Abstract
Subependymal giant cell astrocytoma (SEGA) is a unique brain tumor arising in tuberous sclerosis complex (TSC), an autosomal dominant inherited phacomatosis. There are several case reports of solitary SEGA without any other manifestations of TSC. Usually these cases are thought to be forme fruste of TSC due to somatic mosaicism. However, no previous reports have used molecular methodology to fully investigate mutations in TSC genes or the possibility of somatic mosaicism. Here, we report a 20-year-old woman with a brain tumor. Pathological diagnosis was consistent with SEGA, but comprehensive clinical screening found no other lesions indicative of TSC. Molecular analysis of the tumor revealed loss of heterozygosity and allelic mutation (5228G>A, R1743Q) of TSC2. To detect the small fraction of mosaic mutation in somatic cells, we developed a highly sensitive new method: triple-nested polymerase chain reaction-restriction fragment length polymorphism. The identical TSC2 missense mutation was not detected in any other tissues from the same patient, including peripheral blood, buccal mucosa, urinary sediment, nail, and hair. According to these results, this patient should be considered as having SEGA that developed from two somatic hit mutations in TSC2, rather than being a TSC2 patient with a very small fraction of somatic mosaicism.
Subependymal giant cell astrocytoma (SEGA) is a unique brain tumor that usually accompanies tuberous sclerosis complex (TSC), an autosomal dominant hereditary phacomatosis. Patients with TSC develop multiple hamartomas, mainly in the brain, heart, kidneys, eyes, and skin.1,2 Two distinctive disease causative genes, TSC1 and TSC2, have been cloned;3,4 and germline and second hit mutations in hamartomas, mostly loss of heterozygosity (LOH), have been well studied.5,6,7,8 Clinical expression of TSC is widely different in each case. Mosaicism is relatively common and well documented in TSC, because up to 10% of patients have somatic and/or gonadal mosaicism.9,10,11,12
Because SEGAs are characteristic lesions of TSC and were once even considered as pathognomonic, patients with isolated SEGA without any other TSC manifestations are usually considered as a forme fruste of TSC with somatic mosaicism. Although there are several case reports of isolated SEGA, no previous case was molecularly analyzed for TSC mutations or somatic mosaicism.13,14,15,16,17,18 Here, we report a 20-year-old woman with isolated SEGA and no other clinical manifestations of TSC. We performed comprehensive molecular analysis of the surgically removed SEGA for both LOH and point mutations in the TSC1 and TSC2 genes. We also examined any available tissues throughout the body from the same patient, including peripheral leukocytes (two separate collections in a two year interval), urine, buccal mucosa, hair, and nail, to determine somatic mosaicism.
Materials and Methods
Case Report
A 20-year-old previously healthy woman presented with sudden onset of headache and vomiting. Brain MRI revealed a large, well-demarcated tumor in the left caudate head. The tumor was 4 cm in diameter and homogeneously enhanced after Gadolinium-diethyltriaminepentaacetic acid (Gd-DTPA) administration. The tumor was located near the foramen of Monro, which resulted in obstructive hydrocephalus (Figure 1A). No subependymal nodule (SEN) or cortical tuber was observed. Preoperative differential diagnosis included astrocytoma, oligodendroglioma, central neurocytoma, meningioma, germ cell tumor, and SEGA. Complete clinical screening for TSC, including dermatological and ophthalmologic exams, abdominal and cardiac ultra-sonography, and whole-body computed tomography found no lesions consistent with TSC. A left frontal craniotomy was performed, and the tumor was completely removed via a transcortical, transventricular approach. Macroscopically, the tumor arose from the caudate head and protruded into the anterior horn of the lateral ventricle. Pathological findings were compatible with SEGA (Figure 1B). Written informed consent for molecular analysis was obtained from the patient after explanation of the study, which was approved by the ethics committee of Kanazawa University Graduate School of Medical Science.
