Abstract
Neonatal hypoxia-ischemia (HI) is an important clinical problem with few effective treatments. Granulocyte-colony stimulating factor (G-CSF) is an endogenous peptide hormone of the hematopoietic system that has been shown to be neuroprotective in focal ischemia in vivo, and is currently in phase I/II clinical trials for ischemic stroke in humans. We tested G-CSF in a rat model of neonatal hypoxia-ischemia in postnatal day 7 unsexed rat pups. Three groups of animals were used: hypoxia ischemia (HI, n=67), hypoxia-ischemia with G-CSF treatment (HI+G, n=65), and healthy control (C, n=53). G-CSF (50 μg/kg, subcutaneous) was administered 1 hour after HI, and given on four subsequent days (five total injections). Animals were euthanized 24 hours, 1, 2, and 3 weeks after HI. Assessment included brain weight, histology, immunohistochemistry, and Western blotting. G-CSF treatment was associated with improved quantitative brain weight and qualitative Nissl histology after hypoxia-ischemia. TUNEL demonstrated reduced apoptosis in group HI+G. Western blot demonstrated decreased expression of Bax and cleaved caspase 3 in group HI+G. G-CSF treatment was also associated with increased expression of STAT3, Bcl-2, and Pim-1, all of which may have participated in the anti-apoptotic effect of the drug. We conclude that G-CSF ameliorates hypoxic-ischemic brain injury, and that this may occur in part by an inhibition of apoptotic cell death.
Keywords: Neonatal hypoxia-ischemia, G-CSF, apoptosis, neuron
1. Introduction
Neonatal hypoxia-ischemia (HI) is an important cause of morbidity and mortality in the perinatal period with few effective treatments (Del Toro et al. 1991; Miller 2000; Ferriero 2001). One of the major mechanisms of neuron loss after HI is apoptotic cell death, and the immature brain exhibits a high degree of apoptotic cell death after ischemia (Sidhu et al., 1997). Recent interest has turned to the possibility of improving outcomes after neonatal hypoxia-ischemia by inhibiting apoptotic neuron loss.
Granulocyte colony-stimulating factor (G-CSF) is a growth factor of the hematopoietic system that stimulates proliferation, survival, and maturation of cells committed to the neutrophilic granulocyte lineage (Demetri and Griffin, 1991). G-CSF has also been shown to exert a powerful neuroprotective effect in a variety of in vivo models of brain injury (Solaroglu et al. 2006). Phase I / II clinical trials of G-CSF in ischemic stroke are ongoing (Schabitz et al., 2006, Shyu et al., 2006, Sprigg et al., 2006), and preliminary work suggests that G-CSF has the potential to make clinically significant improvements in outcome after ischemic stroke in adult human patients (Shyu et al., 2006). In animal studies, the neuroprotective effects of G-CSF have been ascribed to anti-apoptotic (Kobayashi et al., 2005; Schäbitz et al., 2003; Schneider et al., 2005), anti-inflammatory (Gibson et al 2005b; Kobayashi et al., 2005), excitoprotective (Schäbitz et al., 2003), and neurotrophic mechanisms (Konishi et al., 1993). In some contexts, however, G-CSF may exacerbate excitotoxic injury (Keller et al., 2006). G-CSF is thought to exert its anti-apoptotic effect through a variety of intracellular cascades including JAK / STAT, PI3-kinase / AKT, and ERK (Solaroglu et al., 2006).
