Abstract
There is now direct evidence that a class of neurons in the rostral ventromedial medulla (RVM) exerts a net facilitatory influence on spinal nociception. The present experiments were designed to test whether activation of these neurons, referred to as “on-cells”, is required as part of a positive feedback loop leading to secondary hyperalgesia in acute inflammation produced by topical application of mustard oil. Activity of a characterized RVM neuron and paw withdrawals to heat (plantar surface) were recorded in barbiturate-anesthetized rats. Following three baseline trials, mustard oil was applied to the skin above the knee. Cell activity and paw withdrawal latencies were monitored for an additional 45 min. Application of mustard oil produced an increase in on-cell discharge that was associated with a substantial decrease in withdrawal latency of the ipsilateral paw. Blocking on-cell activation using local infusion of the NMDA-receptor antagonist AP5 into the RVM prevented hyperalgesia. Secondary thermal hyperalgesia following mustard oil was also associated with a significant decrease in the firing of “off-cells”, a cell population thought to exert a net inhibitory influence on nociception. Depression of off-cell firing was unaffected by AP5 microinjection. The firing of “neutral cells”, which have no documented role in nociceptive modulation, was unchanged following mustard oil and also unaffected by AP5 infusion in the RVM. Brainstem descending controls are receiving increasing attention in efforts to understand hyperalgesia and persistent pain states. The present experiments demonstrate that a novel, NMDA-mediated activation of on-cells is required for secondary thermal hyperalgesia in acute inflammation.
Keywords: Rostral ventromedial medulla, Descending control, On-cells, Pain modulation
1. Introduction
The best characterized and arguably functionally most significant brainstem nociceptive modulatory system has major links in the midbrain periaqueductal gray and rostral ventromedial medulla (RVM). Although originally regarded as mediating analgesia, this system is now widely recognized to exert bidirectional control. Thus, although it is a critical substrate for opioid and stress-induced analgesia, the PAG/RVM system also contributes to enhanced nociception in models of nerve injury and sickness, prolonged opioid administration and acute opioid withdrawal, and inflammation (Porreca et al., 2002; Ren and Dubner, 2002; Heinricher et al., 2003).
Establishing the relative contribution of descending facilitation and inhibition to altered nociceptive behavior requires that the facilitating and inhibiting outputs from the RVM be differentiated experimentally. In principle, bidirectional control from the RVM could be explained by the recruitment of “on-cells” and “off-cells” to produce hyperalgesia and hypoalgesia, respectively. On-cells are defined by a burst of activity associated with withdrawal reflexes, and direct selective activation of on-cells facilitates nociceptive withdrawals (Heinricher and Neubert, 2004; Neubert et al., 2004). Off-cells are defined by a reflex-related pause in firing. Activation of off-cells is sufficient to produce reflex inhibition, and necessary for opioid analgesia (Heinricher and Tortorici, 1994; Heinricher et al., 2001a,b; Heinricher and Neubert, 2004; Neubert et al., 2004). On-cells therefore exert a net facilitatory influence on nociception, and off-cells a net inhibitory influence. There is also indirect evidence that “neutral cells” have a role in nociceptive modulation, although these neurons do not respond to noxious stimulation or opioids (Barbaro et al., 1986; Miki et al., 2002; Suzuki et al., 2004). The contribution of each cell class must therefore be delineated in order to explain the net influence of descending controls from the RVM on nociceptive behavior.
We recently showed that secondary thermal hyperalgesia induced by topical application of mustard oil is associated with a shift in the balance of activity of RVM on-cells and off-cells, so that the on-cell population is more likely to be active at any given time, and off-cells less active. Neutral cell firing is unchanged (Kincaid et al., 2006). These correlative data suggested that secondary thermal hyperalgesia is mediated by an increase in descending facilitation from on-cells, with a possible further potentiation due to decreased descending inhibition. However, although firing of neutral cells was unchanged following mustard oil, the possibility that ongoing activity of neutral cells is relevant to hyper-algesia cannot be ruled out, especially since dorsal horn circuitry may be more sensitive to descending control during inflammation (Vanegas and Schaible, 2004).
The goal of the present studies was to determine if on-cells are the RVM output mediating secondary thermal hyperalgesia in acute inflammation. We combined behavioral testing with single cell recording to show that topical application of mustard oil leads to a novel NMDA-mediated activation of on-cells that is necessary for secondary thermal hyperalgesia in this model. On-cells are thus recruited to facilitate nociception as part of a positive-feedback loop triggered by acute cutaneous inflammation.
2. Experimental procedures
2.1. Animals, surgical preparation and nociceptive testing
All experimental procedures followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain, and were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Male Sprague–Dawley rats (Sasco, 250–300 g) were anesthetized with pento-barbital (60 mg/kg, i.p.), and a catheter inserted into an external jugular vein for administration of sodium methohexital. Each rat was placed in a stereotaxic apparatus, a hole drilled in the skull over the cerebellum, and the dura removed to allow placement of an electrode assembly in the RVM. Body temperature was maintained at approximately 37 °C by a circulating water pad.
Following surgery, the anesthetic level was allowed to lighten until a paw withdrawal reflex (PW) could be elicited by application of heat to the left hindpaw using a feedback-controlled projector lamp focused on the blackened plantar surface of the paw. Following surgical preparation, the animals were maintained in a lightly anesthetized state using a continuous infusion of methohexital at a rate (15–30 mg/kg per h, i.v.) that allowed a stable withdrawal latency and prevented any signs of discomfort. The animals did not move spontaneously, nor did they vocalize or produce vigorous or prolonged withdrawal reflexes following noxious pinch. The rate was adjusted for each animal to allow a baseline paw withdrawal latency of approximately 3 s. The protocol was begun after a stabilization period of at least 30 min, and the infusion rate was not altered during the protocol.
Latency of the paw withdrawal to heat was used as a measure of nociceptive responsiveness, as described previously (Barbaro et al., 1989; Neubert et al., 2004). Each trial consisted of a linear increase in temperature at approximately 1.8 C/s from a holding temperature of 34 °C until the reflex occurred or to a maximum of 53 °C at 10.6 s. Trials were carried out at 5 min intervals throughout the experiment. The holding temperature obviates any concern that apparent effects on reflex latency could be attributed to changes in skin temperature.
