SYNOPSIS
Modified directly observed therapy (mDOT), in which a portion of total doses of a medication regime is ingested under supervision, has demonstrated efficacy as an intervention to assist patients in maintaining adherence to complicated antiretroviral therapy (ART). Although findings are favorable, existing efficacy studies fail to provide sufficient detail to guide others who wish to implement mDOT interventions. The aim of this article is to provide a primer for practitioners and researchers who wish to implement mDOT interventions.
Drawing on the experience of 10 federally funded research projects, we provide guidance on critical questions for program implementation, including: who should be targeted, length/duration/content/location/tapering of sessions, staffing, incentives, and approaches to data collection. In addition, guidance on staff training and minimum requirements for mDOT interventions is offered along with real-world examples of mDOT interventions.
mDOT is feasible and easily adapted to many settings and target populations. Interventions should match the specific needs of the target population and setting and be flexible in terms of design and delivery. mDOT should be considered among the spectrum of adherence interventions.
Numerous randomized controlled clinical trials of intensive antiretroviral therapy (ART) have demonstrated that adherent patients can achieve durable viral load suppression with sustained increases in CD4 lymphocyte counts1–4 and reduced morbidity and mortality.5–8 The benefits of ART are clear; however, its initial success and long-term effectiveness require high levels of adherence, which is challenging and not often accomplished by patients.9–11 Directly observed therapy (DOT), in which all doses of a medication regime are ingested under supervision, has been successful in improving adherence to ART;12–14 however, it is often not practical in real-world settings and existing efficacy studies fail to provide sufficient detail to guide others who wish to implement such interventions.
An alternative to DOT, modified directly observed therapy (mDOT, also known as directly administered antiretroviral therapy [DAART]) has been offered as a potential solution to the challenge of observing every dose. mDOT differs from DOT in that only a portion of total doses are directly observed. Mitty and colleagues have completed a series of studies investigating outreach-based mDOT for ART.15,16 In their most recent inquiry, an intent-to-treat analysis revealed that patients who received an mDOT intervention evidenced greater reduction in viral load than those receiving standard care.16 This effect was most pronounced among individuals who were ART experienced. Several other studies that have evaluated different models of observed therapy also support its use.17–19 However, one controlled study20 did not support its efficacy.
While not definitive, mDOT has promise as an intervention for ART adherence. However, existing efficacy studies fail to provide sufficient detail about the logistics and challenges to guide others in successfully implementing mDOT interventions. Nevertheless, practitioners and researchers who wish to employ this approach face several important questions: (1) Who should be targeted? (2) What should be included in an mDOT intervention? (3) How many weeks of intervention should be offered and where should they be conducted? (4) How long should the mDOT contacts be? (5) How many patients can receive services each day? (6) How should patients be weaned from mDOT interventions? (7) Should incentives for participation in mDOT intervention be offered? and (8) What approaches have been used to collect data about mDOT interventions?
The aim of this article is to address this lack of information by providing a resource for practitioners and researchers who wish to implement mDOT interventions. Specifically, we answered the previous questions by describing how mDOT has been applied in 10 research contexts with population samples of diverse human immunodeficiency virus (HIV)-infected individuals and across a variety of settings. We also identified minimum requirements for the translation and implementation of mDOT and detailed minimum requirements for mDOT staff training.
METHODS
Participants
Respondents in this study were 10 research groups that had employed mDOT interventions for adherence to ART medications in their research or treatment programs.15,18,19,21–26 Respondents spanned the U.S. and had a wide range of experience working with samples of diverse HIV-infected individuals with several sites specifically targeting patients who were experiencing comorbid disorders (e.g., substance abuse) and/or significant psychosocial stressors (e.g., homelessness).
Potential respondents for the study were identified via professional relationships with other researchers. The snowball technique, in which all respondents were asked to recommend additional researchers who employ mDOT for ART adherence in their programs, was used. Finally, a search of the Computer Retrieval of Information on Scientific Projects database of funded studies was completed to identify any additional researchers not yet contacted. Respondents were asked for verbal informed consent to participate during the initial contact and followed up with additional phone or e-mail contact(s). All aspects of this voluntary study were approved by the University of Missouri–Kansas City (UMKC) Institutional Review Board, and all investigators who were approached agreed to participate.