In the present study we tested G-CSF treatment in a model of neonatal hypoxia-ischemia in rats (described below). Three groups of rats were used: neonatal hypoxia-ischemia (HI), neonatal hypoxia-ischemia with G-CSF treatment (HI+G), and healthy control (C). Rats were euthanized 24 hours, 1, 2, and 3 weeks after hypoxia-ischemia for further analysis. We hypothesize that administration of G-CSF after neonatal hypoxia-ischemia will attenuate brain injury, in part by inhibition of apoptosis.
2. Results
2.1 Brain Weight and Nissl histology
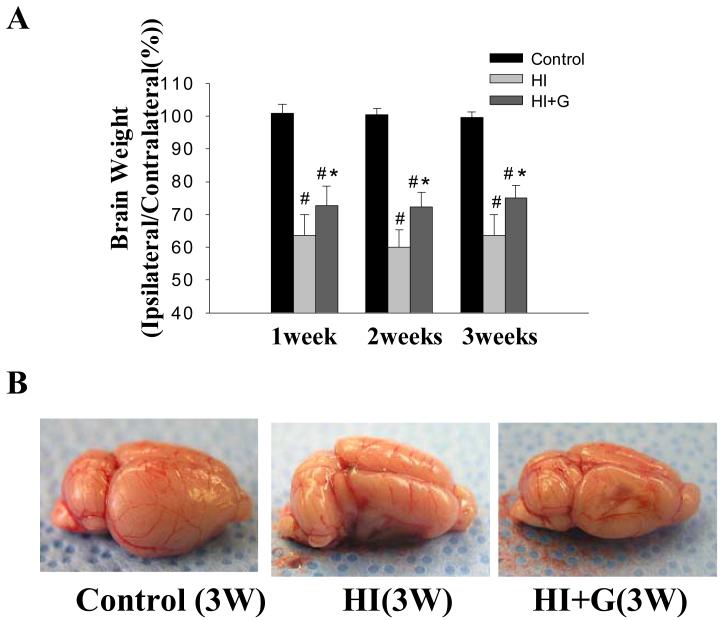
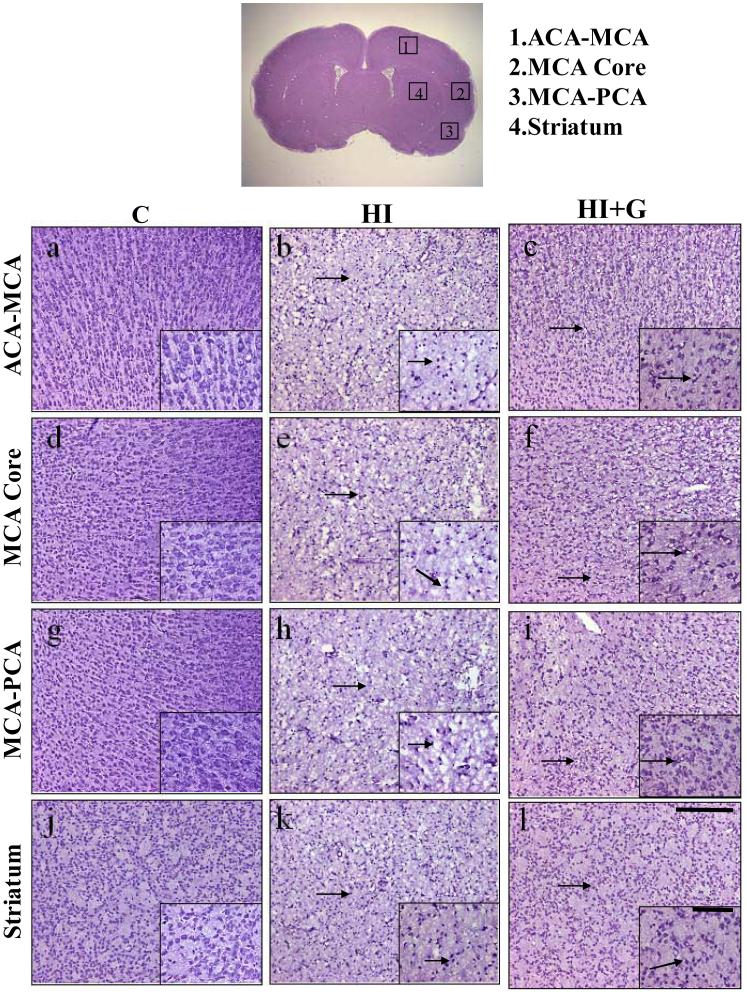
G-CSF treatment was associated with less gross tissue loss on brain weight analysis. On Analysis of Variance (ANOVA), G-CSF treatment was associated with a statistically significant increase in brain weight compared to the untreated group, p<0.05. The healthy control animals demonstrated no tissue loss, as expected (Figure 1A-B). Nissl histology of peri-infarct and infracted regions demonstrated increased vacuolization, neuron loss, and tissue breakdown in brain regions in the untreated animals (Figure 2). Large numbers of neurons in the untreated group were pyknotic and dead (Figure 2). Conversely, G-CSF treatment was associated with qualitatively less vacuolization, neuron loss, and tissue breakdown. The control animals appeared morphologically normal on Nissl stain (Figure 2).
Figure 1.
Brain weight. (A) brain weight expressed as a percentage (ipsilateral hemisphere/contralateral hemisphere) at 1, 2, and 3 weeks after HI. Data are mean ± SD of 12 or 13 pups in each group. On Analysis of Variance (ANOVA), p< 0.05 for both # and * when compared to Group C and HI respectively. (B) Representative pictures of brains taken from Groups C, HI and HI+G 3 weeks after hypoxia-ischemia. The lateral view of the ipsilateral hemisphere at 3 weeks shows that HI leads to a significant loss of brain tissue, and that G-CSF treatment is associated with statistically significant improvements in gross brain weight.
Figure 2.
Photomicrographs of Nissl stained brain sections demonstrate qualitative reduction in damage in group HI+G in a variety of brain regions including the MCA supplied core area (panels d,e,f), ACA-MCA peri-infarct area (panels a,b,c), MCA-PCA peri-infarct area (panels g,h,i) and striatum (panels j,k,l) from C group (panels a,d,g,j), HI group (panels b,e,h,k), and HI+G (panels c,f,i,l) 24 hours after neonatal hypoxia-ischemia (Scale bar = 200μm). The topmost figure depicts the regions of interest. The insets in all panels depict brain tissue at higher magnification (Scale = 100μm). The arrows indicate shrunken and damaged neurons.
2.2 Immunofluorsecence for the G-CSF Receptor
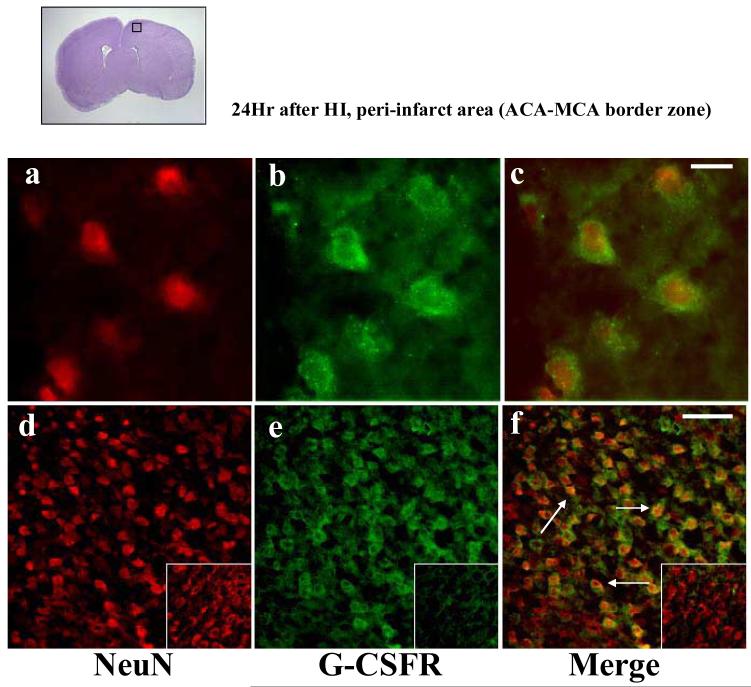
Double immunofluorescence of the G-CSF receptor (G-CSFR) and NeuN (a marker for neurons) demonstrated the G-CSF receptor on neurons after neonatal hypoxia-ischemia (Figure 3). Expression of the G-CSF receptor was qualitatively greater after ischemia compared to the non-ischemic control animals (Figure 3, inset).
Figure 3.