2.2. RVM recording and microinjection
A gold- and platinum-plated stainless steel recording micro-electrode (Frederick Haer Co., Brunswick, ME) was glued parallel to a single-barrel glass infusion pipette with an outer diameter of 75–80 μm in such a way that the tips were separated laterally by 100–300 μm. This separation allowed us to maintain well-isolated recordings of single neurons during and after infusions of 200 nl of saline vehicle or drug solution in the RVM in a reasonable number of experiments. The assembly was oriented in the electrode carrier so that it straddled the mid-line, with both electrode and infusion pipette within the RVM. The infusion pipette was attached to a 1 μl Hamilton syringe with a length of PE-50 tubing for drug infusion.
RVM neurons were classified as previously described (Fields et al., 1983). Spike waveforms were monitored and stored for off-line analysis (Datawave Systems, Thornton, CO) to ensure that the unit under study was unambiguously discriminated throughout the experiment, and to verify that the microinjected solutions did not have local anesthetic effects. Spike times were stored with a temporal resolution of 0.1 ms. Off-cells were characterized by an abrupt pause in ongoing activity beginning just prior to the occurrence of the withdrawal. On-cells were identified by a sudden burst of activity beginning just prior to the occurrence of the withdrawal. Neutral cells were identified by a lack of response during paw withdrawal, and they did not typically respond to noxious or innocuous cutaneous stimulation. Because the reflex-related on-cell activation cannot normally be detected when the neuron is already spontaneously active (which could cause misclassification as a neutral cell), and because the reflex-related off-cell pause can be seen only during active periods, we attempted to test each neuron during both active and silent periods. The behavioral testing protocol was initiated once a well-isolated cell with a robust reflex-related change in activity (or lack of response, in the case of a neutral cell) was identified.
2.3. Protocol and data analysis
Initial experiments monitored paw withdrawal only, without recording, to determine the effects of AP5 and CNQX on hyperalgesia, and to identify appropriate doses for electrophysiological experiments. Experimenters were blinded as to drug identity in this phase. Following three baseline PW trials, saline vehicle (200 nl), AP5 (0.5, 1 or 2 nmol/200 nl) or CNQX (250 or 500 pmol/200 nl) was infused into the RVM. Withdrawal latency was tested once again, and then mustard oil (100%) or mineral oil was applied to the shaved surface of the left or right lateral thigh just above the knee (200 μl saturating a filter paper pledget, approx. 15 × 12 mm for 2 min). Withdrawal latency (left hindpaw) was then monitored for an additional 45 min. Only one protocol was performed in each animal.
Once it was determined that 2 nmol AP5 microinjected into the RVM blocked secondary thermal hyperalgesia (Section 3.1), a second series of studies was performed in which cell recording was combined with behavioral testing. The effects of saline were compared with those of 2 nmol AP5. Three cell parameters were analyzed. (1) Ongoing activity. Because off-cells and on-cells often show irregular alternations between periods of silence and activity, cell activity integrated over the 30 s prior to each withdrawal trial was used as an overall index of ongoing firing. (2) On-cell PW-related burst. Average firing rate in the 3 s period beginning 0.5 s before the PW was recorded for all PW trials. This approach, rather than counting the number of spikes or duration of the reflex-related burst, was necessary because a burst can only be identified in cases in which the neuron happens to be inactive at the time of heat onset. (3) Cycling. The proportion of time that a given cell was considered to be in an “active” or “silent” period was defined as described previously (Barbaro et al., 1989). Briefly, an active period was defined as any epoch lasting at least 2 s with a minimum of 1 spike/s, and a silent period as any epoch of at least 2 s without any cell activity. The proportion of time during which each on- or off-cell was “active” or “silent” was then calculated for the baseline and post-treatment periods. Only one protocol was performed in each animal.
Data are presented as means ± SEM. Non-parametric statistics (Wilcoxon's signed ranks test, Mann–Whitney test) were used for statistical comparison of cell parameters; ANOVA for repeated measures followed by Dunnett's test or t-test for correlated means was used to compare baseline and post-inflammation withdrawal latencies. Comparisons of paw withdrawal latencies among groups were performed using ANOVA followed by Dunnett's test. p < 0.05 was considered statistically significant.
2.4. Histology
At the conclusion of the experiments, recording sites were marked with an electrolytic lesion, and infusion sites by injection of pontamine sky blue dye. Animals were euthanized with an overdose of methohexital, and perfused intracardially with physiological saline followed by 10% formalin. Recording and infusion sites were histologically verified. The RVM was defined as the nucleus raphe magnus and adjacent reticular formation at the level of the facial nucleus. Recording sites were distributed in the RVM as in previous publications from this laboratory (Heinricher and Tortorici, 1994; Heinricher and Roychowdhury, 1997).
3. Results
3.1. Secondary thermal hyperalgesia evoked by application of mustard oil, and dose-related block by AP5 in the RVM
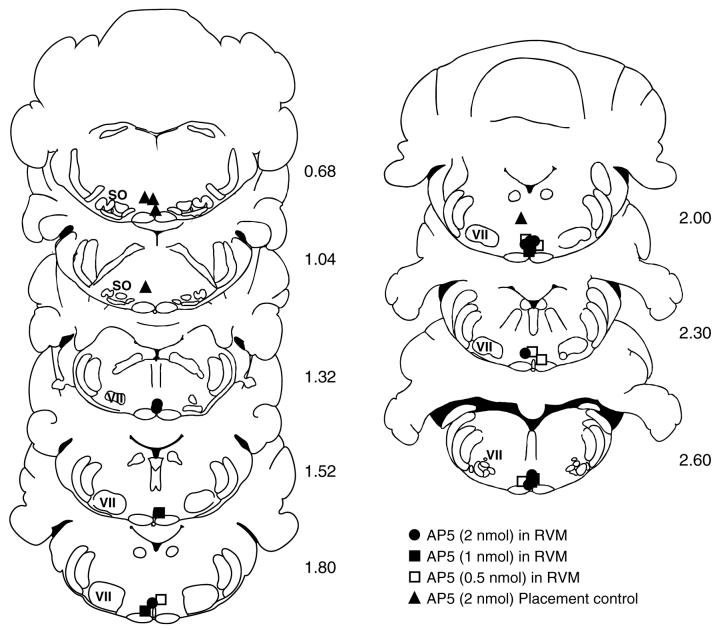
Topical application of mustard oil on the left leg produced a significant decrease in response latency to heat in control animals treated with saline vehicle in the RVM. This hyperalgesia was attenuated in a dose-dependent manner by microinjection of the NMDA-receptor antagonist AP5 into the RVM (Fig. 1A). The effect of AP5 was due to an action in the RVM because microinjections in immediately adjacent regions failed to attenuate the hyperalgesia (Fig. 1A, Placement Control). AP5 had no effect on paw withdrawal latency in animals subsequently treated with mineral oil rather than mustard oil (Fig. 1A, AP5 + Min. Oil). Microinjection sites for AP5 in this set of experiments are shown in Fig. 2.
