SYNOPSIS
Objective.
The purpose of this study was to assess the validity of self-reported history for varicella disease relative to serological evidence of varicella immunity in pregnant women attending antenatal care at clinics located in two diverse geographical locations in the U.S. (Antelope Valley, California, and Philadelphia) with high varicella vaccination coverage.
Methods.
Pregnant women attending prenatal care appointments who needed blood drawn as part of their routine care were eligible to participate. Self-reported varicella disease history was obtained via questionnaire. Varicella serostatus was determined using a whole-cell enzyme-linked immunosorbent assay to test for varicella zoster virus-specific immunoglobulin G (VZV IgG) antibodies.
Results.
Of the 309 study participants from Antelope Valley and the 528 participants from Philadelphia who self-reported having had chickenpox disease, 308 (99.7%; 95% confidence interval [CI]: 98.2, 100) and 517 (97.9%; 95% CI: 96.3, 99.0), respectively, had serological evidence of immunity to varicella. Only 6.8% (95% CI: 3.9, 11.0) and 17.4% (95% CI: 13.1, 22.5) of women who self-reported having a negative or uncertain varicella disease history in Antelope Valley and Philadelphia, respectively, were seronegative for varicella antibodies.
Conclusion.
Despite the dramatic changes in the epidemiology of varicella that have occurred since 1995 due to the introduction and subsequent widespread use of the varicella vaccine, self-reported history of varicella continues to be a strong predictor of VZV IgG antibodies in pregnant women. Negative or uncertain history remains poorly predictive of negative serostatus.
In June 2005 and June 2006, the Advisory Committee on Immunization Practices (ACIP) recommended universal prenatal assessment of pregnant women for evidence of varicella immunity and established guidelines for evidence of immunity.1 According to the new provisional recommendations, what constitutes evidence of immunity to varicella varies based on a person's year of birth, location of birth (U.S. vs. foreign-born), and prior disease presentation (typical vs. atypical). However, pregnant women could meet the criteria for evidence of immunity for prenatal assessment based on a positive self-reported typical varicella disease history. It is left up to clinicians to decide whether to rely on a negative or uncertain disease history as sufficient evidence of susceptibility or to do serologic testing.
These new and revised provisional ACIP recommendations largely reflect the dramatic reductions (about 90%) in varicella incidence that have changed the epidemiology of varicella following the introduction of the varicella vaccine in 1995 and its subsequent widespread use in the U.S.2 In the prevaccine era, as virtually everyone growing up in the U.S. developed chickenpox prior to adulthood, a positive self-reported history of varicella disease proved to be an extremely accurate proxy for serological evidence of varicella immunity in adults, while a negative or uncertain disease history report was typically inaccurate. For example, a study performed in Philadelphia from 1992–1994 found serological evidence of varicella immunity among 100% of a sample of 100 pregnant women who self-reported having had varicella disease.3
In the post-varicella vaccine era (starting in 1995), it is possible that the significant reduction in varicella zoster virus (VZV) circulation in communities with high vaccination coverage may affect the validity of self-reported disease history, as prevalence is one of the determinants of the predictive value of a screening test along with sensitivity and specificity.4 Given that the success of the new prenatal screening recommendation largely rests on the continued accuracy of a positive self-reported disease history, it is important to periodically monitor the accuracy of such a history relative to serological evidence of immunity.
Varicella in pregnancy can have serious adverse effects on both maternal and infant health. Teratogenic effects to the fetus are generally associated with maternal infection during the first and second trimester, particularly the latter. Neonatal varicella can result in death in as many as 20% of cases when maternal rash onset is between five days antepartum and two days postpartum. Increased morbidity and mortality from varicella and varicella pneumonia among pregnant women has also been reported.5–12 Varicella disease at the time of delivery also results in extra public health interventions. Infants whose mothers have signs and symptoms of varicella around the time of delivery, or premature infants exposed during the neonatal period, may need varicella zoster immune globulin, which is now an investigational drug preparation called VariZIG, as VZIG (previously produced by the Massachusetts Biologics Laboratories) is no longer available.13 Additionally, susceptible hospital staff may be subject to furlough for three weeks (one incubation period) after a known exposure to varicella.14
We assessed the validity of self-reported history for varicella disease relative to serological evidence of immunity in pregnant women receiving antenatal care at clinics located in two diverse geographical locations in the U.S. (Antelope Valley, California, and Philadelphia) with high varicella vaccination coverage. We also considered how changes in community-level varicella susceptibility rates would alter the validity of positive self-reported history.