Procedure and measures
Respondents were asked to participate in a semistructured qualitative interview, using a set of standard questions, via phone or e-mail. Additional prompt questions were used in the few instances when respondents did not offer unsolicited opinions about how important they felt mDOT methods were in assisting patients in adhering to ART, whether they thought certain patients differentially benefited from mDOT, how patients felt about regular visits, and what they believed were necessary aspect(s) of an mDOT intervention.
RESULTS
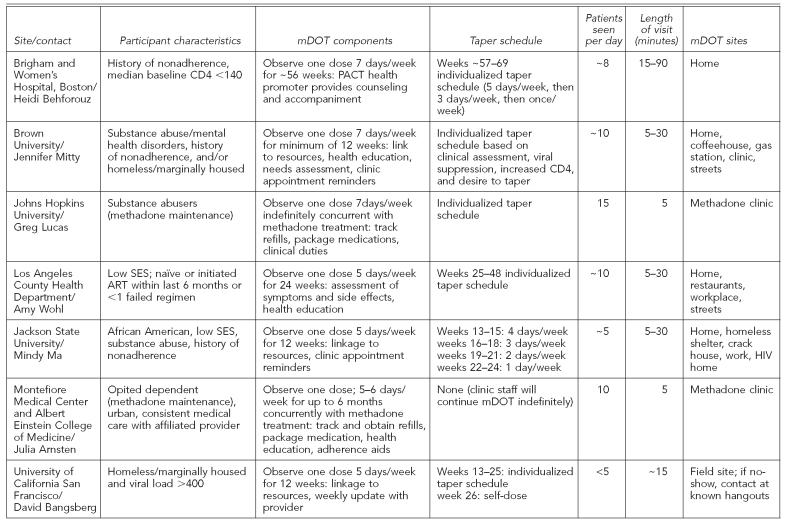
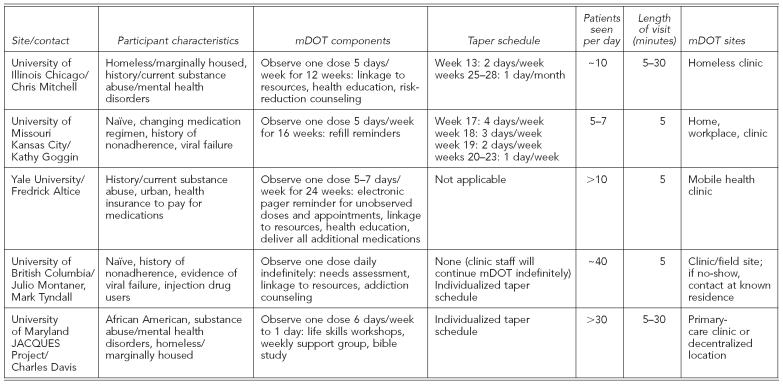
Respondents' answers to the standardized questions are summarized in the Table and detailed in this article.
Table.
Summary of mDOT components from research and real-world sites
mDOT = modified directly observed therapy
ART = antiretroviral therapy
HIV = human immunodeficiency virus
JACQUES = Joint AIDS Community-wide Quest for Unique and Effective treatment Strategies
PACT = Prevention and Access to Care and Treatment
SES = social economic status
Who should be targeted?
Overall, respondents reported working with a wide range of HIV+ individuals, including participants who were dually diagnosed with mental illness and/or substance abuse, individuals receiving methadone maintenance, those who were homeless/marginally housed, and individuals residing in rural as well as urban communities. Several sites targeted individuals with a history of nonadherence and/or evidence of disease progression (e.g., viral load >400), which suggested that participants might benefit from an adherence intervention.
What should be included in an mDOT intervention?
All respondents reported that mDOT included physically observing at least one dose of ART medication on visit days. However, the array of additional mDOT components provided by staff varied greatly and ranged from very minimal (i.e., providing refill reminders) to multicomponent comprehensive interventions (i.e., link to resources, health education, needs assessment, clinic appointment reminders, electronic paging device to prompt unobserved doses, and health-care visits). What constituted health education varied by site, but most included comprehensive adherence and basic HIV disease-management counseling. Many sites with multicomponent mDOT interventions reported that they were fulfilling a need in their communities by providing services that were not readily available to patients.
How many weeks of intervention should be offered and where should they be conducted?