Fig. 1.
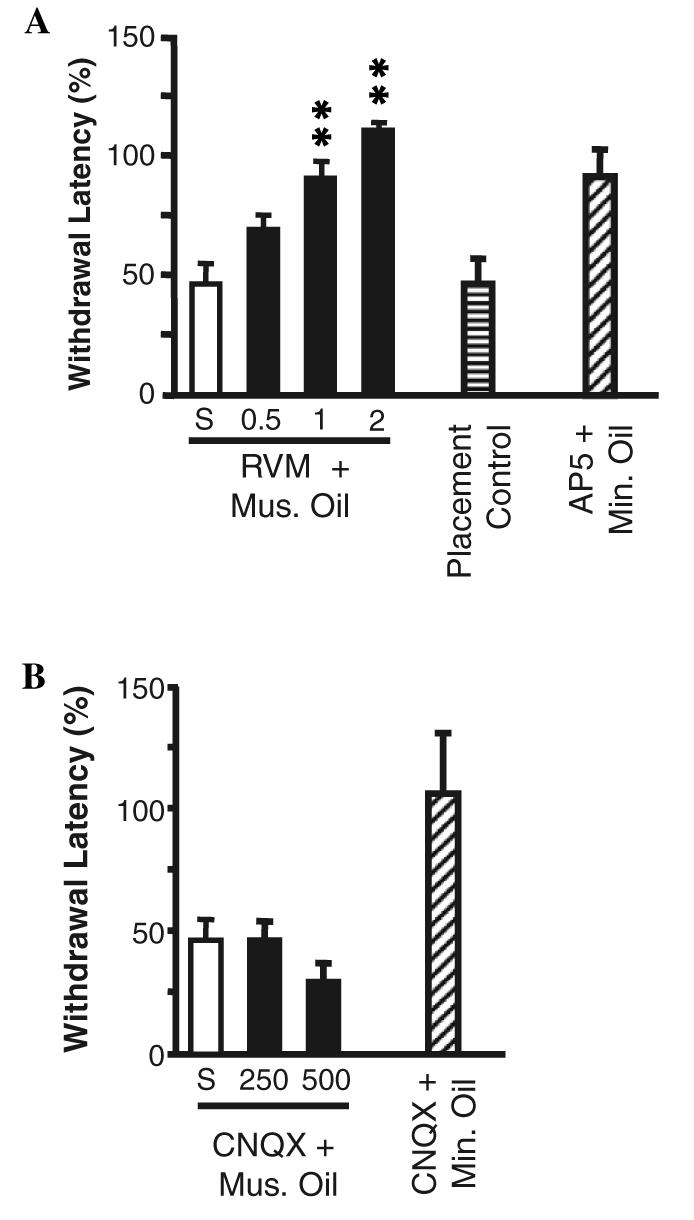
(A) Dose-dependent block of mustard oil-induced secondary hyperalgesia by microinjection of NMDA receptor antagonist AP5 into the RVM. Paw withdrawal latency following RVM microinjection combined with topical application of mustard oil (Mus. Oil) or mineral oil (Min. Oil) is expressed as percent of pre-microinjection baseline latency. Animals in which saline vehicle (S, n = 9) was injected into the RVM showed a significant hyperalgesia. This was attenuated in a dose-dependent manner by microinjection of 0.5, 1 or 2 nmol AP5 into the RVM prior to application of mustard oil (6–9 animals/group, ANOVA followed by Dunnett's test for comparison to saline-treated controls, **p < 0.01). Microinjection of AP5 adjacent to the RVM failed to block the mustard oil-induced hyperalgesia (Placement Control, n =5, p > 0.05 compared to saline). AP5 (2 nmol) had no effect on paw withdrawal latency in animals in which mineral oil, rather than mustard oil, was applied to the limb (p > 0.05 compared to baseline, n = 4). There were no differences in baseline latencies among the groups (ANOVA, p > 0.05). (B) Microinjection of non-NMDA receptor antagonist CNQX into the RVM does not interfere with mustard oil-induced secondary hyperalgesia. Paw withdrawal latency following RVM microinjection of CNQX and application of mustard oil (Mus. Oil) or mineral oil (Min. Oil) is expressed as percent of premicroinjection baseline latency. Saline-treated animals (S, n = 9, from previous figure) showed significant hyperalgesia, and this was not attenuated by microinjection of 250 (n = 5) or 500 (n = 8) pmol CNQX (p > 0.05 compared to baseline). CNQX (500 pmol) did not alter paw withdrawal latencies in control animals treated with mineral oil rather than mustard oil. There were no differences in baseline latencies among the groups (ANOVA, p > 0.05).
Fig. 2.
Histologically verified locations of infusion sites in experiments in Fig. 1A in which different doses of AP5 were infused into the RVM or adjacent medulla. Placement controls were rostral or dorsal to the RVM. VII, facial nucleus; SO, superior olive. Distance from the interaural line is indicated.