MATERIALS AND METHODS
This study was carried out as part of the Varicella Active Surveillance Project, a cooperative agreement between the Centers for Disease Control and Prevention (CDC), the Philadelphia Department of Public Health (PDPH), and the Los Angeles Department of Public Health (LADPH) to monitor the impact of the varicella vaccination program. Details of this surveillance project have been described elsewhere.15
Between December 2001 and March 2004, participants in Philadelphia were recruited from six publicly funded prenatal clinics. Participants in Antelope Valley (AV) were recruited from two publicly funded clinics between March 2003 and March 2004. Only women scheduled to have blood drawn for routine prenatal laboratory tests were eligible to participate, thus no additional needle stick was incurred by patients for the purpose of this study. Because Hispanics comprise a large portion of the population in AV, Spanish-language materials were developed for use at this site, and both English and Spanish speakers were eligible to participate. However, English-speaking ability was a requirement for study participation in Philadelphia. The intention to deliver at either the Hospital of the University of Pennsylvania (HUP) or the Albert Einstein Medical Center (AEMC) was a requirement for study participation in Philadelphia, due to post-delivery follow-up considerations; women intending to deliver elsewhere were not eligible to participate. Follow-up of subjects determined to be susceptible to varicella was conducted by study staff to help ensure that these women were fully vaccinated with two doses post-delivery.
Subject recruitment occurred in the participating clinics, where recruitment information was prominently displayed and clinic staff members were trained to enroll subjects. Subjects completed a brief questionnaire containing demographic information, varicella disease history, and varicella vaccination history. Subjects in Philadelphia were also asked whether they knew what chickenpox disease was. Spanish-speaking participants in AV were provided with study materials, including a consent form, in Spanish.
Serologic specimens were sent to the CDC and tested for VZV-specific immunoglobulin G (VZV-IgG) antibodies using a whole-cell enzyme-linked immunosorbent assay (wc-ELISA), which has been described previously.14 Specimens testing negative or equivocal by wc-ELISA were retested using Glycoprotein-ELISA (gp-ELISA), which is a more sensitive test for detecting VZV-IgG antibodies and was the test used to license the varicella vaccine by the Food and Drug Administration15,16 (unpublished study, Scott Schmid, PhD, Chief of the Herpes Viral Laboratory, CDC, Atlanta). For the purpose of follow-up vaccination and informing subjects of their serostatus, gp-ELISA test results superseded wc-ELISA results for all specimens that were dual tested. For the purposes of data analysis, serological immune status was also determined based on wc-ELISA results only, as this is the only method available to clinics for screening patients in the real world. Findings based on wc-ELISA test results only compared to results based on dual testing are presented.
Participants were notified of their serological test results by their respective local health departments. Susceptible participants were counseled on the risks of varicella during pregnancy, prevention of exposures to VZV infections while pregnant, what to do should an exposure occur, and the need for postpartum vaccination. Additionally, clinics were provided with regularly updated lists of their susceptible patients and stickers to flag the medical records of these patients regarding their need for post-delivery varicella vaccination.
All susceptible participants were followed by study staff to verify vaccination at the hospital (using vaccine donated by Merck Pharmaceuticals) or during a follow-up visit with the prenatal clinic. In Philadelphia, those not receiving full vaccination through the hospital or clinic were individually contacted by the health department to arrange vaccination, including home visits when necessary.