The length of mDOT interventions varied greatly, ranging from 12 to 56 weeks, with one site providing intervention in conjunction with methadone treatment for as long as the participant wanted to continue. Most sites delivered doses to participants' homes or other mutually agreed upon locations in the community (e.g., a coffeehouse). However, several studies were successful in getting participants to come to a single location (e.g., methadone clinic, homeless shelter) or used a mobile van to meet participants in the community.
How long should the mDOT contacts be?
The average length of an mDOT visit varied greatly by site (from 5 to 30 minutes) with differences mostly being attributed to the number of mDOT components provided by outreach workers during a standard visit. Sites with the lowest contact time tended to provide tightly controlled, direct observation of medication ingestion, whereas those with longer average visits offered many additional services (e.g., linkage to care, health education, and/or secondary HIV prevention interventions).
How many patients can receive services each day?
The number of patients seen per day by a single outreach worker varied greatly by site and was directly related to the average length of the mDOT visit and patient regime factors. Not surprisingly, sites that enrolled patients with once daily regimes were able to have longer visits and see more patients per day, as visits could be spread out across the workday without interfering with patients' preferred dosing time. Sites where the majority of patients were on twice to three times per-day regimes had less flexibility in observing doses.
While most sites were able to accommodate patients' individual preferred dosing schedules, there was a limit to what could be done. Creative solutions were found to these types of logistic problems, including hiring more mDOT outreach workers, asking patients to adjust their preferred dosing time, and/or changing the site of the mDOT visit to reduce staff travel time. Despite being effective, all of these potential solutions brought their own problems in terms of patient willingness to comply and generalizability to real-world settings.
How should patients be weaned from mDOT interventions?
Deciding how and when to taper mDOT interventions is difficult and many sites reported changing their approach to this as they gained more experience with their target population. Sites that enrolled a majority of dual-diagnosed, especially current substance-abusing participants, tended to employ more flexible tapering strategies.
Should incentives for participation in mDOT intervention be offered?
Some sites offered incentives (e.g., food, hot showers, money) for participating in mDOT treatment, while others offered enticements only when participants provided evaluation data. While not necessarily attempting to provide incentives for participation in mDOT treatment, some sites did choose to colocate their interventions in environments that already provided reinforcement for participants (e.g., methadone maintenance clinic, homeless clinic).
What approaches have been used to collect data about mDOT interventions?
All sites attempted to evaluate the efficacy of their mDOT intervention. In addition to the doses that were directly observed, most sites relied on self-report of adherence to unobserved doses, often confirming with pill counts. Only a few sites attempted to track adherence using objective measures like medication event monitoring (MEM) caps, and only one used computer-assisted approaches to reduce demand characteristics (e.g., audio computer-assisted self-interview, handheld devices). All sites collected disease markers (e.g., viral load, viral resistance, CD4) to explore mDOT's impact.
In addition, all sites collected data on the number of mDOT sessions completed. Some also tracked “planned misses” to account for unobserved mDOT visits where patients knew in advance that they would have to miss a visit (e.g., doctor's appointment, travel) but wanted to plan for that dose. “Directly delivered doses” were also tracked by some sites that had patients on once per day regimes, where the only dose was taken at bedtime when a visit was impractical. Finally, all sites made attempts to minimize participant burden by combining evaluation sessions with clinic appointments or other services.
MINIMUM REQUIREMENTS FOR MDOT
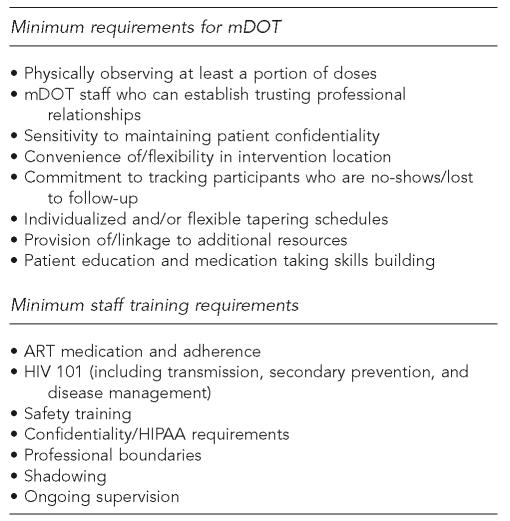
Reporting outcome data for the mDOT interventions described here was outside the scope of this article. Further, most of the studies did not attempt to break apart their mDOT interventions to establish the efficaciousness of particular aspects. However, drawing on the wealth of knowledge provided by the expert respondents, a review of relevant literature, and our own experience, we attempted to identify components that are likely important for producing high-quality mDOT interventions (Figure).