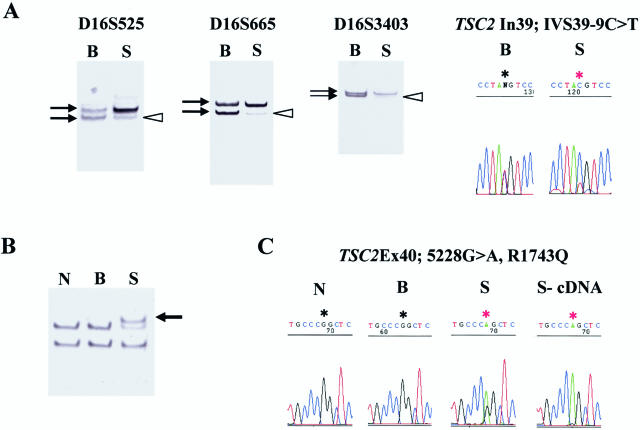
Figure 1.
MRI and pathological findings of the patient. A: Preoperative MRI revealed a large, well-demarcated tumor protruding into the left lateral ventricle. The tumor homogeneously enhanced after Gd-DTPA administration. Neither SEN nor cortical tuber was observed. B: Photomicrograph of the tumor specimen; hematoxylin and eosin staining. The tumor was composed mainly of large polygonal cells resembling gemistocytic astrocytes. Mitotic figures were rare, and no endothelial proliferation or necrosis was seen. These findings were compatible with SEGA.
DNA and RNA Extraction
Peripheral blood leukocyte DNA and SEGA DNA were extracted by standard method. At the same time, peripheral leukocyte DNA from a healthy volunteer was extracted and used as a normal control. DNA was also extracted from a buccal mucosa swab, urinary sediments, hair, and nail by ISOHAIR (Nippon Gene, Toyama, Japan), according to the manufacturer’s instructions. Total RNA of SEGA was extracted by Trizol (Invitrogen, Carlsbad, CA), and cDNA was synthesized by standard method with Rivatra Ace reverse transcriptase (TOYOBO, Tokyo, Japan).
LOH Analysis
Both TSC1 and TSC2 loci of the SEGA and blood DNA were tested for LOH, as previously described.19 Chromosomal markers D9S2126, D9S1830, D9S1199, and D9S1198 were used for detecting TSC1 LOH; and D16S525, D16S3252, D16S665, D16S3403, D16S663, and D16S283 were used for TSC2. All polymerase chain reaction (PCR) products were run on 13.5% nondenatured polyacrylamide gel and developed with a silver stain kit (Bio-Rad, Hercules, CA). The sample was scored as positive for LOH only when the intensity of one allele of the SEGA was decreased more than 50% when compared with that of the blood control. Single-strand conformational polymorphism (SSCP) and sequence analysis of blood DNA revealed a polymorphism in TSC2 intron 39, thus, we also added this polymorphism to TSC2 LOH marker and determined LOH by direct sequencing.
TSC Mutation Screening
The coding exons of both TSC1 and TSC2 in blood and SEGA DNA were screened by SSCP as previously described.19 All PCR products were separated on a SSCP gel (12% polyacrylamide, C = 2, with 8% glycerol) and developed with silver staining. Any samples with detected band shifts were confirmed with the BigDye Terminator v3.1 cycle sequencing kit and ABI PRISM 3100 Genetic analyzer (Applied Biosystems, Foster City, CA).
Investigation of Somatic Mosaicim by Single and Triple-Nested PCR-Restriction Fragment Length Polymorphism (RFLP)
With the above screening method, a missense mutation in TSC2 was detected in SEGA (see Results). To explore the possibility of TSC somatic mosaicism, we tested every available tissue from the patient, ie, peripheral blood leukocyte (two independent collections in a 2-year interval), buccal mucosa, urinary sediments, nail, and hair. Because the detected TSC2 missense mutation destroys the MspI site of the wild-type allele, we set up triple-nested PCR-RFLP (restriction fragment length polymorphism) to detect the small fraction of somatic mosaic mutation and compared its sensitivity with conventional single-step PCR-RFLP. Because only wild-type allele is digested by MspI, a combination of nested PCR and MspI digestion of PCR products can effectively concentrate the mutant allele at each step. A total of 0.1 μg of each DNA sample was completely digested by MspI (TaKaRa, Tokyo, Japan) and then amplified for 10 cycles of 95°C for 10 seconds and 60°C for 5 seconds with the first PCR primer set 40 (5′-TGGCCAAGATCGTGTCTGAC-3′) and 67 (5′-TTCCGCTGGCCCACCTCATAGCCA-3′).