Seroprevalence rates were calculated within self-reported disease history groups. Positive predictive value (PPV) of self-reported history was defined as the proportion of seropositive women among those who self-reported having had chickenpox (total number of women who self-reported positive varicella disease history and who tested seropositive for varicella titers divided by the total number of women who self-reported positive varicella disease history). Negative predictive value (NPV) was defined as the proportion of seronegative women among those who self-reported not ever having had chickenpox or were uncertain as to whether they had a history of disease (total number of women who self-reported negative varicella disease history and who tested seronegative for varicella titers divided by the total number of women who self-reported negative varicella disease history).
To investigate how much varicella susceptibility rates would need to change to impact validity of positive self-reported disease history as a screening method for immune status, we calculated PPV for a hypothetical population of 1,000 women, varying the susceptibility rate from 0% to 100% under three scenarios appropriate for the postvaccine-licensure era that differed with respect to the level of specificity assumed for self-reported disease history (60%, 70%, or 80%) and holding sensitivity constant at 80%.
All analyses were performed using SAS, version 9.1.17 This study was approved by the Institutional Review Boards of the CDC, PDPH, LADHS, HUP, and AEMC.
RESULTS
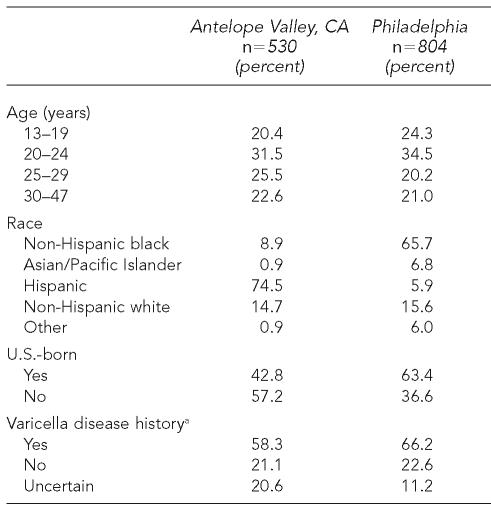
A total of 530 participants were enrolled from AV and 804 from Philadelphia. The demographic profiles of the two groups of subjects are presented in Table 1. While subjects from the two areas were roughly similar with respect to age distribution, they were quite different in terms of race, country of birth, and self-reported history of chickenpox. Subjects from AV were largely Hispanic (75%) and of non-U.S. origin (57%), while subjects from Philadelphia tended to be non-Hispanic black (66%) and U.S.-born (63%). A history of chickenpox was reported by 58% of subjects from AV and 66% of subjects from Philadelphia. Only 7% of the subjects from each site reported that they had received varicella vaccine.
Table 1.
Demographic characteristics of subjects
Excludes six subjects in Philadelphia with missing data on self-reported disease history
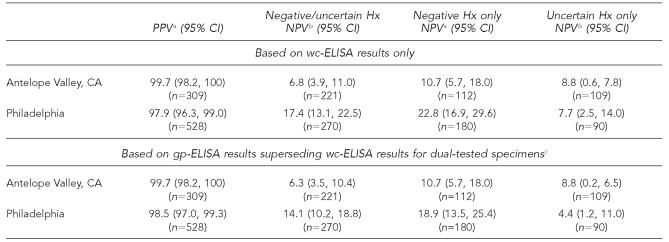
Of the 309 participants from AV who reported a positive varicella disease history, 308 (99.7%; CI: 98.2, 100) were seropositive, while 517 (97.9%; 95% CI: 96.3, 99.0) of the 528 subjects reporting varicella disease history in Philadelphia were seropositive (Table 2). The PPV of self-reported disease history did not vary significantly by age, race, or country of birth for either of the samples. The NPV of a negative or uncertain disease history was 6.8% (95% CI: 3.9, 11.0) for the AV sample (n=221) and 17.4% (95% CI: 13.1, 22.5) for the Philadelphia sample (n=270). Higher NPVs were obtained for negative disease history only (excluding uncertain history) for both samples. Using the gp-ELISA results for specimens initially testing negative/equivocal for calculating PPV and NPV resulted in slight reductions in NPV.
Table 2.
Predictive value of maternal self-reported varicella disease history on varicella immune status in pregnant women
The proportion of seropositive subjects among those who self-reported having had varicella
The proportion of seronegative subjects among those who self-reported a negative or uncertain history of varicella
Only specimens initially testing negative or equivocal by wc-ELISA were retested using gp-ELISA.