Figure.
Minimum mDOT and staff training requirements
mDOT = modified directly observed therapy
ART = antiretroviral therapy
HIV = human immunodeficiency virus
HIPAA = Health Insurance Portability and Accountability Act
While there was great variation in the professional background of individuals hired to perform mDOT interventions across studies, respondents clearly stated that these individuals must be capable of making strong interpersonal connections with participants. While proper training to increase understanding of HIV disease management and the challenges of adherence as well as ongoing supervision were cited as ways to help this occur, respondents were clear that the selection of staff who exhibited empathy with patients while maintaining professional boundaries was critical.
Another aspect of the participant-provider relationship highlighted by many sites entailed taking reasonable steps to ensure patient confidentiality in the field. For example, mDOT workers need to be prepared to creatively deliver medication (e.g., palm medications and hand-off to participant in a handshake) and/or allow participants to explain the mDOT worker's presence in creative ways (e.g., parole officers, friends, visiting nurses, etc.) that allow patients to maintain their confidentiality. This was especially important in some of the methadone maintenance clinics where individuals expressed concern about keeping their HIV+ status private. Given space limitations in many settings, careful planning is necessary to provide privacy that does not inadvertently attract attention to mDOT participants.
Flexibility in all aspects of the mDOT intervention was a recurrent theme. In describing flexibility, respondents were clearly focused on making their mDOT intervention as accessible for participants as possible. For example, given the already chaotic lives of many patients, respondents stressed the importance of working together to identify a convenient location for the intervention to occur. While several groups were successful in using a single community site, this might not work in all instances and ongoing verification that an agreed upon site was still convenient appeared to improve active participation. Respondents also stressed the importance of being flexible with how the intervention was delivered. For example, taking steps to contact patients who failed to show up for their mDOT intervention was stressed, with some sites sending staff out into the community to attempt to make contact with participants in known hangouts.
Respondents indicated that patient education and adherence skill development, as well as linkages to care, were important aspects of mDOT interventions. While these were consistent recommendations, the exact content suggested varied by site and appeared to be directly linked to patient populations and the existing services available. For example, some sites incorporated side-effect management into their mDOT interventions, whereas others suggested developing patients' ability to identify side effects and encouraging them to talk directly with their providers for management advice. The consistent theme was the importance of matching the intervention components to population needs and community resources.
Minimum staff training requirements
As highlighted in the Figure, respondents described what they felt were minimum training needs of mDOT staff. While much of the training was consistent with that which would be necessary for any intervention involving HIV+ individuals, mDOT did present some unique challenges. For example, because so many of the sites made contact with participants in their homes and communities, respondents felt that staff needed to receive high-quality safety training. Specifically, staff needed to be sufficiently “street smart” and effectively equipped to feel confident in these settings to make a connection with patients. The wide range of individuals who can be trained to perform mDOT interventions is a plus of this approach; however, respondents stressed proper training in HIV transmission, secondary prevention, disease management, and confidentiality/Health Insurance Portability and Accountability Act (HIPAA) issues to ensure that appropriate empathy can be communicated.
Respondents emphasized the importance of developing clear procedures for addressing medication side effects, nonadherence, and notification of providers. Depending on the study/program design, this can be a particularly thorny area; however, collaboration with providers and patients in the development of these procedures can help balance patient autonomy and provider responsibility concerns.
DISCUSSION
As is apparent from the diversity of the sites reviewed, mDOT for ART is feasible and easily adapted to many settings and target populations. More research is needed to ascertain which patients need and will benefit most from mDOT interventions; however, emerging data indicate that this approach may be particularly helpful for patients with a history of nonadherence.16
Despite concerns that daily visits would be seen as intrusive, results indicated that patients reported that the mDOT contacts were desirable and frequently requested to continue with visits past the active intervention period. Despite favorable views about mDOT, many respondents indicated that patients routinely expressed concerns about maintaining confidentiality, especially when services were provided in a methadone maintenance clinic. Respondents indicated that in most cases, minimal environmental adjustments, such as conducting mDOT in a private room and/or ensuring that other clients are unaware of what qualifies patients for specific programs, are sufficient to address these concerns.