To eliminate the possibility of amplification of nucleotide change caused by polymerase error, we used a high-fidelity enzyme, KOD-Plus DNA polymerase (TOYOBO). The initial PCR products were digested with MspI again and then amplified for 10 cycles with the second PCR primer set 40 and 66R (5′-AGGTGGCTTGGGCAGTAAGT-3′). The second PCR products were digested with MspI again and a final PCR was performed with primer set 66 (5′-CCACCGATATCTACCCCTCCAAGT-3′) and 66R for 25 cycles. Final products were digested by MspI and separated on 13.5% nondenatured polyacrylamide gel and developed by silver staining. Through these cycles, the wild-type allele was completely removed, and only the mutated allele was amplified. To compare the sensitivity of this analysis, we also performed conventional PCR-RFLP, ie, all samples were amplified by primer set 66 and 66R for 30 cycles and then digested by MspI.
Results
LOH and Mutation Analysis
Through the LOH analysis of the SEGA, all TSC1 markers were found to be informative and detected heterozygosity. On the other hand, TSC2 LOH was detected with D16S525, D16S665, D16S3403, and TSC2 intron 39 polymorphism (IVS39-9C>T) (Figure 2A). The centromere side-two TSC2 markers D16S663 and D16S283 maintained heterozygosity. SSCP analysis revealed no band shift in the TSC1 coding exons, whereas band shifts were detected at TSC2 around exon 40 in SEGA DNA (Figure 2B). Direct sequencing confirmed exon 40 missense change 5228G>A, R1743Q in the SEGA DNA (nucleotide number denotes A of the start codon ATG as nucleotide 1 and includes the sequence of exon 31; GenBank accession no. X75621) but not in blood DNA. Sequence analysis also revealed a polymorphism at intron 39 only in blood DNA, as mentioned above. However, SSCP could not detect this nucleotide substitution. The TSC2 exon 40 missense change was previously reported,20 and a different missense mutation in the same codon (R1743P) was also reported in patients with TSC.21 The SEGA preferentially contained mutant allele both at the DNA and message level (Figure 2C). In conclusion, the SEGA contained both TSC2 missense mutation and TSC2 LOH, and the tumor lost wild-type allele by LOH.
Figure 2.
TSC2 mutations in the SEGA. A: TSC2 LOH was shown with three markers, D16S525, D16S665, and D16S3403, and the TSC2 intron 39 polymorphism. Black arrows indicate the two alleles of each marker, and white arrowheads indicate the lost allele. B: SSCP analysis of TSC2 exon 40 (primer set 66 and 66R) shows clear band shift (arrow) in the SEGA. C: Sequence analysis shows TSC2 exon 40 missense mutation, 5228G>A, R1743Q, in the SEGA. B, blood DNA; S, SEGA DNA; N, normal control DNA; S-cDNA, SEGA cDNA.
Analysis of TSC2 Somatic Mosaicism
Somatic mosaicism of the TSC2 mutation was examined by single and triple-nested PCR-RFLP. To determine the sensitivity of these methods, SEGA DNA was serially diluted with normal genomic DNA from a healthy volunteer to 10, 1, 0.1, and 0.01% SEGA DNA concentration. Using these sensitivity control DNA, conventional single PCR-RFLP detected as little as 10% of SEGA DNA in the sample. On the other hand, triple-nested PCR-RFLP could detect as little as 0.1% of SEGA DNA (Figure 3A). Amplified final products of triple-nested PCR were sequenced and confirmed to contain only mutant allele (Figure 3B). Mutant allele was only detected in the SEGA. These results showed that the patient does not have higher than 0.1% somatic mosaicism in these tested tissues.
Figure 3.
Analysis of somatic mosaicism. A: Comparison of single and triple-nested PCR-RFLP. Triple-nested PCR-RFLP can detect 0.1% of tumor DNA in the sample. B: Sequence analysis of the final PCR products of triple-nested PCR-RFLP demonstrated only mutated allele. C: Schematic representation of primer and mutation position for triple-nested PCR-RFLP. (+) uncut, normal control DNA PCR products not digested with MspI; (+), normal control DNA; PB1, peripheral blood collected in October 2001; PB2, peripheral blood collected in February 2003; Buc, buccal mucosa; Urine, urinary sediment; C/T mix, mixture of control and SEGA DNA with the percentage of SEGA DNA indicated; (−), H2O (negative control).