PPV = positive predictive value
NPV = negative predictive value
CI = confidence interval
Hx = history of disease
wc-ELISA = whole-cell enzyme-linked immunosorbent assay
gp-ELISA = glycoprotein enzyme-linked immunosorbent assay
In terms of knowledge of varicella, 10% of 801 subjects in Philadelphia initially indicated that they did not know (n=53) or were uncertain as to (n=29) what chickenpox disease was. Of these women, 65% gave an unequivocal answer to the question, “Have you ever had chickenpox?” (18 indicated “yes” and 35 indicated “no”), rather than saying they were uncertain. Foreign-born women were more likely to indicate that they did not know or were uncertain as to what chickenpox was compared to U.S.-born women (19% vs. 5%, χ2=39.6, p<0.001).
Successful retesting of the 74 specimens that tested negative or equivocal by wc-ELISA revealed that 14 of them were in fact positive for VZV IgG antibodies by gp-ELISA. Thus, for the purpose of follow-up vaccination, 60 women were considered to have no evidence of immunity to varicella. Efforts to vaccinate these women during regular postpartum follow-up visits were largely successful in AV. Of the 14 susceptible women in AV, one developed chickenpox during the course of her pregnancy and 11 of the remaining 13 (85%) were fully immunized with two doses during their postnatal care appointments. Of the 46 susceptible women in Philadelphia, only 32 (70%) were fully immunized with two doses and another six (13%) received one dose, with 81% of vaccinations occurring outside of regularly scheduled postnatal care and administered by public health nurses affiliated with the study.
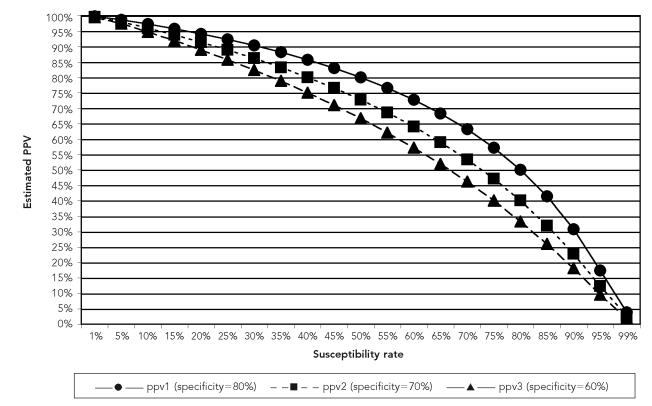
The Figure displays the impact that changing susceptibility rates in a population have on PPV for a hypothetical sample of 1,000 people, conditional on the specificity of self-report disease history to be either 60%, 70%, or 80%, and holding sensitivity constant at 80%. Under these conditions, PPV rates range from 92% to 96% if the varicella susceptibility rate in the population is 15%. Altering the sensitivity of the test to 70% results in even lower PPV rates, especially after susceptibility rates exceed 20% (data not shown).
Figure.
PPV of self-reported varicella history at 80% sensitivity and varying specificity and VZV susceptibility rates
PPV = positive predictive value
VZV = varicella zoster virus
DISCUSSION
Results from two demographically diverse samples of mostly unvaccinated pregnant women seeking antenatal care at publicly funded clinics demonstrate that self-reported history of varicella continues to be a strong predictor of serological evidence of varicella immunity, whereas a negative or uncertain history is a poor predictor of negative serology. These results are largely consistent with those of previous studies and suggest that reliance on self-reported positive disease history is still a good strategy for predicting varicella immunity among women of reproductive age, despite the changing epidemiology of the disease following vaccine introduction in 1995. An examination of 1,799 pregnant women in Los Angeles between April of 1998 and March of 1999 found serological evidence of varicella immunity among 96.1% of women who self-reported having had chickenpox.16 More recently, Plourd and Austin reported a PPV of 96.2% of 1,085 patients registering for prenatal care during the first half of 2002.17
PPV will decline if varicella susceptibility in the population increases. While the level at which PPV rates of self-reported disease history are deemed to be too low to justify using self-reported history for initial screening purposes is debatable, we note that PPV drops below 95% at a susceptibility rate of 15%, assuming sensitivity of 80% and specificity of either 70% or 60%. When vaccine-derived VZV immunity predominates among cohorts in their child-bearing years, screening methods based on varicella disease history alone will no longer be appropriate, and evaluations will need to include a history of varicella vaccination.