G-CSF receptor (G-CSFR) is expressed in the neonatal rat brain neurons after hypoxia-ischemia. Double immunofluorescence photomicrographs (n=4) of NeuN immunoreactivites (red, neuronal marker, panels a,d), G-CSFR immunoreactivites (green, panels b,e) and their merged images (panels c,f) from brain sections taken from the peri-infarct area at 24hr after hypoxia-ischemia at high magnification (x100, Scale bar 10μm, panels a,b,c) and low magnification (x20, Scale bar 20μm, panels d,e,f). The top left picture with denotes the region of interest. The insets show respective immunoreactivities from Group C.
2.3 Evaluation of Apoptosis
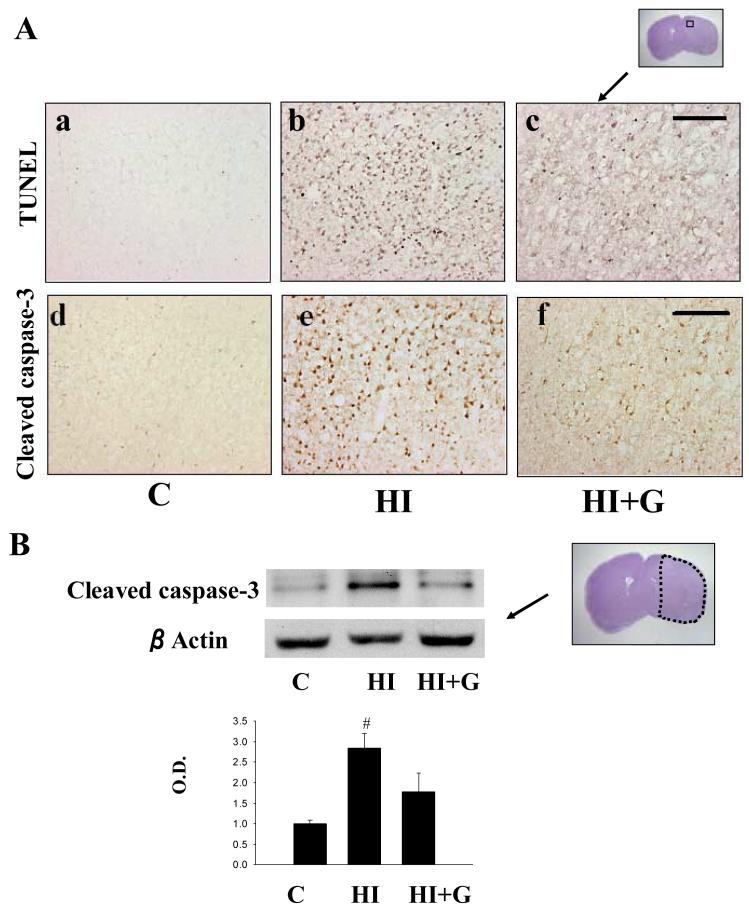
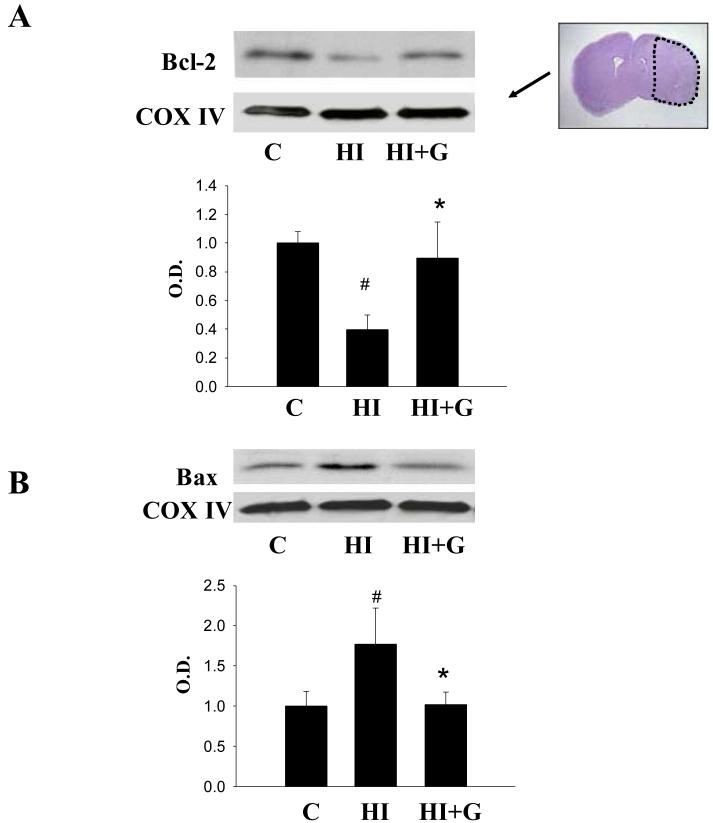
G-CSF treatment was associated with decreased expression of pro-apoptotic markers and increased expression of anti-apoptotic markers. Apoptotic cell death was qualitatively reduced in the G-CSF treatment group as indicated by TUNEL and cleaved caspase 3 immunohistochemical stain (Figure 4A). Western blot of cleaved caspase 3 supported these findings quantitatively (Figure 4B). G-CSF treatment was associated with a statistically significant reduction in cleaved caspase-3 activity on ANOVA, p<0.05, when compared to the untreated group (Figure 4B). Western blot analysis of Bcl-2 (an anti-apoptotic factor) demonstrated a statistically significant increase in the G-CSF group compared to the untreated group, p<0.05 on ANOVA (Figure 5A). Analysis of BAX (a pro-apoptotic factor) demonstrated a statistically significant reduction in the G-CSF group compared to the untreated group, p<0.05 on ANOVA (Figure 5B).
Figure 4.
