Abstract
We describe a multicomponent antigen display and delivery system using bacteriophage T4. Two dispensable outer capsid proteins, Hoc (highly antigenic outer capsid protein, 155 copies) and Soc (small outer capsid protein, 810 copies), decorate phage T4 capsid. These proteins bind to the symmetrically localized capsid sites, which appear following prohead assembly and expansion. We hypothesized that multiple antigens fused to Hoc can be displayed on the same capsid and such particles can elicit broad immunological responses. Anthrax toxin proteins, protective antigen (PA), lethal factor (LF), and edema factor (EF), and their functional domains, were fused to Hoc with an N-terminal hexa-histidine tag and the recombinant proteins were over-expressed in E. coli and purified. Using a defined in vitro assembly system, the anthrax-Hoc fusion proteins were efficiently displayed on T4 capsid, either individually or in combinations. All of the 155 Hoc binding sites can be occupied by one antigen, or they can be split among two or more antigens by varying their molar ratio in the binding reaction. Immunization of mice with T4 phage carrying PA, LF, and EF elicited strong antigen-specific antibodies against all antigens as well as lethal toxin neutralization titers. The triple antigen T4 phage elicited stronger PA-specific immune responses than the phage displaying PA alone. These features offer novel avenues to develop customized multicomponent vaccines against anthrax and other pathogenic diseases.
Keywords: Bacteriophage T4, virus assembly, phage display, anthrax toxin, Hoc, multi-component vaccine
1. Introduction
Most pathogens encode more than one virulence factor. A vaccine must induce antibodies against multiple targets to achieve effective protection against the pathogen. However the majority of subunit vaccine strategies focus on a single immunodominant component [1]. Although complex vaccine formulations such as mixtures of recombinant proteins, synthetic peptides, protein-polysaccharide conjugates, and plasmid DNA have been developed, these have inherent limitations [2]. Thus there is a critical need to develop a broadly applicable multicomponent antigen delivery system.
The unique architecture of bacteriophage T4 capsid (T (triangulation number) =20; width, 86 nm; length, 119.5 nm) with two non-essential outer capsid proteins, Hoc (highly antigenic outer capsid protein; 39 kDa) and Soc (small outer capsid protein; 9 kDa), provides a suitable platform for multicomponent antigen display. Hoc and Soc decorate the capsid, which is composed of the major capsid protein, gp23* (“*” represents the cleaved mature capsid protein) (49 kDa, 930 copies;), the vertex protein, gp24* (46 kDa, 55 copies), and the portal protein, gp20 (61 kDa, 12 copies) [3; see Fig. 1B]. Hoc, a dumbbell shaped protrusion at the centre of the gp23* hexon, is a monomer present at up to 155 copies per capsid, whereas Soc, a small protrusion at the interface of adjacent hexons, is a trimer present at up to 810 copies per capsid [4-7]. These proteins bind to the capsid sites, which appear after maturation cleavages of the capsid proteins and capsid expansion. These proteins, especially Soc, stabilize the capsid against extremes of pH (>10.5), but are not required for phage viability or infectivity [4,6].
Fig. 1.
Schematics of the anthrax toxin-Hoc recombinants. A. Anthrax toxin antigens and their domains (red, green and blue) were fused to the N- or C-termini of Hoc (yellow) via a linker (pink) by the PCR-based SOE strategy [24,25]. Insertion of the fusions into the plasmid vector resulted in the attachment of hexa-histidine tag (grey) to the N-terminus of each recombinant. The basic aa sequence features of each segment of the recombinants are shown in rectangular boxes. The LF and EF clones have null mutations, E687C and K346R, respectively. See Materials and Methods for additional information. B. Multicomponent phage T4 displaying three anthrax toxin antigens. The hypothetical structure is generated by merging the x-ray structures of PA (red), LF (green) and EF (blue) to the Hoc monomer (yellow spikes) of phage T4 cryo-EM reconstruction [3]. The gp23* protrusions are shown in aqua, gp24* protrusions in purple, and Soc subunits in white.
Hoc and Soc can be modified to display foreign peptides and antigens. In addressing certain drawbacks of the classic in vivo phage display systems; M13 [8], λ [9,10], T3/T7 [11] and T4 [12,13], we recently developed a novel in vitro approach to assemble1 foreign proteins on phage T4. Full-length genes corresponding to the anthrax protective antigen (PA), HIV p24, Nef, and gp41, were fused to the Hoc gene, over-expressed in E. coli, purified and assembled in vitro through Hoc-capsid interactions [14,15]. Preliminary data indicated that the in vitro system can display more than one HIV antigen on the same capsid [15].