For programs in which mDOT takes place in patients' homes, confidentiality concerns often centered on neighbors observing the visits. Nevertheless, when given the option, results indicated that most patients chose to receive mDOT visits at home. When questions were asked, patients tended to use creative explanations to account for the mDOT visits, and interventionists needed to be ready and willing to go along with patients' descriptions. With a little planning and willingness to adjust procedures to fit new contexts, mDOT is a viable treatment option in almost any context.
In this article, we have identified key elements of an mDOT intervention, but studies are still necessary to empirically test the effectiveness of components. Despite the lack of empirical data, it is clear from our expert respondents that components should match the needs of the target population and setting, and should include some direct observation of the ingestion of ART medications. However, providing direct observation often presents logistical challenges. For example, as patients stabilize on methadone treatment, they may be given take-home doses, meaning that they will not attend clinic daily. For community-based programs, patients may live outside of a reasonable catchment area. Creative solutions to address logistical challenges, such as observations via camera phones, video Internet, family members, or phone verification, were suggested by our respondents and should be explored in future studies. These solutions may be especially important for those who work with patients in rural communities or large metropolitan areas where urban sprawl is a problem.
Currently, there is no empirical evidence to guide important decisions about the structure of mDOT interventions (e.g., the ideal length of sessions, duration of intervention, content, tapering plan). However, what is clear from the results of this inquiry is that these decisions should match the specific needs of the target population and setting. Flexibility in the design and delivery of mDOT was a consistent theme. Several sites utilize a flexible model where patients can enter and leave mDOT as needed to maintain adherence. Outside of the research context, this may be the ideal approach. Despite the lack of empirical evidence to assist practitioners/researchers in making these decisions, the studies described in this article provide a preliminary guide and should assist in program design, staffing plans, and budgeting.
Offering incentives for participation in intervention is still controversial; however, some studies have found that it improves adherence to intervention and outcomes.27 Historically, paying for participation in interventions has been seen as problematic, as it might lead to compliance with an intervention that is not really feasible and, therefore, result in misleading findings. In addition, behavioral research indicates that extrinsic reinforcement for desired behaviors may actually decrease individuals' internal motivation, leading to a drop-off in the desired behavior.28 However, a recent meta-analysis refutes this claim, leading many to believe that providing incentives may maintain patient involvement in interventions long enough for them to experience intrinsic benefits.29 As more data emerge in the next few years, future studies/programs may find more support for decisions to offer incentives for intervention participation.
Collection of outcome data is important for improving our understanding of the impact of mDOT interventions. Most of the studies described here have used self-report measures of adherence to unobserved doses; however, more objective approaches (i.e., MEMs) may be particularly helpful in detecting subtle changes in behavioral adherence.
Outside of a research context, there are still good reasons to collect outcome data on mDOT interventions. In addition to contributing to the limited amount of effectiveness data, practice settings may be more successful in securing financial support for mDOT interventions if they collect data to demonstrate the impact on their patients. For example, some studies have observed that mDOT has an unexpected positive impact on other health behaviors, such as adherence to non-ART medications or other health behaviors.24 Others have reported improvements in patient mood and overall hope, which patients attributed to the daily contact with an mDOT interventionist.15,16 Data are also emerging that demonstrate the cost-effectiveness of mDOT for ART interventions;31 however, these additional benefits need to be documented and considered in the overall assessment of the viability of this labor-intensive intervention.
The significant contact with patients inherent in mDOT interventions may provide an ideal opportunity for the delivery of other important services, like secondary HIV prevention, smoking cessation, employment reintegration, or engagement in case management and overall health care. Not surprisingly, several groups are exploring the feasibility and efficacy of these types of combined interventions.32
Regardless of the intervention focus, respondents indicated that careful planning and collaboration regarding intervention components and educational materials is important to ensure that the content is consistent with providers' recommendations. Close collaboration with providers is always a challenge, as their time is limited and their responsibilities are vast. But most are deeply committed to providing patients with the best resources possible and will be eager to ensure that program materials are consistent with that goal. Just as with the establishment of any intervention program, mDOT programs present numerous logistical challenges. Careful planning and consultation with successful programs, such as those highlighted in this article, may offer potential solutions to these challenges.