Apoptotic cell death after neonatal HI. A. representative photomicrographs of TUNEL stained brain sections and cleaved caspase-3 stained sections from the peri-infarct zone demonstrate fewer markers of apoptotic cell death in group HI+G compared to group HI 24 hours after hypoxia-ischemia. Scale bar = 200μm. B. Western blot for cleaved caspase-3 protein expression demonstrates less cleaved caspase-3 in group HI+G compared to group HI 24 hours after hypoxia-ischemia. The lower panel depicts cleaved caspase-3 protein expression measured by densitometry analysis. Values represent mean ± S.D. with 5 samples in C group and 8 samples in HI, HI+G group each, expressed as percent change over control group. On ANOVA, p< 0.05 for # when compared to Group C. O.D., optical density.
Figure 5.
Bcl-2 and Bax expression. A. Group HI+G demonstrated increased levels of Bcl-2 in mitochondrial fractions compared to group HI 24 hours after hypoxia-ischemia. B. Group HI+G demonstrated reduced levels of Bax in mitochondrial fractions compared to group HI 24 hours after hypoxia-ischemia. For both A and B values represent mean ± S.D. with 5 samples in C group and 8 samples in HI, HI+G group each, expressed as percent change over control group. On ANOVA, p< 0.05 for both # and * when compared to Group C and HI, respectively (ANOVA). O.D., optical density. The Nissl stained photomicrograph depicts the area of interest.
2.4 Evaluation of STAT3 and Pim-1
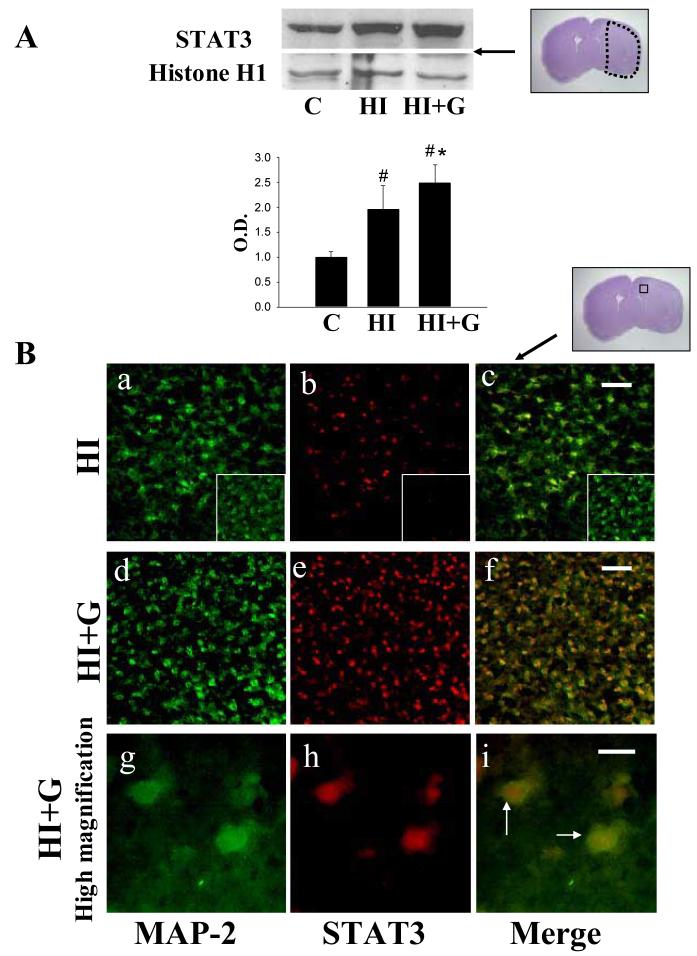
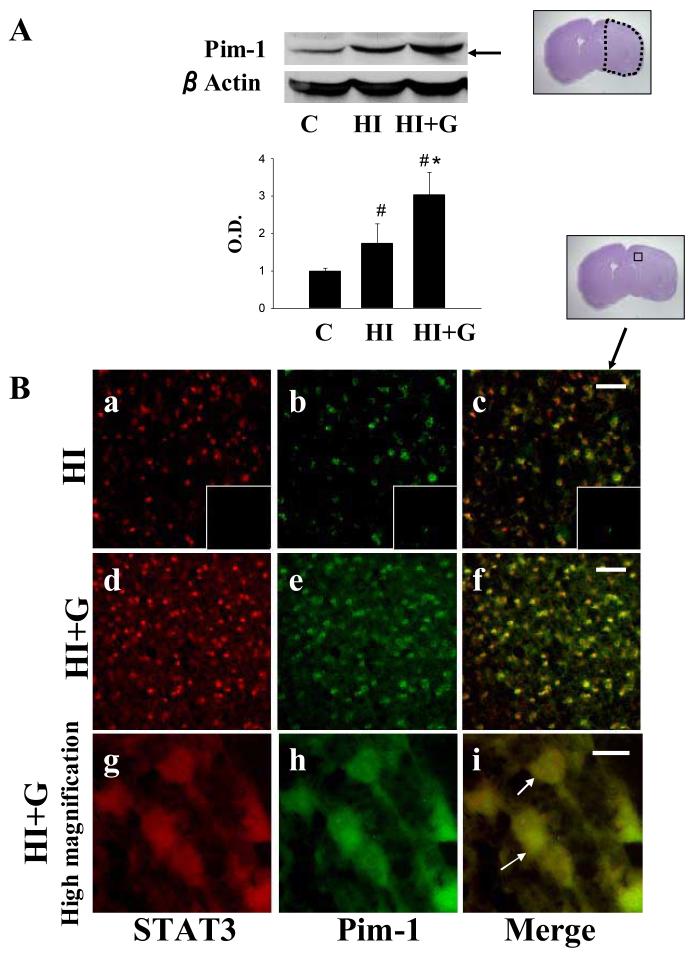
G-CSF treatment was associated with a statistically significant increase in expression of intracellular mediators of anti-apoptotic pathways including STAT3 (Figure 6A) and Pim-1 (Figure 7A) compared to the untreated group (p<0.05 on ANOVA). Double immunofluorescence for STAT3 and MAP2 (a marker of neurons) demonstrated expression of STAT3 in neurons in the G-CSF treatment group, while untreated animals demonstrated less STAT3 (Figure 6B). Double immunofluorescence for STAT3 and Pim-1 demonstrated co-expression of these two proteins (Figure 7B).