Here we describe the use of phage T4 as a multicomponent anthrax toxin display and delivery system (see Fig. 1B). The tripartite anthrax toxin is comprised of three interacting partners, protective antigen (PA, 83 kDa), lethal factor (LF, 90 kDa) and edema factor (EF, 89 kDa). Although PA has been the principal target for numerous vaccine development strategies [16,17], inclusion of LF and EF could elicit additional protective immune responses [18,19], which may be necessary to develop a more robust next generation anthrax vaccine. We fused multiple anthrax toxins and their domains to Hoc and assembled them in vitro on phage T4 in various combinations. Manipulation of binding parameters allowed good copy number control of the antigens as well as complete saturation of the capsid binding sites. Immunization of mice with the triple anthrax toxin-T4 elicited broad and strong antigen-specific antibodies as well as lethal toxin neutralization titers. This study represents the first detailed description of a phage display system with multicomponent capability, which should lead to the development of a more effective anthrax vaccine as well as vaccines against an array of pathogens.
2. Materials and Methods
2.1 Bacteriophage, bacteria, and plasmids
The hoc-soc- T4 phage (hoc-Q21am; soc-deletion) was used to prepare phage stocks by infecting Escherichia coli P301 (sup-) cells. For construction of anthrax toxin-Hoc fusion recombinants, the T7 expression plasmids, pET-15b (Ampr) and pET-28b (Kanr) (EMD Biosciences, Inc) were used as cloning vectors. Plasmids pPA26, pLF7 (LF-E687C)2, pEF-K346R and purified phage T4 genomic DNA were used as templates for amplification of the PA gene (pagA) [20], LF gene (lef) [21], EF gene (cya), [22] and Hoc gene respectively. E. coli XL-10 Gold was used for initial transformation and E. coli codon-plus BL21 (DE3) RIL/RIPL (Stratagene) was used for IPTG-induced over-expression of toxin-Hoc fusion proteins [23].
2.2 Construction of anthrax toxin-Hoc fusion recombinants
Toxin-Hoc fusions were generated by employing the basic principles of PCR-based Splicing by Overlap Extension (SOE) strategy as described previously [24,25]. The N-terminal Hoc fusions include full-length clones, PA-Hoc, LF-Hoc, and EF-Hoc, and domain clones, LFn-Hoc and LFc-Hoc (LFn refers to the N-terminal domain of LF that interacts with PA63; LFc refers to the C-terminal domain of LF that has the MAPKK protease activity; see Fig. 1 for further details). The C-terminal fusions include Hoc-PA (full-length cleavage-minus, i.e., the cleavage site RKKR164-167 of PA was mutated to PGG), and Hoc-PA domain-4 (PA4); all full-length clones corresponded to the mature protein that is devoid of the N-terminal signal sequence (Fig. 1A). A linker sequence corresponding to Factor Xa protease cleavage site, Ile-Glu-Gly-Arg, and the flexible sequence, Pro-Gly-Gly, was incorporated between the toxin and Hoc genes. Recombinant gene fusions following amplification and purification were inserted in-frame into the BamH1 site of pET-15b or NheI-BamHI fragment of pET-28b vector (EMD Biosciences, Inc). This resulted in an in-frame fusion with the upstream hexa-histidine sequence of pET-15b and pET-28b vectors [14].
2.3 Purification and characterization of recombinant anthrax toxin-Hoc fusion proteins
Both the N-terminal (PA-Hoc, LF-Hoc, LFn-Hoc, LFc-Hoc and EF-Hoc) and C-terminal (Hoc-PA and Hoc-PA4) Hoc fusions were over-expressed in E. coli BL21 (DE3) codon-plus RIL/RIPL by 1 mM IPTG induction and purified as per the basic procedure described below. The cells containing the over-expressed fusion proteins were lysed by French-press (Aminco, USA) and the soluble fraction of each lysate was subjected to purification by HisTrap affinity chromatography (AKTA-prime, GE Healthcare) followed by Superdex-200 gel permeation chromatography (AKTA-FPLC, GE Healthcare). Recombinant PA and LF, produced in B. anthracis strain BH445, were purified by the procedures described earlier [27,28], whereas the EF null mutant K346R was expressed in E. coli and purified by the procedure described above.