mDOT interventions in the real world
Despite the limited empirical evidence for the efficacy of mDOT for ART, many health-care providers have embraced its potential and found ways to integrate it into their clinical settings. For example, the Joint AIDS Community-wide Quest for Unique and Effective Treatment Strategies (JACQUES) Demonstrative Project at the University of Maryland's Institute of Human Virology provides services for African American, HIV+, homeless, or marginally housed individuals with co-occurring substance abuse and/or other mental health disorders (Table).33 Participants are offered an array of services, including life skills workshops, support groups, bible study, and mDOT. Their flexible approach allows patients to choose how many daily mDOT contacts they want per week, and patients can start, stop, and restart any of the available services (including mDOT) at will for up to 96 weeks.
Another example of mDOT in the real world is a program directed by the British Columbia Center for Excellence in HIV/acquired immunodeficiency syndrome.34 This program provides daily DOT for patients on once per day ART regimes. In addition to DOT, needs assessments, linkages to other resources, and addictions counseling are provided during brief visits at a convenient clinic or field site. Patients create individualized tapering schedules and all services, including daily DOT, are provided for as long as patients are interested and benefiting from services. This program is offered free of charge by the British Columbia Ministry of Health to all who need it.
Clearly, mDOT has promise and should be considered among the spectrum of adherence interventions for HIV+ individuals.
Acknowledgments
The authors wish to acknowledge the important contributions of the researchers and project directors who generously shared their experience. The authors also thank Dr. Linda Garavalia and colleagues from the University of Missouri Kansas City Human Immunodeficiency Virus Research Group and Project MOTIV8 for their helpful comments and feedback on earlier versions of this article. The authors also thank the Canadian Acquired Immunodeficiency Syndrome Treatment Information Exchange (www.catie.ca) for sharing its expertise.
Footnotes
This work was supported by National Institute of Mental Health grant RO1 MH68197.
REFERENCES
- 1.Staszewski S, Morales-Ramirez J, Tashima KT, Rachlis A, Skiest D, Stanford J, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. N Engl J Med. 1999;341:1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 2.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 3.Saag MS, Tebas P, Sension M, Conant M, Myers R, Chapman SK, et al. Randomized, double-blind comparison of two nelfinavir doses plus nucleosides in HIV-infected patients (Agouron study 511) AIDS. 2001;15:1971–8. doi: 10.1097/00002030-200110190-00009. [DOI] [PubMed] [Google Scholar]
- 4.McMahon D, Lederman M, Haas DW, Haubrich R, Stanford J, Cooney E, et al. Antiretroviral activity and safety of abacavir in combination with selected HIV-1 protease inhibitors in therapy-naïve HIV-1 infected adults. Antivir Ther. 2001;6:105–14. [PubMed] [Google Scholar]
- 5.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–33. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 6.Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1998;351:543–9. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 7.Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DL, Swindells S, Mohr J, Brester M, Virgis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Shafer RW, Winters MA, Palmer S, Merigan TC. Multiple concurrent reverse transcriptase and protease mutations and multidrug resistance of HIV-1 isolates from heavily treated patients. Ann Intern Med. 1998;128:906–11. doi: 10.7326/0003-4819-128-11-199806010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Tuldra A, Fumaz CR, Ferrer MJ, Bayes R, Arno A, Balague M, et al. Prospective randomized two-arm controlled study to determine the efficacy of a specific intervention to improve long-term adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:221–8. doi: 10.1097/00126334-200011010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Fischl M, Rodriguez A, Scerpella E, Mondroig R, Thompson L, Rechtine D. Impact of directly observed therapy on outcomes in HIV clinical trials. Proceedings of the 7th Conference on Retroviruses and Opportunistic Infections; 2000 Jan 30–Feb 2; San Francisco. [Google Scholar]
- 13.Babudieri S, Aceti A, D'Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284:179–80. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Lanzafame M, Trevenzoli M, Cattelan AM, Rovere P, Parrinello A. Directly observed therapy in HIV therapy: a realistic perspective? J Acquir Immune Defic Syndr. 2000;25:200–1. doi: 10.1097/00042560-200010010-00018. [DOI] [PubMed] [Google Scholar]