Figure 6.
A. G-CSF treatment was associated with increased nuclear expression of STAT3 on Western blot analysis 24 hours after hypoxia-ischemia. Values represent mean ± S.D. with 5 samples in C group and 8 samples in HI, HI+G group each, expressed as percent change over control group. On ANOVA, p< 0.05 for both # and * when compared to Group C and HI, respectively (ANOVA). O.D., optical density. The Nissl stained photomicrograph depicts the area of interest. B. Double immunofluorescence for STAT3 and MAP-2 (a marker of neurons) demonstrated expression of STAT3 by neurons in the peri-infarct region 24 hours after hypoxia-ischemia. Panels a-f, low magnification ×20, Scale bar = 50μm. Panels g-i, high magnification, ×100, Scale bar = 10μm.
Figure 7.
A. G-CSF treatment was associated with significantly increased expression of Pim-1 on Western blot analysis 24 hours after hypoxia-ischemia. values represent mean ± S.D. with 5 samples in C group and 8 samples in HI, HI+G group each, expressed as percent change over control group. On ANOVA, p< 0.05 for both # and * when compared to Group C and HI, respectively. O.D., optical density. The Nissl stained photomicrograph depicts the area of interest. B. Double immunofluorescence for STAT3 and Pim-1 demonstrated coexpression of these two proteins 24 hours after hypoxia-ischemia, with qualitative increased expression in group HI+G. Panels a-f, low magnification ×20, Scale bar 50μm. Panels g-i, high magnification ×100, Scale bar = 10μm.
3. Discussion
In this study we found that G-CSF treatment was associated with improved brain weight (Figure 1), improved qualitative histological outcomes (Figure 2), decreased apoptotic neuron loss (Figure 4), decreased apoptotic signaling (Figure 4, 5B), and increased expression of proteins associated with cell survival (Figure 5A, 6, and 7). The hypoxia-ischemia insult was associated with a qualitative increase in expression of the G-CSF receptor (Figure 3).
The G-CSF receptor (G-CSFR) is expressed by neurons and glial cells in the adult brain after injury (Kobayashi et al., 2005; Schäbitz et al., 2003; Schneider et al., 2005). Quantitative upregulation of the G-CSF receptor after ischemic brain injury has been demonstrated in post-mortem analysis of human brain tissue (Hasselblatt et al., 2007). We found that the G-CSF receptor is also expressed by the neonatal brain after hypoxia-ischemia (Figure 3). This suggests that hypoxia-ischemia may activate intrinsic protective mechanisms to combat neonatal brain injury, which is consistent with prior observations (Schneider et al., 2005).
In prior reports the neuroprotective effects of G-CSF have been ascribed to anti-apoptotic (Kobayashi et al., 2005; Schäbitz et al., 2003; Schneider et al., 2005), anti-inflammatory (Gibson et al 2005b; Kobayashi et al., 2005), excitoprotective (Schäbitz et al., 2003), and neurotrophic (Konishi et al., 1993) effects. In vitro studies have reported that the G-CSF inhibits apoptosis by activation of the JAK/STAT3 (Shimozaki et al., 1997), phospatidylinositol 3'kinase (PI3K) / Akt (Dong and Lamer, 2000; Schneider et al., 2005), and mitogen-activated protein kinase (MAPK)/ERK pathways (Schneider et al., 2005), and these results have been largely confirmed in brain ischemia models in vivo (Kobayashi et al., 2005; Schäbitz et al., 2003). Our results suggest that G-CSF treatment inhibited apoptotic neuron loss, and this may have occurred partly through JAK/STAT3 signaling (Figure 6). We did not study the potential neurotrophic or excitoprotective effects of G-CSF, and it is possible that these mechanisms also played a role in the G-CSF-mediated neuroprotection observed in this study.
Pim-1, a downstream effector of many cytokine signaling pathways, has been shown to play a role in the control of cell growth and differentiation of hematopoietic cells (Wang Z et al 2001, Bachman and Moroy 2005). The expression of the Pim-1 gene is induced by a large set of cytokines, including G-CSF, GM-CSF, IL-3, IFN-α, and IL-6 (Lilly et al 1992; Matikainen et al 1999; Rahman et al., 2001). STAT3 can bind directly to the Pim-1 promoter sequence and upregulate pim-1 gene expression. Reports have noted that Pim-1 is elevated after seizure (Feldman et al., 1998). We found an upregulation of Pim-1 after hypoxia-ischemia, and that this was enhanced by G-CSF treatment (Figure 7A). Pim-1 expression seems to parallel STAT3 expression, suggesting an association between the two in ischemic pathology (Figure 7B).
While the anti-apoptotic effects of Pim-1 are still largely unknown, prior reports have shown that Pim-1 is associated with sustained expression of Bcl-2 mRNA and protein levels (Lilly et al., 1999, Rahman et al., 2001; Sakai et al., 1997). Bcl-2 is an anti-apoptotic protein present at relatively high levels in the neonatal brain and declines significantly in the postnatal brain (Abe-Dohmae et al., 1993). The expression of Bcl-2 in neonatal brain injury remains unclear (Akhtar et al., 2004; Hagberg, 2004), as several studies have shown that Bcl-2 levels may not be elevated in rodent models of neonatal brain injury (Chen et al., 2001; Northington et al., 2001), whereas others have reported contrary results (Ferrer et al., 1997; Hu et al., 2003; Nunez and McCarthy, 2004). One study reported that Bcl-2 was expressed in normal neurons, but that the expression was decreased in apoptotic neurons and further decreased in necrotic neurons following neonatal hypoxia-ischemia (Ferrer at al., 1997). In the current study, we found that the expression of Bcl-2 decreased in the mitochondrial fraction following hypoxia-ischemia, and that this was reversed with G-CSF treatment (Figure 5).