We previously showed that secondary hyperalgesia in this paradigm was limited to the treated limb (Kincaid et al., 2006). In the present study, we tested the possibility that this was because topical mustard oil recruited a “diffuse noxious inhibitory control” mechanism that balanced the pro-nociceptive influence from the RVM. Mustard oil was applied to the limb contralateral to the tested paw following microinjection of AP5 into the RVM. No increase in paw withdrawal latency was seen under these conditions (PW latency 2.41 ± 0.18 s in baseline vs. 2.00 ± 0.33 s following mustard oil application to the contralateral hindlimb in animals in which AP5 was microinjected into the RVM, p > 0.05). This rules out a remote inhibition, which presumably would have been revealed following block of descending facilitation from the RVM.
By contrast with the actions of AP5, microinjection of the non-NMDA receptor antagonist CNQX into the RVM (Fig. 1B) did not interfere with the secondary thermal hyperalgesia produced by mustard oil. CNQX by itself had no effect on paw withdrawal latencies in control animals treated with mineral oil.
3.2. AP5-induced blockade of prolonged on-cell activation produced by cutaneous application of mustard oil
We previously showed that application of mustard oil to the skin evoked a substantial and sustained increase in the firing of on-cells, decrease in the firing of off-cells and no change in the firing of neutral cells (Kincaid et al., 2006). This activation was correlated with decreased paw withdrawal latency. In order to determine whether block of on-cell activation could explain the ability of AP5 to prevent hyperalgesia in this model, we next recorded the activity of identified on-cells, off-cells and neutral cells in animals pre-treated with AP5 (2 nmol) or saline vehicle prior to application of mustard oil. As in the more extensive dose–response studies outlined above, hyperalgesia was blocked by microinjection of this dose of AP5 in the RVM (Fig. 3). Consistent with a previous report from this laboratory (Kincaid et al., 2006), thermal hyperalgesia was evident at the first test after removal of the mustard oil-soaked pledget, and was maintained for the entire 45 min monitoring period in control animals (Fig. 3, saline).
Fig. 3.
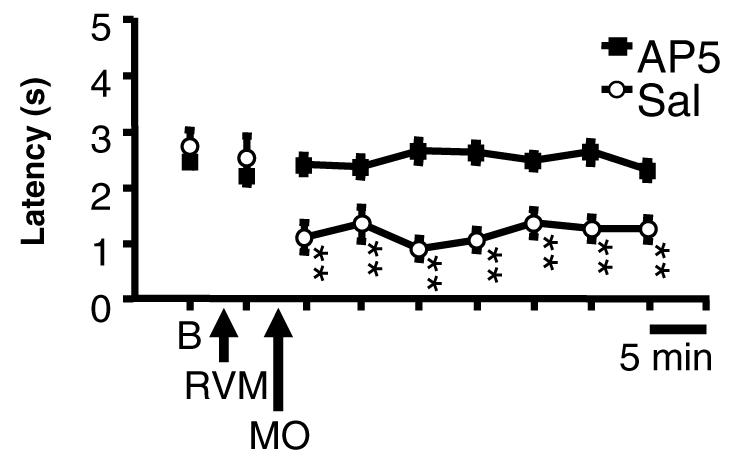
Time course of changes in paw withdrawal latencies of animals treated with 2 nmol AP5 vs. saline in recording experiments. B, Baseline; RVM, microinjection of AP5 (2 nmol, n = 41) or saline (n = 16); MO, application of mustard oil. (p < 0.01 ANOVA for repeated measures followed by Dunnett's test for comparison of each time point to baseline). There was no difference in baseline latencies between the groups (t-test, p > 0.05).
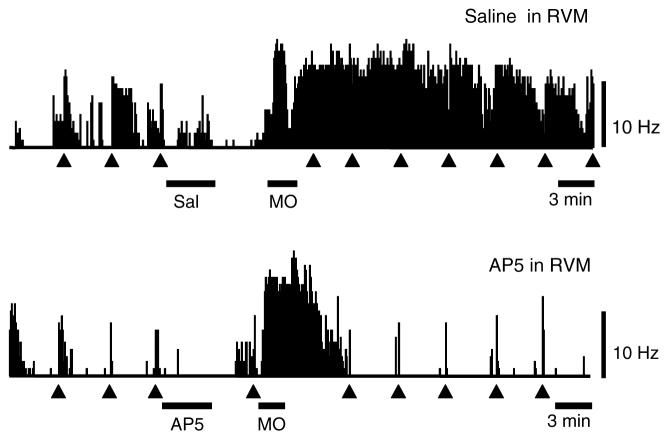
On-cells recorded from control animals pre-treated with saline in the RVM showed a robust, long-lasting activation following application of mustard oil. This was prevented in animals in which AP5 was microinjected into the RVM. Ratemeter records in Fig. 4 show two representative cells. The effect of AP5 is quantified in group data presented in Fig. 5, which shows ongoing firing in saline- and AP5-treated animals. On-cells recorded in control animals showed a significant activation associated with the period of mustard oil application, and firing rate remained significantly elevated for at least 20 min. The greater level of overall activity was due to an increase in the percentage of time that the neurons were active, with no change in firing rate during active periods (Fig. 6). By contrast, on-cells recorded in animals pre-treated with AP5 did not show a significant increase in ongoing firing (Fig. 5), reflecting the fact that both percentage of time in active periods and firing rate during active periods were unchanged following mustard oil (Fig. 6). Ongoing firing prior to mustard oil application was unaffected by RVM microinjection of AP5 or saline (Fig. 5, On-Cells, RVM and Fig. 6, RVM).
Fig. 4.
Ratemeter records illustrate effect of AP5 on the on-cell response to cutaneous mustard oil. Saline control, cell recorded before and after microinjection of saline (200 nl) into the RVM illustrates typical response to mustard oil, with a prolonged elevation of ongoing firing. The cell was active 38.7% of the baseline period, but 98.4% of the time following mustard oil application. Firing rate during active periods was increased slightly, from 4.5 spikes/s in baseline, to 6.5 spikes/s following removal of the mustard oil. Paw withdrawal latency in this animal was 49% of baseline following mustard oil. AP5 in RVM, cell recorded before and after microinjection of AP5 (2 nmol/200 nl). The cell was activated in association with vigorous hindlimb withdrawals during the period in which mustard oil was on the left hindlimb. However, this elevated firing was not maintained upon removal of the mustard oil-soaked pledget. The cell was active 14.8% of the baseline period, and there was substantial activation that outlasted the period of mustard oil application by a little more than 4 min. However, once that burst of activity was complete, the cell returned to a pattern of relative inactivity, firing only 6.2% of the remaining time. Paw withdrawal latency in this animal was 140% of baseline following mustard oil. MO, mustard oil applied to hindlimb ipsilateral to reflex testing. Triangles indicate PW trials. 1 s bins.