Follow-up gp-ELISA testing of specimens that tested negative or equivocal by wc-ELISA revealed that 19% did in fact have VZV-IgG antibodies. Thus, had these women been evaluated for postdelivery vaccination under typical clinic conditions (wc-ELISA only), they would have been unnecessarily vaccinated. However, the vaccination of some people who are mistakenly identified as susceptible should not result in untoward effects, considering the excellent safety profile of varicella vaccination.18 Of greater public health importance is the possibility of false-positive test results by wc-ELISA, which would lead to women being erroneously informed that they are not susceptible to varicella. While we did not evaluate the rate of false-positives among wc-ELISA seropositive specimens, prior research indicates that false positive results are rare using this assay (<0.01% of specimens, unpublished study comparing wc-ELISA, gp-ELISA, latex bead agglutination, and commercial ELISAs, Scott Schmid, PhD, Chief of the Herpes Viral Laboratory, CDC, Atlanta).
Despite extensive training of residents and attending nursing staff in Philadelphia, health-care providers did not provide the necessary postpartum vaccination to their patients who were determined to be susceptible to varicella; the study staff administered 81% of the postpartum vaccinations. It is possible that health-care providers will be less motivated to carry through on postpartum vaccination if the need for vaccination is based on self-reported disease history of untested validity as opposed to the actual serological evidence of lack of immunity. Regardless, instituting a method for postpartum vaccination of women deemed to be susceptible (e.g., standing orders for vaccination of women with negative/uncertain histories or with negative VZV serology) will be a key component to the ultimate success of any screening program.
Both the PPV and NPV of self-reported varicella disease history are impacted by the prevalence of varicella in the population, which is associated with age, race, and geography/climate of birth, among other factors. Prior to the introduction of the varicella vaccine in the U.S., increasing age was found consistently to be the most important factor determining infection with VZV. Nationally representative data from the National Health and Nutrition Examination Surveys (NHANES) administered from 1988–1994 showed varicella seroprevalence rates steadily increasing from 86% among 6- to 11-year-olds to 99.6% among adults aged 40 to 49 years.19 In AV, the median age of the seropositive subjects was 24 years compared to 21 years among those who were seronegative; in Philadelphia, the median age was 23 years for the seropositive subjects and 24.5 years for the seronegative subjects (data not shown).
NHANES data also demonstrated that race was associated with naturally acquired varicella infection independent of age, with non-Hispanic black women aged 20 to 39 years being 60% less likely to be varicella seropositive compared to their non-Hispanic white counterparts. Several studies have documented lower seropositive rates among adults born in tropical and semitropical environments compared with temperate zones.16,20–22 The current study did not have sufficient sample size or variability with respect to these three variables to consider their independent impact on PPV or NPV. Our study had higher proportions of women who traditionally have lower seropositive rates, including non-Hispanic black women (Philadelphia), women born outside the U.S., and women born in countries having subtropical/tropical climates (Table 1). Therefore, our results are likely to underestimate PPV of self-reported history compared to all women of child-bearing age in the U.S. Moreover, our findings may only be applicable to similar populations of women seeking prenatal care at publicly funded clinics.
Due to the possibility of selection bias in our samples, seroprevalence rates were not calculated independent of self-reported disease history. Study recruitment materials advertised that participants needing varicella vaccination would receive it for free as compensation for study participation. This incentive may have resulted in the disproportionate enrollment of women with no or uncertain history of varicella disease relative to women with a positive disease history, which would result in an inflated seronegative rate in this sample. As a clinical educational measure taken in light of the potentially serious ramifications of varicella disease in pregnancy, the study consent form framed the purpose of the study as identifying women who have never had chickenpox. This also may have resulted in biased recruitment toward women with no or uncertain history of varicella. Additionally, the sensitivity of reported varicella history would have been underestimated, while specificity would have been overestimated as a result of this bias.