In the present study Bax demonstrated an inverse relationship with Bcl-2 (Figure 5). Bax levels were increased in mitochondrial fraction following hypoxia-ischemia, and this was reversed with G-CSF treatment. Bax, a pro-apoptotic protein, is expressed in both the embryonic and adult brain and can heterodimerize with Bcl-2, Bcl-Xl, Mcl-1 (Bcl-2 family proteins). Bax regulates cytochrome c release from the mitochondria, perhaps via the formation of the mitochondrial transition pore, resulting in the activation of caspase 3 and apoptotic cell death (Northington et al., 2001). We observed that an increase in Bax correlated with an increase in Cleaved Caspase 3. Thus, the present data suggests that the apoptotic cell death following neonatal hypoxia-ischemia is likely associated with Bax translocation to the mitochondria with a concomitant decrease in Bcl-2, resulting in activation of caspase-3. However, the changes in expression of Bcl-2 and Bax probably do not fully explain the anti-apoptotic effects of G-CSF. A prior report noted that upregulation in Bcl-2 may be observed in apoptotic neurons after hypoxia-ischemia, and that Bcl-2 by itself is not sufficient to prevent cell death. Likewise, upregulation of Bax by itself may not be sufficient to cause apoptotic death (Ferrer et al., 1997).
We did not study the extent to which G-CSF treatment modulated excitotoxic injury in the neonatal hypoxia-ischemia. A recent study of G-CSF in a neonatal ibotenate injection (excitotoxic) model demonstrated that G-CSF has the potential to exacerbate excitotoxic injury in the dose range used in the present study (Keller et al., 2006), and it is possible that this attenuated the beneficial effects of the drug. We also did not study possible neurotrophic effects of G-CSF. G-CSF has been reported to have neurotrophic effects on neurons in vitro and in vivo (Konishi et al., 1993), and these effects may have contributed to the beneficial outcomes observed in our study. G-CSF has been reported to promote brain repair through the stimulation of intrinsic neural stem cells (Kobayashi et al., 2005; Schneider et al., 2005). Others studies have suggested G-CSF-mediated brain protection by recruitment of bone marrow-derived stem cells (Shyu et al., 2004; Kawada et al 2006). Future studies will address these possible mechanisms of neuroprotection in neonatal hypoxia-ischemia.
Animals in the G-CSF treatment group received the same dose of G-CSF per body weight, and future studies will address the dose-response relationship, as well as the time course of treatment necessary for optimal effect. Future studies of G-CSF and neonatal hypoxia-ischemia will emphasize long-term behavioral and functional outcomes after G-CSF administration and long-term histological outcomes.
We conclude that G-CSF treatment ameliorates brain tissue loss after neonatal hypoxia-ischemia, and that this may occur in part through an inhibition of apoptosis.
4. Experimental Procedures
4.1 Animal Model and Experimental Protocol
The neonatal hypoxia-ischemia protocol used in this study was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Pregnant Sprague-Dawley rats (Harlan Labs, Indianapolis, IN, USA) were housed in individual cages. After birth, pups were housed with their dam under a 12 hour light/dark cycle, with food and water available ad libitum. All surgical procedures were performed on postnatal day 7 unsexed pups. The animals were subjected to a well-characterized neonatal hypoxia-ischemia model (Vannucci RC and Vannucci JS, 1997). The pups were anesthetized with isoflurane (3% in mixed air and oxygen) by inhalation induction and placed on a surgical table maintained at 37°C. The right common carotid artery of each pup was exposed, carefully isolated from the vagus nerve and jugular vein, and ligated with 5-0 surgical silk. The incision was sutured and the pups recovered with their dam in a stable thermal environment. After a recovery period of 2 hours, the pups were placed in a glass chamber, which was submerged in a water bath (37.5°C), and exposed to mixture of 8% O2 and 92% N2 for 2 hours. After the hypoxic stress, pups were randomly assigned to one of the following groups: Hypoxia-ischemia (Group HI, n=67), or hypoxia-ischemia with G-CSF treatment (Group HI+G, n=65). Healthy control animals (group C, n=53) were also used in this study. Rat pups were euthanized under general anesthesia 24 hours, 1, 2, or 3 weeks after hypoxia-ischemia.
4.2 G-CSF Treatment
Pups in the HI+G group received G-CSF (50μg/kg, subcutaneous, Amgen, Thousand Oaks, CA) 1 hour after HI and on four subsequent days (total of 5 doses). Groups C and HI received phosphate-buffered saline (PBS, Aldrich, St. Louis, MO) injections of similar volume. The dose of G-CSF agrees with previously published reports (Lee et al., 2005).
4.3 Brain weight
Gross tissue loss was assessed by brain weight analysis 1 week, 2 weeks, and 3 weeks after hypoxia-ischemia, as described previously (Calvert et al., 2002). The pups were euthanized under general anesthesia [Ketamine (80mg/kg)/xylazine (10mg/kg)]. After removal of the brain, the cerebellum and brain stem were dissected from the forebrain. The hemispheres were separated by a midline incision and then weighed on a high precision balance (sensitivity ±0.001g). Damage is expressed as the percent reduction in weight of the ipsilateral hemisphere compared to the contralateral hemisphere.
4.4 Histology
The pups (under deep anesthesia) were transcardially perfused with ice-cold PBS followed by phosphate buffered formalin (Fisher Scientific, Cat #245-684). The whole brain was subsequently removed and immersed in the same fixative for 12 hours. After removal of paraformaldehyde the brains were cryoprotected and then rapidly frozen by 2-methylbutane chilled in liquid nitrogen. Coronal brain sections with 10μm thickness at the level of the striatum were prepared using a cryostat and mounted on poly-l-lysine-coated slides (Richard-Allan Scientific). Nissl stain followed standard protocols (Calvert et al., 2002).
4.5 Immunohistochemistry
Immunohistochemical analysis followed standard protocols as described elsewhere (Hayashi et al., 2005). The diaminobenzidine (DAB) staining method (ABC Staining Kit, Santa Cruz Biotech, Santa Cruz, CA) was used for detection of cleaved caspase 3 immunohistochemistry. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling immunohistochemistry (TUNEL) staining using DAB substrate was performed per manufacturer's instructions using an in situ cell death detection kit (Roche Diagnostic,IN). Antibodies included rabbit anti-cleaved caspase-3 (1:200) (Cell Signaling Technology, MA), and appropriate secondary anti-rabbit antibodies (1:200) (Santa Cruz Biotech, Santa Cruz CA).