Discussion
SEGA is a rare and unique astrocytoma that usually arises in patients with TSC and was once regarded as pathognomonic for TSC.22 According to the diagnostic criteria of the National Tuberous Sclerosis Association in 1992, histologically confirmed SEGA alone fulfills the criteria of definite TSC.1 There are several case reports of SEGA without any other clinical manifestation of TSC; these cases have been thought as forme fruste of TSC with somatic mosaicism.
The diagnostic criteria were revised at 1998 because no single major feature fulfills the diagnosis of definite TSC. According to these new criteria, our patient is diagnosed with possible TSC.2 Molecular analysis of our patient revealed that the SEGA possessed both TSC2 missense mutation and TSC2 LOH according to Knudson’s two-hit theory. To detect the small fraction of mosaic mutation in somatic cells, we have developed a new method, triple-nested PCR-RFLP. This method detects as little as 0.1% of mutated DNA in samples. Using this highly sensitive detection system, we demonstrated that all samples other than SEGA (peripheral blood, buccal mucosa, urinary sediment, nail, and hair) did not contain the same mutation detected in SEGA.
Because we could not check every cell of the human body, it is theoretically possible to miss a very small fraction of somatic mosaicism. Meanwhile, natural somatic mutation occurs constantly at some frequency. In fact, the somatic mutation rate in vivo has been experimentally determined for some genes. LOH ratio of adenine phosphoribosyltransferase gene in normal human T lymphocyte is estimated as 2 to 15 × 10−5;23 somatic mutation frequencies of the hypoxanthine phosphoribosyltransferase gene in human kidney cortical epithelial cells are ∼5 × 10−5 in the first decade of life and ∼2.5 × 10−4 in the eighth and later decades of life.24 Although the natural somatic mutation frequency of TSC genes has not been determined, every person has TSC somatic mosaicism at a certain small ratio. Furthermore, the new mutation ratio of TSC is estimated as 2.5 to 16 × 10−6 per gamete per generation, thus every person also possesses germline mosaicism of TSC with this ratio.25
On the other hand, we also have to consider technical limitations. Although we used KOD-Plus DNA polymerase, with fidelity 82 times higher than TaqDNA polymerase, misincorporation of nucleotide can occur during the nested PCR cycles. In addition, the amount of template DNA used in the first PCR cycle is 0.1 μg, which is equivalent to 2 × 104 cells on the assumption that each cell contains 5 pg of genomic DNA. According to these reasons, we could not improve the detection sensitivity of triple-nested PCR-RFLP infinitely. The actual sensitivity of this method, using the mixture of control and SEGA DNA, was 10−3. So, the sensitivity is not only acceptably high enough to detect a small fraction of mosaicism, but also reliably low from the point of view of both the natural somatic mutation ratio and experimental artifact. In summary, our data strongly suggest that TSC2 mutations of the patient are well localized to SEGA. Thus, it is reasonable to think that this patient has isolated SEGA without TSC, rather than a forme fruste of TSC with a very small fraction of somatic mosaicism. In the case of retinoblastoma, another autosomal dominant inherited tumor disease, it is well known that sporadic cases have developed their tumors through two successive somatic hit mutations of RB1 gene.26 Therefore, we can think similarly in cases of sporadic SEGA.
Among TSC lesions, LOHs were detected frequently in renal angiomyolipoma but less frequently in brain lesions, eg, SEGA and cortical tuber.27 A recent study showed that some SEGAs do not have TSC2 LOH but rather have phosphorylation of TSC2 protein tuberin.28 These data suggest that TSC2 LOH is not an absolutely necessary condition for development of TSC brain lesions. Our current data do not conflict with this previous data. Because there is some evidence that SEN and SEGA are consecutive lesions and SEN may grow to SEGA,29 it is hypothesized that SEN is developed by a single-hit mutation of TSC2 and tuberin phosphorylation, then TSC2 LOH accelerates tumor growth and transforms SEN to SEGA.