When examining estimated PPVs at varying susceptibility levels, we selected a sensitivity and range of specificity values that would account for the potential bias instead of using values directly from the study. Although the potential for this kind of selection bias precludes the use of the study data for estimating overall seroprevalence rates as well as the sensitivity and specificity of varicella history in this sample, it did not result in biased estimations of seroprevalence rates within self-reported disease history groups.
A crude estimate of the number of women giving birth each year who are susceptible to varicella infection can be calculated by applying age-specific varicella susceptibility rates for women obtained from NHANES data to the estimated number of women who gave birth to a live-born infant annually from the Vital Statistics Natality Report. This calculation (data not shown) suggests that approximately 94,000 women aged 15 to 44 years in the U.S. who delivered a live-born infant in 2004 may have been susceptible to varicella disease during their pregnancy.
From a prevention standpoint, it is of course preferable to identify and vaccinate all susceptible women prior to their becoming pregnant. In the absence of a mechanism for doing so, the prenatal screening for varicella susceptibility, as provisionally recommended by ACIP, is a feasible next-best alternative that will likely prove to be efficacious in preventing the morbidity and mortality associated with maternal, fetal, and perinatal chickenpox. However, whether this strategy will remain efficacious and cost-effective until vaccine-derived immunity predominates among the cohort of women of reproductive age will in part depend on changes in population susceptibility and in the PPV of self-reported disease history. Both of these should continue to be monitored periodically.
A formal economic analysis of prevaccination serotesting compared with presumptive immunization of those self-reporting a negative or equivocal history of disease that considers a range of seroprevalence rates in sensitivity analyses would help determine the levels of population susceptibility and PPV of self-reported disease history at which this provisional policy is cost-effective. In situations in which serological testing followed by vaccination of susceptible women at subsequent visits is not feasible due to limited availability of laboratory services, poor rates of return for additional visits, or other constraints, presumptive varicella vaccination for women who lack a positive history of disease and are not pregnant or planning to become pregnant within the next month should be considered.
Acknowledgments
The authors gratefully acknowledge the antenatal clinic and laboratory staff in Antelope Valley, California, and Philadelphia for their dedication and continued hard work and support throughout the project. The authors especially thank Bruce Barlow, Val Leyden, Teresa Maupin, Sandra Dos Santos Chaves, Dalya Guris, Paul Gargiullo, C. Victor Spain, and Jane Seward for their valuable assistance.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) Prevention of varicella—provisional updated ACIP recommendations for varicella vaccine use. [cited 2007 Mar 9]. Available from: URL: http://www.cdc.gov/nip/vaccine/varicella/varicella_acip_recs_prov_june_2006.pdf.
