Abstract
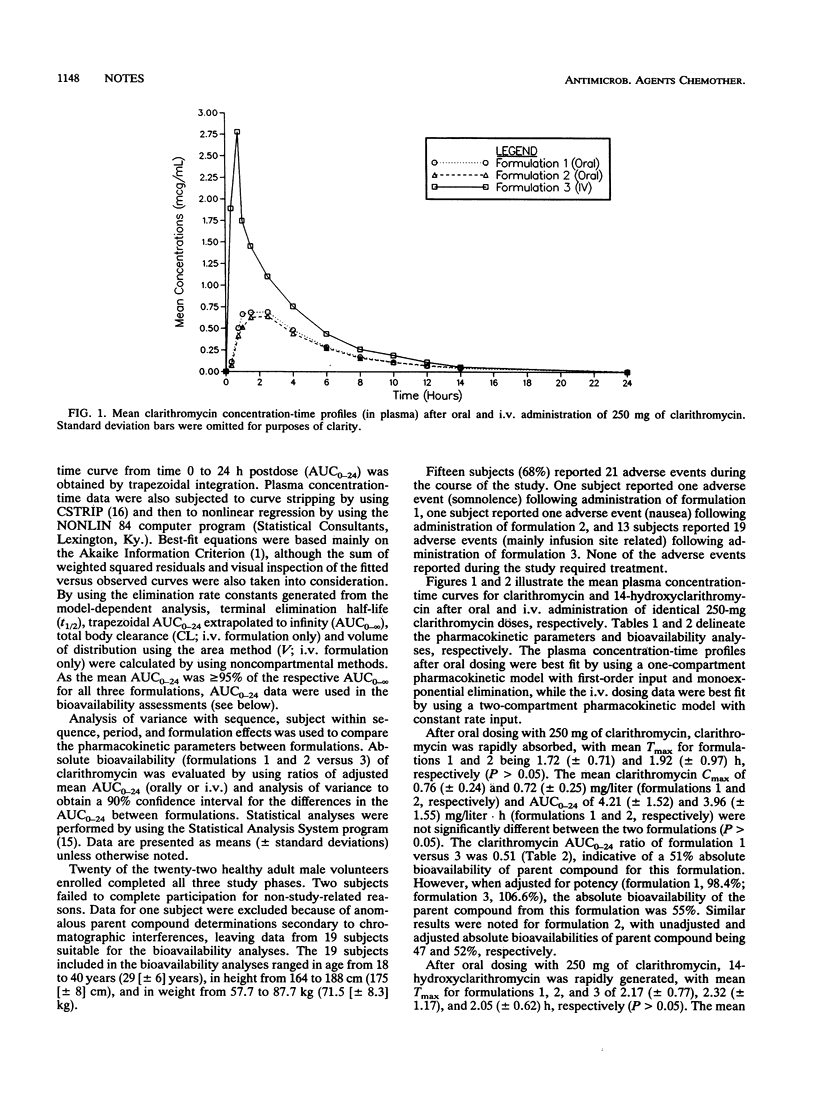
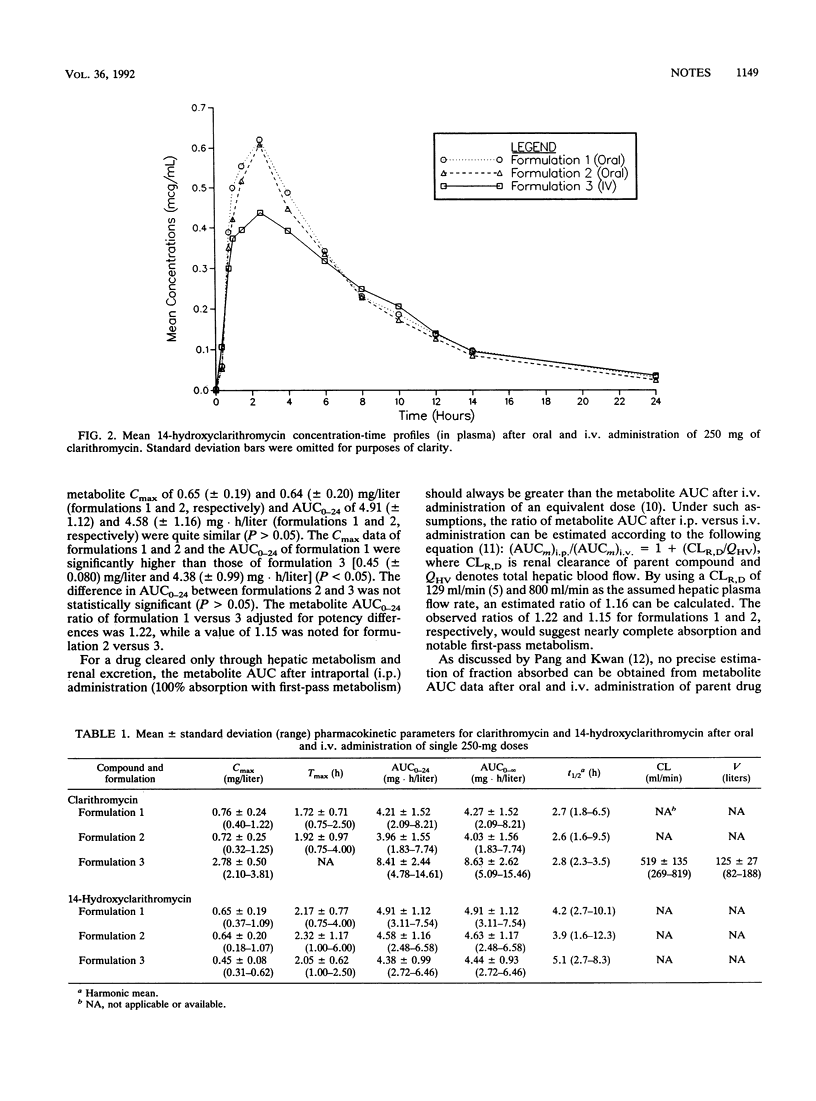
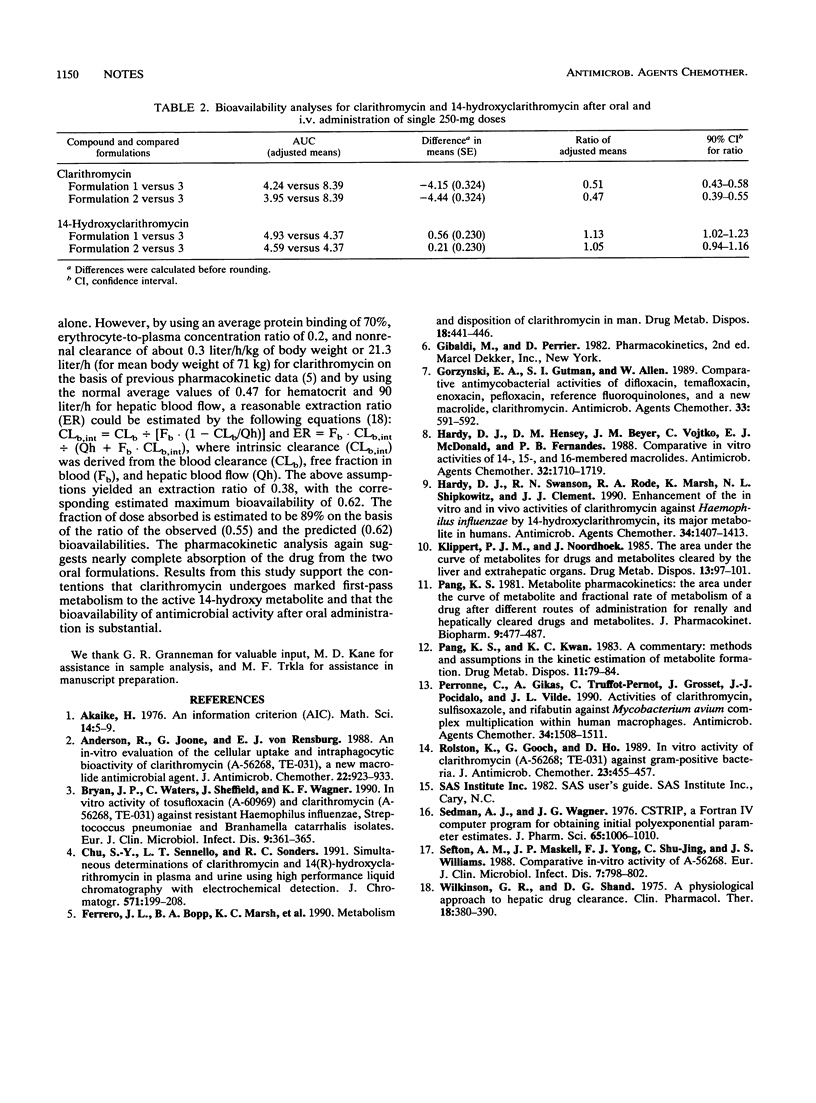
The absolute bioavailability of clarithromycin, a new macrolide antimicrobial agent, was assessed in a three-way, randomized, single-dose, crossover study conducted with 22 healthy volunteers, 19 of whom provided analyzable study data. The bioavailability parameters of two 250-mg oral tablet formulations were calculated with reference to an identical dose administered by intravenous infusion of the lactobionate salt. After adjustment for formulation potency, the mean absolute bioavailabilities of the two oral formulations were 52 and 55%, on the basis of the appearance of parent compound in the systemic circulation. Metabolite peak concentration and area under the plasma concentration-time curve data after oral dosing were generally greater than those after intravenous infusion, suggesting that marked first-pass metabolism of clarithromycin occurs after oral administration. Pharmacokinetic analysis of the parent drug and the active 14-hydroxy metabolite data suggests complete (or nearly complete) absorption of the drug after oral administration.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Joone G., van Rensburg C. E. An in-vitro evaluation of the cellular uptake and intraphagocytic bioactivity of clarithromycin (A-56268, TE-031), a new macrolide antimicrobial agent. J Antimicrob Chemother. 1988 Dec;22(6):923–933. doi: 10.1093/jac/22.6.923. [DOI] [PubMed] [Google Scholar]