Fig. 5.
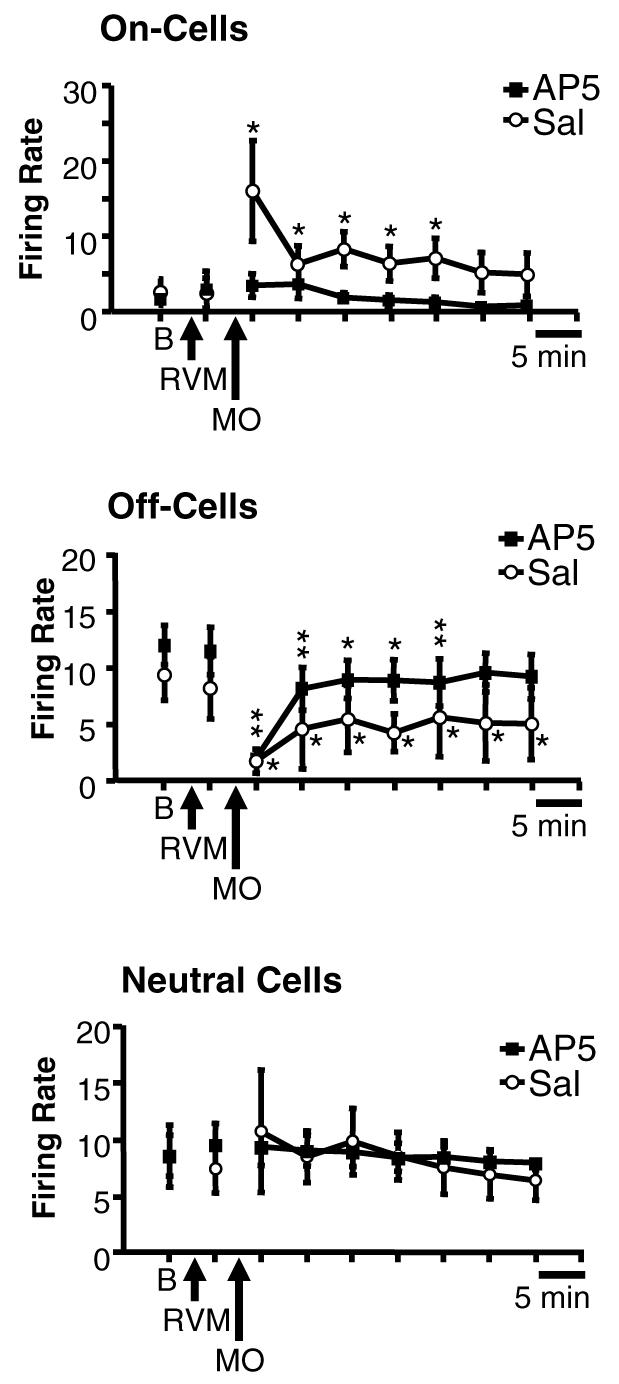
Group data illustrate that block of NMDA-mediated transmission within the RVM with AP5 prevents sustained activation of on-cells following mustard oil, without altering the suppression of off-cell firing. Mean (±SEM) overall ongoing discharge of RVM neurons in baseline (B), immediately following RVM microinjection of AP5 or saline (RVM), and for 30 min following removal of the mustard oil-soaked pledget (MO). On-cells showed a dramatic increase in ongoing discharge during application of mustard oil in saline-treated control animals (7 neurons), and firing was maintained significantly above baseline for at least 20 min, as previously reported (Kincaid et al., 2006). This activation was prevented by pre-treatment with AP5 (15 neurons). Off-cell discharge was significantly depressed following mustard oil in both AP5 (17 neurons) and saline-treated (5 neurons) animals. Neutral cell discharge was unchanged in both groups (5 in AP5 group, 4 in saline group). *p < 0.05, **p < 0.01, compared to baseline.
Fig. 6.
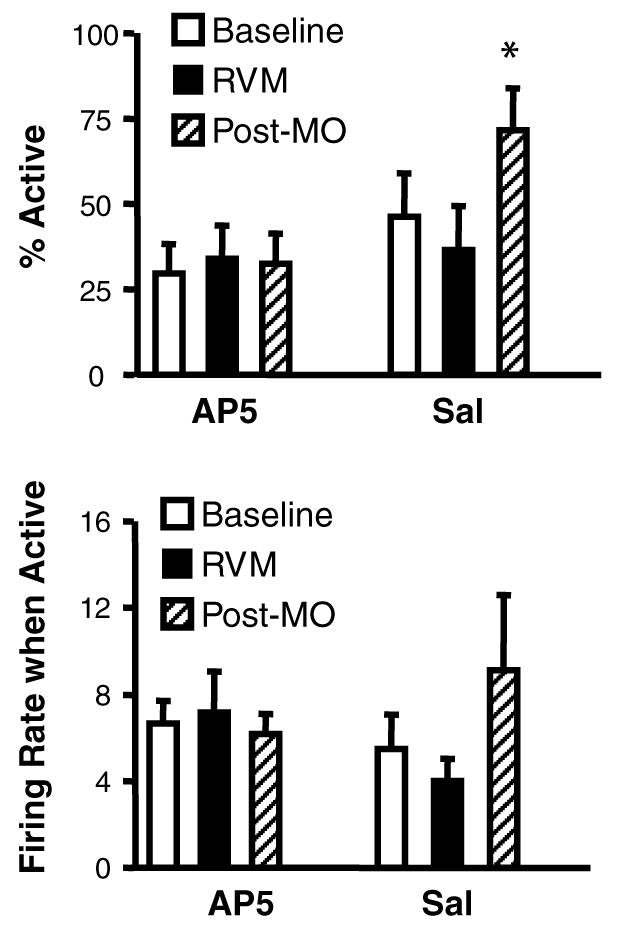
Mustard oil causes a shift in the firing pattern of on-cells that is blocked by AP5 in the RVM. Both the percentage of time that the cells were active and firing rate during active periods are plotted for baseline, post-RVM microinjection (RVM), and following application and removal of the mustard oil (Post-MO). In control animals, mustard oil caused a shift in the firing pattern so that on-cells were active a greater percentage of the time than during baseline. This shift was prevented by AP5 in the RVM. Firing rate during active periods was unchanged following mustard oil in both groups. This figure also demonstrates the lack of effect of AP5 microinjection on on-cell firing pattern or rate (RVM). Means ± SEM are presented, *p < 0.05 compared to baseline.