- 2.Guris D, Jumaan AO, Mascola L, Watson B, Zhang JX, Chaves SS, et al. Changing varicella epidemiology in active surveillance sites—United States, 1995–2005. J Infect Dis. 2007 doi: 10.1086/522156. In press. [DOI] [PubMed] [Google Scholar]
- 3.Silverman NS, Ewing SH, Todi N, Montgomery OC. Maternal varicella history as a predictor of varicella immune status. J Perinatol. 1996;16:35–8. [PubMed] [Google Scholar]
- 4.Rothman KJ, Greenland S. 2nd edition. Philadelpha: Lippincott, Williams, and Wilkins; 1998. Modern epidemiology; pp. 509–10. [Google Scholar]
- 5.Hollier LM, Grissom H. Human herpes viruses in pregnancy: cytomegalovirus, Epstein-Barr virus, and varicella zoster virus. Clin Perinatol. 2005;32:671–96. doi: 10.1016/j.clp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Tan MP, Koren G. Chickenpox in pregnancy: revisited. Reprod Toxicol. 2006;21:410–20. doi: 10.1016/j.reprotox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Sauerbrei A, Wutzler P. Neonatal varicella. J Perinatol. 2001;21:545–9. doi: 10.1038/sj.jp.7210599. [DOI] [PubMed] [Google Scholar]
- 8.Enders G, Miller E. Varicella and herpes-zoster in pregnancy and the newborn. In: Arvin AM, Gershon AA, editors. Varicella-zoster virus. Cambridge (England): Cambridge University Press; 2000. pp. 317–47. [Google Scholar]
- 9.Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548–51. doi: 10.1016/s0140-6736(94)92943-2. [DOI] [PubMed] [Google Scholar]
- 10.Harger JH, Ernest JM, Thurnau GR, Moawad A, Thom E, Landon MB, et al. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Frequency of congenital varicella syndrome in a prospective cohort of 347 pregnant women. Obstet Gynecol. 2002;100:260–5. doi: 10.1016/s0029-7844(02)02059-8. [DOI] [PubMed] [Google Scholar]
- 11.Harger JH, Ernest JM, Thurnau GR, Moawad A, Momirova V, Landon MB, et al. National Institute of Child Health and Human Development, Network of Maternal-Fetal Medicine Units. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis. 2002;185:422–7. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- 12.Gershon A, Takahashi M, Seward J. Varicella vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4th edition. Philadelphia: Elsevier, Inc.; 2004. pp. 783–824. [Google Scholar]
- 13.A new product (VariZIG) for postexposure prophylaxis of varicella available under an investigational new drug application expanded access protocol. MMWR Morb Mortal Wkly Rep. 2006;55(8):209–10. [PubMed] [Google Scholar]
- 14.Behrman A, Schmid S, Crivaro A, Watson B. A cluster of primary varicella cases among healthcare workers with false-positive varicella zoster virus titers. Infect Control Hosp Epidemiol. 2003;24:202–6. doi: 10.1086/502187. [DOI] [PubMed] [Google Scholar]
- 15.West Point (PA): Merck & Co., Inc.; 1996. Jul, VARIVAX package inserts. [Google Scholar]
- 16.White CJ, Kuter BJ, Hildebrand CS, Isganitis KL, Matthews H, Miller WJ, et al. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics. 1991;87:604–10. [PubMed] [Google Scholar]
- 17.SAS Institute Inc. SAS: Version 9.1 for Windows. Cary (NC): SAS Institute Inc.; 2006. [Google Scholar]
- 18.Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002;287:606–11. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg JM, Ziel HK, Burchette R. Evaluation of varicella immune status in an obstetric population in relation to place of birth. Am J Perinatol. 2002;19:387–94. doi: 10.1055/s-2002-35613. [DOI] [PubMed] [Google Scholar]
- 20.Plourd DM, Austin K. Correlation of a reported history of chickenpox with seropositive immunity in pregnant women. J Reprod Med. 2005;50:779–83. [PubMed] [Google Scholar]
- 21.Wise RP, Salive ME, Braun MM, Mootrey GT, Seward JF, Rider LG, et al. Postlicensure safety surveillance for varicella vaccine. JAMA. 2000;284:1271–9. doi: 10.1001/jama.284.10.1271. [DOI] [PubMed] [Google Scholar]
- 22.Kilgore PE, Kruszon-Moran D, Seward JF, Jumaan A, Van Loon FP, Forghani B, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(Suppl 1):S111–8. doi: 10.1002/jmv.10364. [DOI] [PubMed] [Google Scholar]
- 23.Barnett ED, Christiansen D, Figueira M. Seroprevalence of measles, rubella, and varicella in refugees. Clin Infect Dis. 2002;35:403–8. doi: 10.1086/341772. [DOI] [PubMed] [Google Scholar]
- 24.Kjersem H, Jepsen S. Varicella among immigrants from the tropics, a health problem. Scand J Soc Med. 1990;18:171–4. doi: 10.1177/140349489001800303. [DOI] [PubMed] [Google Scholar]
- 25.Maretic Z, Cooray MP. Comparisons between chickenpox in a tropical and a European country. J Trop Med Hyg. 1963;66:311–5. [PubMed] [Google Scholar]