- Bryan J. P., Waters C., Sheffield J., Wagner K. F. In vitro activity of tosufloxacin (A-60969) and clarithromycin (A-56268, TE-031) against resistant Haemophilus influenzae, Streptococcus pneumoniae and Branhamella catarrhalis isolates. Eur J Clin Microbiol Infect Dis. 1990 May;9(5):361–365. doi: 10.1007/BF01973747. [DOI] [PubMed] [Google Scholar]
- Chu S. Y., Sennello L. T., Sonders R. C. Simultaneous determination of clarithromycin and 14(R)-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1991 Nov 15;571(1-2):199–208. doi: 10.1016/0378-4347(91)80446-j. [DOI] [PubMed] [Google Scholar]
- Ferrero J. L., Bopp B. A., Marsh K. C., Quigley S. C., Johnson M. J., Anderson D. J., Lamm J. E., Tolman K. G., Sanders S. W., Cavanaugh J. H. Metabolism and disposition of clarithromycin in man. Drug Metab Dispos. 1990 Jul-Aug;18(4):441–446. [PubMed] [Google Scholar]
- Gorzynski E. A., Gutman S. I., Allen W. Comparative antimycobacterial activities of difloxacin, temafloxacin, enoxacin, pefloxacin, reference fluoroquinolones, and a new macrolide, clarithromycin. Antimicrob Agents Chemother. 1989 Apr;33(4):591–592. doi: 10.1128/aac.33.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Hensey D. M., Beyer J. M., Vojtko C., McDonald E. J., Fernandes P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988 Nov;32(11):1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Swanson R. N., Rode R. A., Marsh K., Shipkowitz N. L., Clement J. J. Enhancement of the in vitro and in vivo activities of clarithromycin against Haemophilus influenzae by 14-hydroxy-clarithromycin, its major metabolite in humans. Antimicrob Agents Chemother. 1990 Jul;34(7):1407–1413. doi: 10.1128/aac.34.7.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippert P. J., Noordhoek J. The area under the curve of metabolites for drugs and metabolites cleared by the liver and extrahepatic organs. Its dependence on the administration route of precursor drug. Drug Metab Dispos. 1985 Jan-Feb;13(1):97–101. [PubMed] [Google Scholar]