On-cells typically began discharging at a high rate when the mustard oil-soaked pledget was applied to the skin, and in most cases continued firing without pause for several minutes after the mustard oil was removed. Although firing rate during mustard oil activation in the two groups was comparable (saline: 10.2 ± 2.8 spikes/s, AP5: 11.3 ± 2.3 spikes/s, p > 0.05), the duration of the mustard oil-evoked firing (the latency from onset of the mustard oil until the first subsequent silent period of at least 2 s) was reduced by over 4 min in the AP5-treated group (saline: 572.2 ± 138.3 s, AP5: 311.8 ± 70.3 s, p < 0.05, Mann–Whitney test). The reflex-related burst was not increased following mustard oil in either group (data not shown).
3.3. AP5 microinjection in the RVM and off-cells and neutral cells
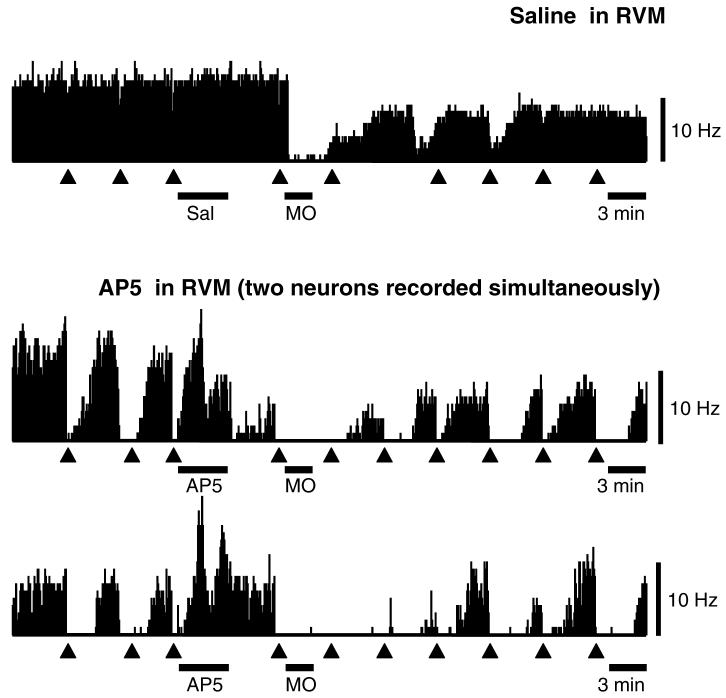
Off-cell firing was significantly depressed following mustard oil application, and this was not attenuated or shifted to activation by pretreatment with AP5 in the RVM. Ratemeter records in Fig. 7 show examples of an off-cell recorded in a saline-treated control animal, and a pair of off-cells recorded on a single electrode in an animal treated with AP5. Cell activity was decreased in both cases. The effect of AP5 in the RVM on the depression of off-cell activity induced by mustard oil is quantified in group data shown in Fig. 5, which shows ongoing firing in saline- and AP5-treated groups. Mustard oil application was followed by a significant reduction in the activity in both groups. Baseline firing rates in the two groups were not significantly different (Mann–Whitney test), and AP5 per se had no effect on off-cell firing, as shown by the time point following the RVM microinjection in Fig. 5 (Off-Cells, RVM).
Fig. 7.
Ratemeter records illustrate lack of effect of AP5 on the off-cell response to cutaneous mustard oil. Saline control, cell recorded before and after microinjection of saline (Sal, 200 nl) into the RVM illustrates typical response to mustard oil, with a marked depression of ongoing firing. Paw withdrawal latency following mustard oil in this animal was 23% of baseline. AP5 in RVM, two off-cells recorded on a single electrode before and after microinjection of AP5 (AP5, 2 nmol/200 nl). These neurons cycled in phase, although the cell in the upper of these two traces exhibited a somewhat higher level of excitability throughout. Both cells were strongly inhibited during application of mustard oil, and demonstrated long silent periods and a lower level of activity during active periods for the remainder of the protocol. Paw withdrawal latency in this animal was 82% of baseline following mustard oil. MO: mustard oil applied to hindlimb ipsilateral to reflex testing. Triangles indicate PW trials. 1 s bins.
The significant decrease in overall activity in both groups reflects a decrease in percentage of time active as well as in firing rate during active periods. Percentage of time active in AP5-treated animals was 89.4 ± 3.1% in baseline, and 72.2 ± 6.8% following mustard oil in AP5-treated animals. A similar change was seen in saline-treated animals, with percentage of time active 84.8 ± 8.5% in baseline, and 57.9 ± 23.5% following mustard oil (baseline and post-mustard oil, AP5 vs. saline groups, p > 0.05, Mann–Whitney tests). Firing rate during active periods in AP5-treated animals was 11.3 ± 1.6 spikes/s in baseline, 9.0 ± 1.5 spikes/s following mustard oil. In saline-treated animals, firing rate during active periods was 9.2 ± 2.9 spikes/s in baseline and 5.8 ± 3.1 spikes/s following mustard oil (baseline and post-mustard oil, AP5 vs. saline groups, p > 0.05, Mann–Whitney tests).
Off-cell activity was depressed during the period when the mustard oil-soaked pledget was applied to the skin, and in most cases the neuron did not resume firing for several minutes after the mustard oil was removed. The duration of the mustard oil-evoked firing (the latency from onset of the mustard oil until the first subsequent active period of at least 2 s) was not significantly different in control and AP5-treated groups (saline: 568.4 ± 357.2 s, AP5: 349.5 ± 124.8 s, p > 0.05, Mann–Whitney test).