Immunofluorescence analysis followed standard protocols (Hayashi et al., 2005). Antibodies included rabbit anti-G-CSF-Receptor (1:200) (G-CSFR), mouse anti-STAT3 (1:200), goat anti-MAP-2 (1:200), goat anti-Pim-1 antibody (1:200), (Santa Cruz Biotech), mouse anti-NeuN antibody (1:400) (CHEMICON International, CA), and appropriate secondary antibodies (1:200) (Jackson Immunoresearch, PA).
4.6 Western blot
The ipsilateral middle cerebral artery (MCA) perfusion region was separated from the rest of the brain (Shimamura et al., 2006), and nuclear, mitochondrial, and cytosolic fractions were prepared for Western blot using techniques described previously (Matsumori et al., 2005). Briefly, whole-cell lysates were obtained by gently homogenizing the brain sample with a homogenizer in 5 volumes of buffer A (20mM HEPES, 1.5mM MgCl2, 10mM KCl, 1mMEDTA,1mM EGTA, 250mM sucrose, 0.1mM PMSF 1mM dithiothreitol (DTT) and proteinase inhibitor cocktail tablets; pH 7.9). Samples were further centrifuged at 750g at 4°C for 15 minutes to separate the sample into supernatant A and pellet A. Pellet A, containing the nuclear fraction, was resuspended in 90μl of buffer B (20mM HEPES, 1.5mM MgCl2, 20mM KCl, 0.2mMEDTA,0.5mM EGTA, 0.2mM PMSF 0.5mM dithiothreitol (DTT) and proteinase inhibitor cocktail tablets; pH 7.9) and mixed with 30μl of buffer C (20mM HEPES, 1.2M KCl, 0.2mM EDTA , 0.2mM PMSF 0.5mM dithiothreitol (DTT) and proteinase inhibitor cocktail tablets; pH 7.9). The samples were placed on ice for 30 minutes during the extraction and then centrifuged at 12,000g. Supernatants containing the nuclear fraction were transferred and stored at −70°C. Supernatant A, containing cytosolic/mitochondrial protein, was further centrifuged at 16,000g for 30min at 4°C to separate supernatant B from pellet B. Supernatant B was used as the cytosolic fraction and pellet B was used as the mitochondrial fraction after resuspension in buffer A. Protein concentrations were then determined by the DC protein assay (Bio-rad, Hercules, CA).
Fifty (50) μg of protein was loaded into each lane of an appropriate SDS-PAGE gel (Bio-rad), and proteins were transferred onto nitrocellulose membranes (Bio-rad). The membranes were blocked with non- fat milk and then incubated with the primary antibodies which included mouse anti-STAT3 (1:200), goat anti-Pim-1 (1:200), rabbit anti-Bcl-2 (1:500), mouse anti-Bax (1:1000) (Santa Cruz Biotechnology, CA), rabbit anti-cleaved caspase-3 (1:1000) (Cell Signaling Technology, MA), goat anti-β-actin antibody (Santa Cruz Biotechnology, CA), rabbit anti-Histone H1 antibody (1:500) (Santa Cruz Biotechnolgy, CA), mouse anti-COX IV (1:2000) (Abcam, MA). After incubation with the appropriate horseradish peroxidase-conjugated secondary antibody (1:2000) (Santa Cruz Biotechnology, CA) for 2 hours at room temperature, immunoreactive bands were visualized in the linear range with the enhanced chemiluminescence ECL Western blotting system (ECL Plus Western blotting Detection Reagents). Quantitative evaluation of Western blot was performed using a computerized digital image system (Image J), and values are expressed relative to Control and an appropriate compartment-specific intracellular protein (cytosolic β-actin, mitochondrial COX IV, or nuclear Histone H1).
4.7 Statistical Analysis
Results are expressed as mean ± standard deviation (SD) and comparisons were made between the different groups using one-way analysis of variance (ANOVA) supported by Sigma Stat (Systat Software, Port Richmond, CA), with p<0.05 considered statistically significant.