Another rare TSC lesion, lymphangioleiomyomatosis (LAM), is a progressive interstitial lung disease characterized by diffuse proliferation of abnormal smooth muscle cells. LAM can occur as an isolated disorder (sporadic LAM) in a non-TSC patient. Recently, it has been proven that sporadic LAM contains two somatic TSC2 mutations.30,31,32 Therefore one could consider solitary SEGA to be similar to sporadic LAM. These are rare events; however, two successive somatic mutations of TSC2 in those tissues create identical lesions to those of patients with TSC. Furthermore, we can consider inherited TSC, sporadic TSC, TSC with somatic and/or gonadal mosaicism, and isolated TSC lesions (SEGA or LAM) to be sequential spectrum phenomenon because these patients only differ as to when the first hit occurred.
Acknowledgments
We give special thanks to our patient. Her cooperation and patience during the completion of the detailed molecular analysis enabled the accomplishment of this study. After the genetic counseling was completed, she had a healthy baby.
Footnotes
Supported by personal grant of Department of Pediatrics, Kanazawa University Graduate School of Medical Science.
References
- Roach ES, Smith M, Huttenlocher P, Bhat M, Alcorn D, Hawley L. Diagnostic criteria: tuberous sclerosis complex. Report of the Diagnostic Criteria Committee of the National Tuberous Sclerosis Association. J Child Neurol. 1992;7:221–224. doi: 10.1177/088307389200700219. [DOI] [PubMed] [Google Scholar]
- Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- The European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- Beauchamp RL, Banwell A, McNamara P, Jacobsen M, Higgins E, Northrup H, Short P, Sims K, Ozelius L, Ramesh V. Exon scanning of the entire TSC2 gene for germline mutations in 40 unrelated patients with tuberous sclerosis. Hum Mutat. 1998;12:408–416. doi: 10.1002/(SICI)1098-1004(1998)12:6<408::AID-HUMU7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Niida Y, Lawrence-Smith N, Banwell A, Hammer E, Lewis J, Beauchamp RL, Sims K, Ramesh V, Ozelius L. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat. 1999;14:412–422. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ, Sims K, MacCollin M, Louis DN, Ramesh V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet. 2001;69:493–503. doi: 10.1086/321972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JF, Banuls J, Ramon R, Guijarro J, Botella R, Betlloch I. Unilateral multiple facial angiofibromas: a mosaic form of tuberous sclerosis. J Am Acad Dermatol. 2000;43:127–129. doi: 10.1067/mjd.2000.106361. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska J, Wigowska-Sowinska J, Napierala D, Slomski R, Kwiatkowski DJ. Mosaicism in tuberous sclerosis as a potential cause of the failure of molecular diagnosis. N Engl J Med. 1999;340:703–707. doi: 10.1056/NEJM199903043400905. [DOI] [PubMed] [Google Scholar]
- Verhoef S, Bakker L, Tempelaars AM, Hesseling-Janssen AL, Mazurczak T, Jozwiak S, Fois A, Bartalini G, Zonnenberg BA, van Essen AJ, Lindhout D, Halley DJ, van den Ouweland AM. High rate of mosaicism in tuberous sclerosis complex. Am J Hum Genet. 1999;64:1632–1637. doi: 10.1086/302412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose VM, Au KS, Pollom G, Roach ES, Prashner HR, Northrup H. Germ-line mosaicism in tuberous sclerosis: how common? Am J Hum Genet. 1999;64:986–992. doi: 10.1086/302322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi N, Yoshihara W, Shimada N, Tanaka H, Fujita N, Hirabuki N, Watanabe Y, Nakamura H. Solitary subependymal giant cell astrocytoma: case report. Eur J Radiol. 2000;33:55–58. doi: 10.1016/s0720-048x(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Bignold LP, Allsop JL. Recurrent subependymal giant-cell astrocytoma in the absence of tuberous sclerosis: case report. J Neurosurg. 1979;50:106–109. doi: 10.3171/jns.1979.50.1.0106. [DOI] [PubMed] [Google Scholar]