Purified toxin-Hoc fusion proteins were analyzed for function by in vitro binding to either trypsin-nicked PA (nPA or PA63) or LF/EF and compared with the native counterparts. Briefly, a reaction mixture containing the target protein (PA/PA-Hoc, ∼2 μg) in cleavage buffer (20 mM Tris, 100 mM NaCl, pH 7.5) was digested with trypsin (1 ng/μg of protein) at room temperature for 30 min and the reaction was stopped by the addition of the trypsin inhibitor, TLCK (200 μg/ng trypsin). In vitro binding of native/fusion proteins was carried out at room temperature for 30 min in binding buffer (25 mM Tris, pH 9.0 and 2 mg/ml CHAPS) containing trypsin nicked PA/PA-Hoc. Finally, all the samples were run on a non-denaturing 4-12% polyacrylamide gradient gel (Invitrogen) and stained with Coomassie blue R-250.
2.4 In vitro display of anthrax toxins on phage T4
The hoc-soc- phage was grown on E. coli P301 and purified by 25-50% linear sucrose gradient centrifugation (Biocomp Inc. NB, Canada, sucrose gradient maker) [29]. The reaction mixture (total volume of 100 μl) consisted of assembly buffer (50 mM sodium phosphate buffer pH 7.0, 75 mM NaCl and 1 mM MgSO4), 2-3 × 1010 phage particles (equivalent to plaque forming units) and an appropriate amount of the recombinant toxin-Hoc fusion protein (in many cases, a 30:1 ratio of fusion protein:capsid binding sites was used) in a low-bind Eppendorf tube (Eppendorf, Germany). For multicomponent display, a mixture of toxin-Hoc fusion proteins was added to the binding reaction. In some experiments, the reaction mixture also included the respective free toxin (not fused to Hoc) and/or BSA to evaluate any nonspecific binding. The reaction mixture was incubated at 37°C for 45 min. Following high-speed centrifugation (13,000 rpm for 1 hr), the supernatant was recovered and the phage pellet was washed twice with the assembly buffer. The final pellet was resuspended in 20 μl buffer and transferred to a new tube. Both the supernatant and pellet were analyzed by SDS-PAGE, Coomassie blue staining and/or Western blotting.
2.5 Western blotting analysis
T4 phage displaying either single or multiple anthrax antigens were electrophoresed on 10% SDS-PAGE and transferred to PVDF membrane as per the standard protocol [30]. Each toxin antigen was detected by using antigen-specific polyclonal rabbit-antibodies (anti-PA, anti-LF and anti-EF) as the primary antibody, horseradish peroxidase-labeled goat anti-rabbit IgG as the secondary antibody, and 3-3’ diaminobenzidine tetrahydrochloride as the substrate.
2.6 Quantification of T4-displayed anthrax toxins
The copy number of bound antigen per phage particle was quantified by scanning the Coomassie blue-stained bands using a PDSI laser density scanner (GE Health Care). The density volumes of the antigen band as well as the control protein, gp23*, in the same lane were determined [14]. The data were analyzed by nonlinear regression analysis using the GraphPad PRISM-4 software (San Diego, CA) and normalized to the well-established copy number of gp23*, 930 copies per phage particle. In some cases, the data were checked with a second internal control, the tail sheath protein, gp18 (71 kDa; 144 copies per particle). The apparent binding constant, Kd, and the maximum copy number per particle, Bmax, were determined from saturation binding curves and Scatchard plots.
2.6 Immunogenicity studies
Female CBA/J mice (6 wk old; Jackson Laboratory, Bar Harbor, ME, USA), 10 mice per group, were immunized i.m. at weeks 0 and 4 with single antigen-T4 (PA-Hoc-T4) or triple antigen-T4 (PA/LF/EF-Hoc-T4). The amount of each antigen(s) was 1.25 μg displayed on 4 × 1010 hoc-soc- phage particles in phosphate buffered saline without any adjuvant. The mice were bled every 2 weeks and sera from individual mice were assayed for the presence of PA, LF, and EF-specific IgG using an enzyme-linked immunosorbent assay (ELISA) [31]. Briefly, 96-well flat-bottomed Nunc Maxisorp plates (VWR, Bridgeport, NJ) coated with 0.1 μg/well of purified recombinant PA, LF or EF were blocked overnight with 0.5% gelatin in PBS, washed and incubated overnight with the test serum followed by HRP-labeled, affinity purified goat anti-mouse IgG (The Binding Site, San Diego, CA) and ABTS substrate (KPL, Gaithersburg, MD). Plates were read at 405 nm on a SpectraMax 250 plate reader (Molecular Devices, Palo Alto, CA). The data are expressed as end point titers, with the titer being defined as the highest dilution that yielded an optical density reading greater than or equal to twice the background values. The titers were calculated after subtracting the mean absorbance of duplicate wells lacking antigen from wells containing antigen.