Neutral cells showed no change in firing following application of mustard oil in animals treated with saline or AP5 (Fig. 5). None of the recorded neurons was likely to be serotonergic, at least based on the criterion of slow, regular firing employed by other groups (Wessendorf and Anderson, 1983; Jacobs and Fornal, 1991; Gao and Mason, 2001). Mean firing rate in baseline of the nine neutral cells studied was 9.1 ± 1.5 spikes/s, minimum rate 4.4 spikes/s, maximum rate 16.3 spikes/s.
4. Discussion
4.1. Relationship between changes in activity of RVM cell populations and secondary hyperalgesia in acute inflammation
The role of descending control from the PAG/RVM system in inflammation has not been fully resolved. Inactivation of the RVM has been reported by some laboratories to interfere with hyperalgesia after inflammation (Mansikka and Pertovaara, 1997; Urban and Gebhart, 1999; Kincaid et al., 2006). This would suggest that the RVM is part of a positive feedback loop triggered by acute inflammation. By contrast, others describe an increase in descending inhibition after inflammation, or increased potency of opioid compounds (Hurley and Hammond, 2001; Ren and Dubner, 2002). This implies a very different scenario in which the RVM is part of a negative feedback loop, which limits the development of sensitization and consequent hyperalgesia. Still others suggest that both descending inhibition and facilitation from the RVM are recruited over time during inflammation, and that the net effect on nociceptive neurons and behaviors reflects a balance between these two influences at different levels of the cord (Vanegas and Schaible, 2004).
The most direct approach to parsing out the relative contribution of descending facilitation and inhibition from the RVM to a net behavioral hypersensitivity is to measure each output directly, and to then determine the behavioral effect of blocking the different outputs. We previously showed that secondary thermal hyperalgesia induced by topical mustard oil is correlated with an acute shift in the firing pattern of on- and off-cells in the RVM, so that the on-cell population is more likely to be active at any given time. Neutral cell firing is unchanged (Kincaid et al., 2006). In the present study, selective block of the activation of on-cells using local infusion of the NMDA receptor antagonist AP5 into the RVM prevented hyperalgesia. This demonstrates that on-cell activation is a necessary link in the neural circuitry mediating secondary hyperalgesia in this acute inflammatory model. The depression of off-cell firing after mustard oil was not reversed by AP5. The decrease in off-cell firing following mustard oil may therefore make some contribution, but is not by itself sufficient to induce measurable behavioral hyperalgesia.
The present data also do not support a role for neutral cells in secondary hyperalgesia in acute inflammation. Nevertheless, neutral cells may become important in long-term modulation of nociception, or in other changes in behavior or physiology during more prolonged inflammation (Miki et al., 2002). Indeed, one limitation of the present experiments is that the protocol was restricted to only one hour. However, in our experience, interpretation of neuronal and behavioral data would have been significantly confounded by the potential for drift in anesthetic depth if a longer protocol had been adopted.
4.2. Descending inhibition is not increased in acute mustard oil inflammation
A number of studies have documented increased descending inhibition during inflammation (Vanegas and Schaible, 2004). However, the source of descending inhibition was not identified in most of those studies. In the present experiments, descending inhibition from the RVM (i.e., off-cell firing) was suppressed, not enhanced, after mustard oil. Moreover, microinjection of AP5 into the RVM did not lead to an increase in withdrawal latency ipsilateral or contralateral to the mustard oil treatment. Thus, blocking the facilitatory output mediated by on-cells did not unmask an inhibitory influence mediated by the RVM or any other circuit. However, descending inhibition may come into play at later time points in this paradigm.
The lack of contralateral hypoalgesia when the descending facilitation from the RVM was suppressed also rules out remote inhibition as an explanation for the limited area of secondary hyperalgesia. The RVM is thought to exert a global influence over nociceptive inputs from all body regions, yet secondary hyperalgesia in the cutaneous mustard oil paradigm is exhibited only on the treated limb. One possible explanation for this discrepancy would have been that local application of mustard oil provokes heterotopic inhibition (Le Bars, 2002), which might have masked descending facilitation from the RVM in regions of the dorsal horn that had not been sensitized. However, blocking the facilitatory output from the RVM did not reveal hypoalgesia on the contralateral hindpaw. Thus a balance between descending inhibition and facilitation cannot explain the limited area of hyperalgesia. It seems more likely that the measurable thermal hyperalgesia seen on the treated limb is due to a convergence of the descending facilitatory influence from the RVM with sensitizing input to the ipsilateral lumbar dorsal horn from the inflamed skin.
4.3. Excitatory amino acid transmission in the RVM
Novel NMDA- and non-NMDA-mediated transmission in the RVM has been implicated in both descending facilitation and enhanced descending inhibition after inflammation (Coutinho et al., 1998; Urban and Gebhart, 1999; Wei and Pertovaara, 1999; Ren and Dubner, 2002). Moreover, upregulation of both NMDA and AMPA receptors, as well as increased AMPA receptor phosphorylation, has been reported during prolonged inflammation induced by complete Freund's adjuvant (Terayama et al., 2000; Miki et al., 2002; Guan et al., 2003, 2004). Interestingly, alterations in AMPA receptor function are delayed relative to changes in NMDA receptors, and the changes in AMPA receptor function were more closely linked to a delayed enhancement of descending inhibition than to descending facilitation (Guan et al., 2003, 2004). Bridging the gap between cellular and behavioral approaches to the function of excitatory amino acid receptors in abnormal pain states thus requires clarifying the opposing pro-nociceptive and anti-nociceptive actions of these receptors at the circuit level.