Acknowledgement
This study was supported by NIH grants NS45694, HD43120, NS43338 and NS53407 to JHZ
Abbreviations
- (G-CSF)
Granulocyte-colony stimulating factor
- (BBB)
blood-brain barrier
- (HI)
hypoxia-ischemia
- (TUNEL)
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
Footnotes
Section: (8) Disease-related neuroscience
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe-Dohmae S, Harada N, Yamada K, Tanaka R. Bcl-2 gene is highly expressed during neurogenesis in the central nervous system. Biochem Biophys Res Commun. 1993;191:915–21. doi: 10.1006/bbrc.1993.1304. [DOI] [PubMed] [Google Scholar]
- Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bachmann M, Moroy T. The serine/threonine kinase Pim-1. Int J Biochem Cell Biol. 2005;37:726–730. doi: 10.1016/j.biocel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Calvert JW, Yin W, Patel M, Badr A, Mychaskiw G, Parent AD, Zhang JH. Hyperbaric oxygenation prevented brain injury induced by hypoxia-ischemia in neonatal rat model. Brain Res. 2002;951:1–8. doi: 10.1016/s0006-8993(02)03094-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ginis I, Hallenbeck JM. The protective effect of ceramide in immature rat brain hypoxia-ischemia involves up-regulation of bcl-2 and reduction of TUNEL-positive cells. J Cereb Blood Flow Metab. 2001;21:34–40. doi: 10.1097/00004647-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Del Toro J, Louis PT, Goddard-Finegold J. Cerebrovascular regulation and neonatal brain injury. Pediatr. Neurol. 1991;7:3–12. doi: 10.1016/0887-8994(91)90098-6. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- Dong F, Lamer AC. Activation of Akt kinase by granulocyte colony-stimulating factor (G-CSF): evidence for the role of a tyrosine kinase activity distinct from the Janus kinases. Blood. 2000;95:1656–62. [PubMed] [Google Scholar]
- Feldman JD, Vician L, Crispino M, Tocco G, Baudry M, Herschman HR. Seizure activity induces PIM-1 expression in brain. J Neurosci Res. 1998;53:502–9. doi: 10.1002/(SICI)1097-4547(19980815)53:4<502::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Pozas E, Lopez E, Ballabriga J. Bcl-2, Bax and Bcl-x expression following hypoxia-ischemia in the infant rat brain. Acta Neuropathol (Berl) 1997;94:583–9. doi: 10.1007/s004010050753. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev. Neurosci. 2001;23:198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005b;64:763–9. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- Hagberg H. Mitochondrial impairment in the developing brain after Hypoxia-ischemia. J Bioenerg Biomembr. 2004;36:369–73. doi: 10.1023/B:JOBB.0000041770.00567.4f. [DOI] [PubMed] [Google Scholar]
- Hasselblatt M, Jeibmann A, Riesmeier B, Maintz D, Schabitz WR. Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke. Acta Neuropathol. 2007;113:45–51. doi: 10.1007/s00401-006-0152-y. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Iwai M, Ikeda T, Jin G, Deguchi K, Nagotani S, Zhang H, Sehara Y, Nagano I, Shoji M, Ikenoue T, Abe K. Neural precursor cells division and migration in neonatal rat brain after ischemia/hypoxic injury. Brain Res. 2005;1038:41–49. doi: 10.1016/j.brainres.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Hu X, Qiu J, Grafe MR, Rea HC, Rassin DK, Perez-Polo JR. Bcl-2 family members make different contributions to cell death in hypoxia and/or hyperoxia in rat cerebral cortex. Int J Dev Neurosci. 2003;21:371–7. doi: 10.1016/s0736-5748(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takahasi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- Keller M, Simbruner G, Urbanek M, Tinhofer I, Griesmaier E, Sarkozy G, Schwendimann L, Gressens P. Systematic application of granulocyte-colony stimulating factor and stem cell factor exacerbates excitotoxic brain injury in newborn mice. Pediatric Res. 2006;59(4):549–553. doi: 10.1203/01.pdr.0000205152.38692.81. [DOI] [PubMed] [Google Scholar]
- Kobayashi MK, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2005 doi: 10.1038/sj.jcbfm.9600195. Jul 27 online. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Chui DH, Hirose H, Kunishita T, Tabira T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and vivo. Brain Res. 1993;609:29–35. doi: 10.1016/0006-8993(93)90850-m. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK. Granulocyte-colony stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain research. 2005;1058:120–128. doi: 10.1016/j.brainres.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Lilly M, Le T, Holland P, Hendrickson SL. Sustained expression of the pim-1 kinase is specifically induced in myeloid cells by cytokines whose receptors are structurally related. Oncogene. 1992;7:727–732. [PubMed] [Google Scholar]
- Lilly M, Sandholm J, Cooper JJ, Koskinen PJ, Kraft A. The PIM-1 serine kinase prolongs survival and inhibits apoptosis-related mitochondrial dysfunction in part through a bcl-2-dependent pathway. Oncogene. 1999;18:4022–31. doi: 10.1038/sj.onc.1202741. [DOI] [PubMed] [Google Scholar]
- Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koshinen PJ, Julkunen I. Interferon-α activates multiple STAT protein and upregulates proliferation-associated IL-2α, c-myc, and pim-1 genes in human T cells. Blood. 1999;93:1980–1991. [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Wenstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxia/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25:889–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- Miller V. Neonatal cerebral infarction. Semin. Pediatr. Neurol. 2000;7:278–288. doi: 10.1053/spen.2000.20076. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Flock DL, Martin LL. Delayed neurodegeneration in neonatal Rat Thalamus after Hypoxia-Ischemia is apoptosis. The Journal of Neuroscience. 2001;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Cell death in the rat hippocampus in a model of prenatal brain injury: time course and expression of death-related proteins. Neuroscience. 2004;129:393–402. doi: 10.1016/j.neuroscience.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Yoshikawa H, Nakajima Y, Tasaka K. Down-regulation of Pim-1 and Bcl-2 is accompanied with apoptosis of interleukin-6-depleted mouse B-cell hybridoma 7TD1 cells. Immunology Letters. 2001;75:199–208. doi: 10.1016/s0165-2478(00)00322-9. [DOI] [PubMed] [Google Scholar]
- Sakai I, Kraff AS. The kinase domain of Jak2 mediates induction of bcl-2 and delays cell death in hematopoietic cells. J Bio Chem. 1997;172:12350–12358. doi: 10.1074/jbc.272.19.12350. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stoke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schneider A. Developing granulocyte-colony stimulating factor for the treatment of stroke: current status of clinical trials. 2006;37(7):1654. doi: 10.1161/01.STR.0000227299.62106.0e. [DOI] [PubMed] [Google Scholar]
- Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR. The hematopoietic factor G-CSF is a neuronal ligand that conteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke. 2006;37(7):1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- Shimozaki K, Nakajima K, Hirano T, Nagata S. Involvement of STAT3 in the granulocyte colony stimulate factor-induced differentiation of myeloid cells. J Biol Chem. 1997;272:25184–9. doi: 10.1074/jbc.272.40.25184. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Lee CC, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174(70):927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006;37(4):1123–?. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, Dennis MS, Russel N. Granulocyte-colony stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery Enhancement after Stroke (STMS) pilot randomized, controlled trial. Stroke. 2006;37(12):2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci JS. A model of perinatal hypoxic-ishemic brain damage. Ann NY Acad Sci. 1997;835:234–249. doi: 10.1111/j.1749-6632.1997.tb48634.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bhattacharya N, Weaver M, Petersen K, Meyer M, Gapter L, Magnuson NS. Pim-1: a serine/threonine kinase with a role in cell survival, proliferation , differentiation and tumorigenesis. J Vet Sci. 2001;2:167–179. [PubMed] [Google Scholar]