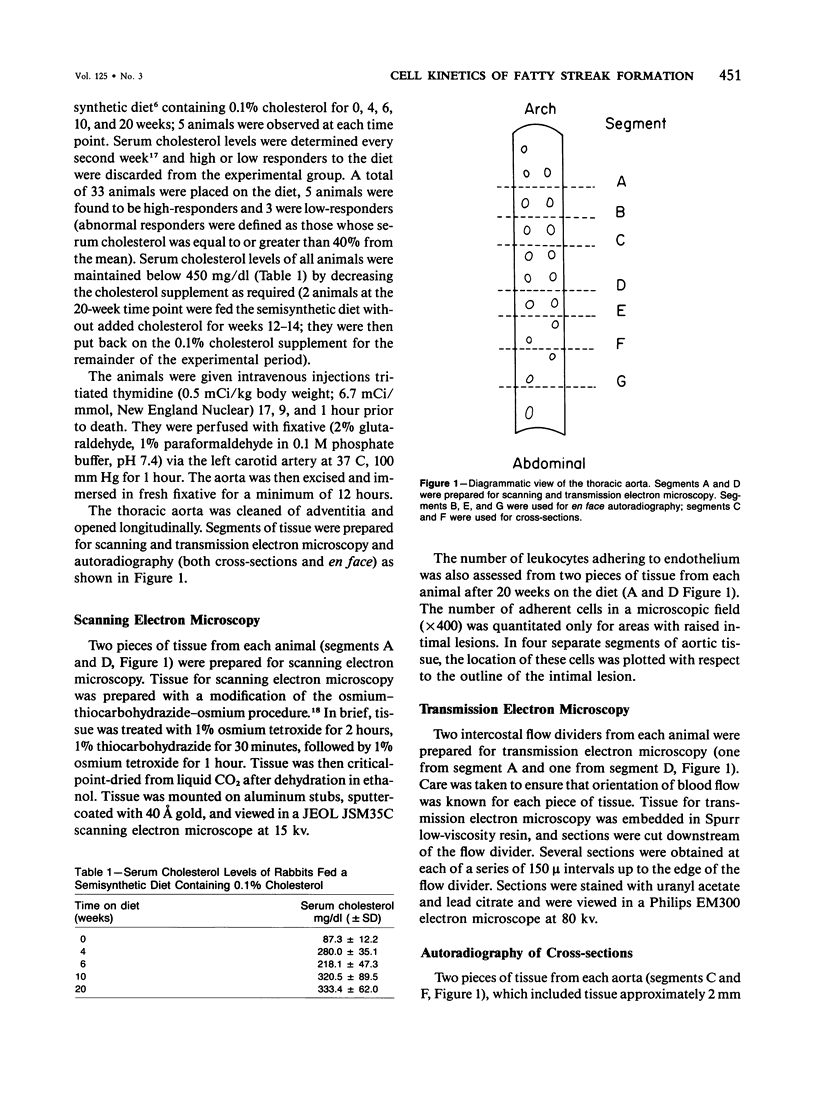
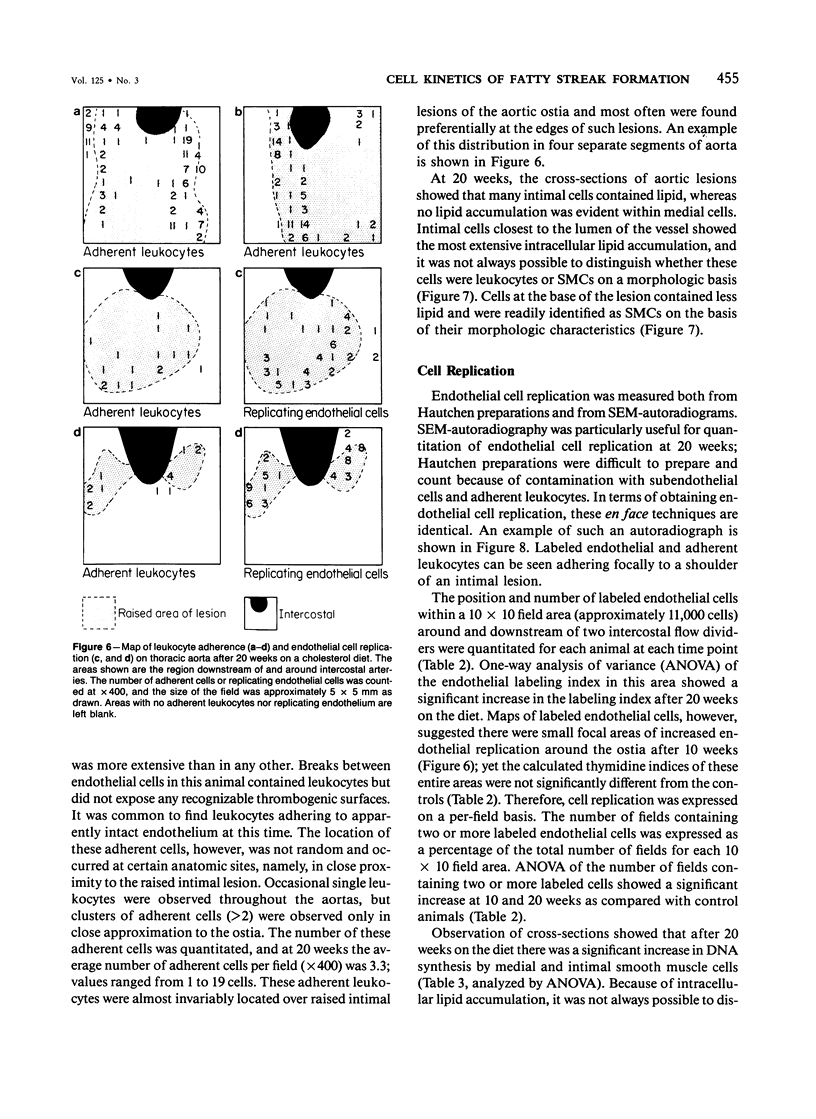
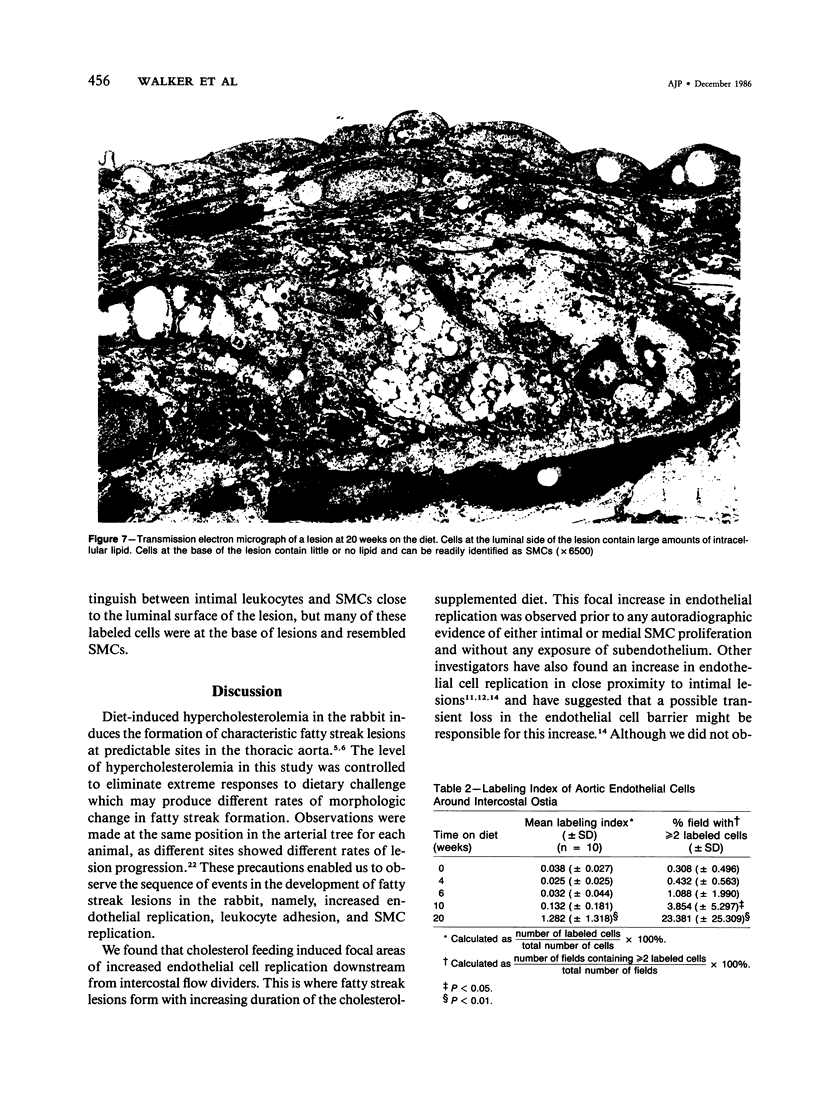
The rationale for this study was to determine whether in the hypercholesterolemic rabbit any evidence of endothelial injury could be detected prior to or during the early phase of fatty intimal lesion formation. The data presented showed that in the first 12 weeks of feeding a 0.1% cholesterol-rich diet, rabbit aortas were covered with an intact endothelium. Focal areas of increased endothelial cell replication were observed adjacent to the aortic ostia at 12 and 20 weeks. These replicating cells were almost exclusively located at the shoulders of large raised lesions. In a similar fashion, adherent leukocytes were observed adjacent to the aortic ostia, and at later times they were concentrated at the periphery of these intimal lesions. Smooth muscle cell replication, as assessed by autoradiography, was found to be significantly increased only after 20 weeks of feeding the lipid-rich diet. These data suggest that an increased endothelial cell turnover and leukocyte adhesion were the first detectable changes induced by cholesterol feeding and that smooth muscle cell proliferation occurred soon after these events.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., de la Motte C. A. Characterization of the adhesion of the human monocytic cell line U937 to cultured endothelial cells. J Clin Invest. 1985 Apr;75(4):1153–1161. doi: 10.1172/JCI111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Regulation of production of a platelet-derived growth factor-like protein by cultured bovine aortic endothelial cells. J Cell Physiol. 