All three RVM cell classes express excitatory amino acid receptors, and respond to exogenous glutamate (Heinricher and Roychowdhury, 1997). Under basal conditions, there appears to be no significant excitatory amino acid-mediated synaptic input to off-cells or neutral cells, whereas the reflex-related on-cell burst is mediated by a non-NMDA receptor (Heinricher and Roychowdhury, 1997; Heinricher et al., 2001b). Consistent with this, we saw no effect of AP5 or CNQX, or of the broad-spectrum excitatory amino acid antagonist kynurenate microinjection on paw withdrawal or tail flick latency in control animals in this or previous work (Heinricher and McGaraughty, 1998; Heinricher et al., 1999, 2001b). This conflicts with previous findings from Urban and colleagues, who saw an acute facilitation of the tail flick reflex and enhancement of mustard oil-induced hyperalgesia following microinjection of the non-NMDA receptor antagonist DNQX (10 nmol) into the RVM (Coutinho et al., 1998; Urban et al., 1999). The reason for the discrepancy between our data and those of Urban and colleagues is unclear. However, the dose of CNQX used here was sufficient to interfere with the reflex-related on-cell burst without producing non-specific effects on neuronal firing (Heinricher et al., 2001b), indicating that non-NMDA receptor block in the present experiments was adequate.
The present experiments show that an NMDA-mediated input to on-cells is brought into play during inflammation, and that the resulting increase in ongoing activity of these neurons is necessary for behavioral hyperalgesia. This novel input to on-cells parallels the well-known recruitment of NMDA-mediated activation at the spinal cord in enhanced pain states (Dickenson, 1990), and complements the NMDA-mediated activation of off-cells implicated in opioid analgesia (Heinricher et al., 2001b).
The mechanism through which inflammation recruits a novel NMDA-mediated increase in the ongoing activity of on-cells remains to be determined. Upregulation of NMDA receptor subunit expression triggered by brain-derived neurotrophic factor release in the RVM has been reported during inflammation induced with complete Freund's adjuvant (Miki et al., 2002; Guo et al., 2006). This raises the possibility that on-cells show a novel or increased expression of NMDA receptor with inflammation. However, network properties may also be a factor. For example, various neuropeptides are known to potentiate responses to NMDA in the dorsal horn (Dougherty et al., 1993; Chizh and Headley, 1994; Chizh et al., 1995; Cumberbatch et al., 1995; Chizh and Headley, 1996), and similar processes may play a role in the RVM, especially in acute, rather than chronic, inflammation.
5. Conclusion
Secondary hyperalgesia produced by cutaneous mustard oil in lightly anesthetized rats is associated with a significant, NMDA-mediated increase in the ongoing discharges of on-cells. Selectively blocking on-cell activation in this paradigm prevents hyperalgesia. On-cells are thus a necessary link in a positive feedback loop triggered by acute peripheral inflammation. NMDA-receptors therefore have two opposing roles in the RVM, producing analgesia when recruited to activate off-cells by opioid administration, and hyperalgesia when contributing to activation of on-cells following an acute inflammatory stimulus.
Acknowledgment
This work was supported by a grant from NIDA (DA05608).
References
- Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–10. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–25. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Cumberbatch MJ, Birch PJ, Headley PM. Endogenous modulation of excitatory amino acid responsiveness by tachykinin NK1 and NK2 receptors in the rat spinal cord. Br J Pharmacol. 1995;115:1013–9. doi: 10.1111/j.1476-5381.1995.tb15912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, Headley PM. Thyrotropin-releasing hormone (TRH)-induced facilitation of spinal neurotransmission: a role for NMDA receptors. Neuropharmacology. 1994;33:115–21. doi: 10.1016/0028-3908(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Headley PM. Thyrotropin-releasing hormone facilitates spinal nociceptive responses by potentiating NMDA receptor-mediated transmission. Eur J Pharmacol. 1996;300:183–9. doi: 10.1016/0014-2999(95)00869-1. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Chizh BA, Headley PM. Modulation of excitatory amino acid responses by tachykinins and selective tachykinin receptor agonists in the rat spinal cord. Br J Pharmacol. 1995;115:1005–12. doi: 10.1111/j.1476-5381.1995.tb15911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson AH. A cure for wind up: NMDA receptor antagonists as potential analgesics. Trends Pharmacol Sci. 1990;11:307–9. doi: 10.1016/0165-6147(90)90228-z. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Zorn S, Willis WD. Combined application of excitatory amino acids and substance P produces long-lasting changes in responses of primate spinothalamic tract neurons. Brain Res Rev. 1993;18:227–46. doi: 10.1016/0165-0173(93)90003-i. [DOI] [PubMed] [Google Scholar]
- Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–52. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Mason P. Physiological and anatomic evidence for functional subclasses of serotonergic raphe magnus cells. J Comp Neurol. 2001;439:426–39. doi: 10.1002/cne.1360. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366:201–5. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Zou S-P, Dubner R, Ren K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain. 2003;104:401–13. doi: 10.1016/s0304-3959(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–37. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain. 1998;75:247–55. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventrome-dial medulla. J Neurophysiol. 2001a;85:280–6. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–9. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Pertovaara A, Ossipov MH. Descending modulation after injury. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Progress in pain research and management 2003. Vol. 24. IASP Press; Seattle: 2003. pp. 251–60. [Google Scholar]
- Heinricher MM, Roychowdhury S. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla (RVM) depends upon excitatory amino acid transmission. Neuro-science. 1997;78:1159–65. doi: 10.1016/s0306-4522(96)00683-5. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-d-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001b;92:129–38. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–46. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal μ-opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–45. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–78. [PubMed] [Google Scholar]
- Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Rev. 2002;40:29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- Mansikka H, Pertovaara A. Supraspinal influence on hindlimb withdrawal thresholds and mustard oil-induced secondary allodynia in rats. Brain Res Bull. 1997;42:359–65. doi: 10.1016/s0361-9230(96)00313-9. [DOI] [PubMed] [Google Scholar]
- Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, et al. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–60. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–65. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–7. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport. 2000;11:1915–9. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wei H, Pertovaara A. MK-801, an NMDA receptor antagonist, in the rostroventromedial medulla attenuates development of neuropathic symptoms in the rat. Neuroreport. 1999;10:2933–7. doi: 10.1097/00001756-199909290-00011. [DOI] [PubMed] [Google Scholar]
- Wessendorf MW, Anderson EG. Single unit studies of identified bulbospinal serotonergic units. Brain Res. 1983;279:93–103. doi: 10.1016/0006-8993(83)90166-x. [DOI] [PubMed] [Google Scholar]