- 15.Mitty JA, Macalino GE, Bazerman LB, Loewenthal HG, Hogan JW, MacLeod CJ, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–50. [PubMed] [Google Scholar]
- 16.Mitty JA, Delong AK, Macalino GE, Hogan JW, Bazerman LB, Loewenthal HG, et al. A randomized controlled trial of modified directly observed HAART (mDOT) vs. standard of care (SOC) among HIV+ active substance users. Proceedings of the 43rd Annual Meeting of the Infectious Diseases Society of America; 2005 Oct 6–9; San Francisco. [Google Scholar]
- 17.Kagay CR, Porco TC, Liechty CA, Charlebois E, Clark R, Guzman D, et al. Modeling the impact of modified directly observed antiretroviral therapy on HIV suppression and resistance, disease progression, and death. Clin Infect Dis. 2004;38(Suppl 5):S414–20. doi: 10.1086/421406. [DOI] [PubMed] [Google Scholar]
- 18.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–35. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 19.Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38(Suppl 5):S376–87. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- 20.Wohl AR, Garland W, Valencia R, Squires K, Witt M, Kovacs A, et al. A randomized trial of directly administered antiretroviral therapy (DAART) and an adherence case management program (IAP). Proceedings of the 43rd Annual Meeting of the Infectious Diseases Society of America; 2005 Oct 6–9; San Francisco. [DOI] [PubMed] [Google Scholar]
- 21.Arnsten JH, Berg KM, Cooperman NA, Villanueva M, Li X, Parker F, et al. A 6-month, randomized controlled trial of directly observed therapy delivered in methadone clinics. Proceedings of the 2nd NIMH/IAPAC International Conference on HIV Treatment Adherence; 2007 Mar 28–30; Jersey City, New Jersey. [Google Scholar]
- 22.Behforouz HL, Kalmus A, Scherz C, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immun Defic Syndr. 2004;36:642–5. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 23.Goggin K, Gerkovich M, Wright J, Catley D, William K. Review of a randomized controlled community trial utilizing mDOT. Proceedings of the NIMH/IAPAC International Conference on HIV Treatment Adherence; 2006 Mar 8–10; Jersey City, New Jersey. [Google Scholar]
- 24.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006;43:234–42. doi: 10.1086/505212. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell CG, Freels S, Creticos CM, Oltean A, Douglas R. Preliminary findings of an intervention integrating modified directly observed therapy and risk reduction counseling. AIDS Care. 2007 doi: 10.1080/09540120601040813. In press. [DOI] [PubMed] [Google Scholar]
- 26.Wohl AR, Garland WH, Valencia R, Squires K, Witt MD, Kovacs A, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42:1619–27. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 27.Bangsberg DR. Can mDOT for adherence to anti-depressant medications impact ART adherence?. Proceedings of the NIMH/IAPAC International Conference on HIV Treatment Adherence; 2006 Mar 8–10; Jersey City, New Jersey. [Google Scholar]
- 28.Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125:627–68. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- 29.Cameron J, Banko KM, Pierce WD. Pervasive negative effects of rewards on intrinsic motivation: the myth continues. Behavior Analyst. 2001;24:1–44. doi: 10.1007/BF03392017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinkston MM, Bradley-Ewing A, Malomo D, Goggin KJ. A qualitative examination of the indirect effects of modified directly observed therapy on health behaviors other than adherence. Annals Behav Med. 2007;33(Suppl):S046. doi: 10.1089/apc.2007.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, III, Kimmel AD, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115:632–41. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell CG, Freels S, Creticos C, Oltean A, Liu H, Douglas R. Preliminary findings of modified directly observed therapy and risk reduction counseling for a population of marginally housed HIV+ persons. AIDS Care. 2007 doi: 10.1080/09540120601040813. In press. [DOI] [PubMed] [Google Scholar]
- 33.Tyndall M, McNally M, Lai C, Zhang R, Wood E, Kerr T, et al. Directly observed therapy programs for antiretroviral treatment among injection drug users in Vancouver: access, adherence and outcomes. Int J Drug Policy. 2007 doi: 10.1016/j.drugpo.2006.11.009. In press. [DOI] [PubMed] [Google Scholar]
- 34.Amoroso A, Spencer DE, Redfield RR. Improving on success: what treating the urban poor in America can teach us about improving antiretroviral programmes in Africa. AIDS. 2004;18(Suppl 3):S39–43. doi: 10.1097/00002030-200406003-00008. [DOI] [PubMed] [Google Scholar]