1984 Nov;121(2):298–308. doi: 10.1002/jcp.1041210206. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goode T. B., Davies P. F., Reidy M. A., Bowyer D. E. Aortic endothelial cell morphology observed in situ by scanning electron microscopy during atherogenesis in the rabbit. Atherosclerosis. 1977 Jun;27(2):235–251. doi: 10.1016/0021-9150(77)90061-2. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Bondjers G. Endothelial proliferation and atherogenesis in rabbits with moderate hypercholesterolemia. Artery. 1980;7(4):316–329. [PubMed] [Google Scholar]
- Hansson G. K., Chao S., Schwartz S. M., Reidy M. A. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am J Pathol. 1985 Oct;121(1):123–127. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Schwartz S. M. Evidence for cell death in the vascular endothelium in vivo and in vitro. Am J Pathol. 1983 Sep;112(3):278–286. [PMC free article] [PubMed] [Google Scholar]
- JAMIESON A. A METHOD FOR THE RAPID DETERMINATION OF SERUM TOTAL CHOLESTEROL USING A MODIFICATION OF THE PEARSON REACTION. Clin Chim Acta. 1964 Dec;10:530–535. doi: 10.1016/0009-8981(64)90190-1. [DOI] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. I. Leukocyte margination and endothelial alterations at the celiac bifurcation. Am J Pathol. 1984 Jul;116(1):56–68. [PMC free article] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C., Taylor R. G., White M. S. Concurrent endothelial cell turnover and leukocyte margination in early atherosclerosis. Scan Electron Microsc. 1983;(Pt 3):1453–1459. [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kelley R. O., Dekker R. A., Bluemink J. G. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res. 1973 Nov;45(3):254–258. doi: 10.1016/s0022-5320(73)80051-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial injury and regeneration. IV. Endotoxin: a nondenuding injury to aortic endothelium. Lab Invest. 1983 Jan;48(1):25–34. [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981 Apr;44(4):301–308. [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Cell replication in the aortic endothelium: a new method for study of the problem. Lab Invest. 1973 Jun;28(6):699–707. [PubMed] [Google Scholar]
- Scott R. F., Thomas W. A., Kim D. N., Schmee J. Endothelial cell labeling indices in swine aortas in relation to intimal cell mass-derived atherosclerotic lesions. Atherosclerosis. 1985 Sep;56(3):263–270. doi: 10.1016/0021-9150(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Stary H. C., McMillan G. C. Kinetics of cellular proliferation in experimental atherosclerosis. Radioautography with grain counts in cholesterol-fed rabbits. Arch Pathol. 1970 Feb;89(2):173–183. [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. N., Bowyer D. E. Endothelial healing in the rabbit aorta and the effect of risk factors for atherosclerosis. Hypercholesterolemia. Arteriosclerosis. 1984 Sep-Oct;4(5):479–488. doi: 10.1161/01.atv.4.5.479. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- de Bono D. P., Green C. The adhesion of different cell types to cultured vascular endothelium: effects of culture density and age. Br J Exp Pathol. 1984 Feb;65(1):145–154. [PMC free article] [PubMed] [Google Scholar]