- Pang K. S., Kwan K. C. A commentary: methods and assumptions in the kinetic estimation of metabolite formation. Drug Metab Dispos. 1983 Mar-Apr;11(2):79–84. [PubMed] [Google Scholar]
- Pang K. S. Metabolite pharmacokinetics: the area under the curve of metabolite and the fractional rate of metabolism of a drug after different routes of administration for renally and hepatically cleared drugs and metabolites. J Pharmacokinet Biopharm. 1981 Aug;9(4):477–487. doi: 10.1007/BF01060890. [DOI] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Pocidalo J. J., Vilde J. L. Activities of clarithromycin, sulfisoxazole, and rifabutin against Mycobacterium avium complex multiplication within human macrophages. Antimicrob Agents Chemother. 1990 Aug;34(8):1508–1511. doi: 10.1128/aac.34.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston K., Gooch G., Ho D. In-vitro activity of clarithromycin (A-56268; TE-031) against gram-positive bacteria. J Antimicrob Chemother. 1989 Mar;23(3):455–457. doi: 10.1093/jac/23.3.455. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Sefton A. M., Maskell J. P., Yong F. J., Chi S. J., Williams J. D. Comparative in vitro activity of A-56268. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):798–802. doi: 10.1007/BF01975054. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Shand D. G. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975 Oct;18(4):377–390. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]