- Prahlow JA, Teot LA, Lantz PE, Stanton CA. Sudden death in epilepsy due to an isolated subependymal giant cell astrocytoma of the septum pellucidum. Am J Forensic Med Pathol. 1995;16:30–37. doi: 10.1097/00000433-199503000-00006. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yamada K, Nakahara T, Ishihara A, Takaki S, Kochi M, Ushio Y. Rapid regrowth of solitary subependymal giant cell astrocytoma: case report. Neurol Med Chir (Tokyo) 2002;42:224–227. doi: 10.2176/nmc.42.224. [DOI] [PubMed] [Google Scholar]
- Tabuchi S, Takigawa H, Oka A, Mizuguchi M, Horie Y, Watanabe T. Subependymal giant cell astrocytoma with positive tuberin expression: case report. Neurol Med Chir (Tokyo) 2003;43:616–618. doi: 10.2176/nmc.43.616. [DOI] [PubMed] [Google Scholar]
- Medhkour A, Traul D, Husain M. Neonatal subependymal giant cell astrocytoma. Pediatr Neurosurg. 2002;36:271–274. doi: 10.1159/000058432. [DOI] [PubMed] [Google Scholar]
- Niida Y, Lawrence-Smith N, Banwell A, Hammer E, Lewis J, Beauchamp RL, Sims K, Ramesh V, Ozelius L. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat. 1999;14:412–422. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gilbert JR, Guy V, Kumar A, Wolpert C, Kandt R, Aylesworth A, Roses AD, Pericak-Vance MA. Mutation and polymorphism analysis in the tuberous sclerosis 2 (TSC2) gene. Neurogenetics. 1998;1:267–272. doi: 10.1007/s100480050039. [DOI] [PubMed] [Google Scholar]
- Jones AC, Shyamsundar MM, Thomas MW, Maynard J, Idziaszczyk S, Tomkins S, Sampson JR, Cheadle JP. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet. 1999;64:1305–1315. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd CW, Scheithauer BW, Gomez MR, Altermatt HJ, Katzmann JA. Subependymal giant cell astrocytoma: a clinical, pathological, and flow cytometric study. Neurosurgery. 1991;28:864–868. [PubMed] [Google Scholar]
- Gupta PK, Sahota A, Boyadjiev SA, Bye S, Shao C, O’Neill JP, Hunter TC, Albertini RJ, Stambrook PJ, Tischfield JA. High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination. Cancer Res. 1997;57:1188–1193. [PubMed] [Google Scholar]
- Martin GM, Ogburn CE, Colgin LM, Gown AM, Edland SD, Monnat RJ., Jr Somatic mutations are frequent and increase with age in human kidney epithelial cells. Hum Mol Genet. 1996;5:215–221. doi: 10.1093/hmg/5.2.215. [DOI] [PubMed] [Google Scholar]
- Gomez MR, Sampson JR, Whittemore VH. New York: Oxford,; Tuberous Sclerosis Complex. (ed 3) 1999:313–323. [Google Scholar]
- Nussbaum RL, McInnes RR, Willard HF. Philadelphia: Saunders,; Thompson & Thompson Genetics in Medicine. (ed 6) 2001:320–323. [Google Scholar]
- Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996;59:400–406. [PMC free article] [PubMed] [Google Scholar]
- Han S, Santos TM, Puga A, Roy J, Thiele EA, McCollin M, Stemmer-Rachamimov A, Ramesh V. Phosphorylation of tuberin as a novel mechanism for somatic inactivation of the tuberous sclerosis complex proteins in brain lesions. Cancer Res. 2004;64:812–816. doi: 10.1158/0008-5472.can-03-3277. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Takaki T, Hikita T, Nishio S. Subependymal giant-cell astrocytoma associated with tuberous sclerosis: do subependymal nodules grow? Childs Nerv Syst. 1989;5:43–44. doi: 10.1007/BF00706748. [DOI] [PubMed] [Google Scholar]
- Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62:810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrinidis A, Khare L, Carsillo T, Smolarek T, Au KS, Northrup H, Henske EP. Mutational analysis of the tuberous sclerosis gene TSC2 in patients with pulmonary lymphangioleiomyomatosis. J Med Genet. 2000;37:55–57. doi: 10.1136/jmg.37.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]