Lethal toxin neutralization titers were measured as the ability of the sera to block the cytotoxicity of lethal toxin in a J774A.1 macrophage cell line (passage 3-20) as described by Hering et al. [32] with some modifications as described below. Antisera diluted in cell culture medium were added in duplicate to 96-well tissue culture-treated flat microtiter plates (Corning costar, Acton, MA) that were pre-incubated with purified recombinant PA and LF at a final concentration of 50 ng/ml each, for 1 h at 37°C. A plate-to-plate transfer of 125 μl from this plate to another 96-well plate containing a monolayer of J774A.1 cells (2 × 105cells/well plated the day before the assay) was performed and incubated for 6-7 hours at 37°C. Thiazolyl Blue Tetrazolium Bromide (MTT) (50 μl) (Sigma Aldrich, St. Louis, MO) was added (0.5 mg/ml final concentration) to each well and the plates were incubated for 1 h at 37°C. Fluid was removed from the wells, 50 μl of formazan solubilization buffer was added, and the plates were shaken for 10 min at RT. Absorbance at 570 nm was read on a SpectraMax 250 plate reader (Molecular Devices, Palo Alto, CA). A 4-parametric sigmoid regression curve (Prism software) was used to determine the dilution of antisera that resulted in 50% neutralization of anthrax lethal toxin (ED50). Each plate contained wells that had toxin only (no antisera) and wells that contained no toxin and no antisera. These were used as the minimum and maximum assay values. Polyclonal rabbit anti-PA antibody was used as a control to monitor day-to-day assay variability.
3. Results
3.1 Purification and characterization of anthrax toxin-Hoc fusion proteins
Seven anthrax toxin-Hoc fusion constructs, five N-terminal fusions (PA-Hoc, 127 kDa; LF-Hoc, 134 kDa; LFn-Hoc, 76 kDa; LFc-Hoc, 103 kDa and EF-Hoc, 133 kDa) and two C-terminal fusions (Hoc-PA, 126 kDa; Hoc-PA4, 60 kDa) (Fig. 1A), each with an N-terminal hexa-Histidine tag, were constructed and expressed in E. coli up to about 8-12% of the total protein. Western blotting with toxin-specific antibodies specifically recognized respective size PA-Hoc, LF-Hoc and EF-Hoc fusion proteins in induced extracts as well as in the purified preparations. A majority of the over-expressed fusion proteins, about 70-90% of the total, remained in the soluble fraction, allowing for rapid purification by HisTrap affinity chromatography and FPLC gel filtration. About 1 mg of PA-Hoc, Hoc-PA, LF-Hoc, and EF-Hoc, and 2-3 mg of LFn-Hoc, LFc-Hoc and Hoc-PA4, up to ∼95% purity were obtained from one liter of induced culture (Fig. 2A).
Fig. 2.
Purification and characterization of anthrax toxin-Hoc fusion proteins. A: 10% SDS-PAGE showing purified toxin-Hoc fusion proteins. B: the same proteins following 4-12% native PAGE. Lanes: 1, Native PA; 2, PA-Hoc; 3, Hoc-PA; 4, Hoc-PA4; 5, LF; 6, LF-Hoc; 7, LFn-Hoc; 8, LFc-Hoc; 9, EF-Hoc; 10, EF; M-mol. wt. standards. C: Trypsin cleavage and in vitro binding analyses. Following trypsin cleavage and/or binding, the samples were electrophoresed on a native 4-12% gradient gel. See Materials and Methods for details of cleavage and binding assays. Lanes: 1, PA; 2, PA-Hoc; 3, nicked PA63; 4, nicked PA63-Hoc; 5, LF; 6, nicked PA63 + LF; 7, nicked PA63 + LF-Hoc; 8, nicked PA63-Hoc + LF; 9, nicked PA63-Hoc + LF-Hoc; 10, EF; 11, nicked PA63 + EF; 12, nicked PA63+EF-Hoc; 13, nicked PA63-Hoc + EF; 14, nicked PA63-Hoc + EF-Hoc. All the gels were stained with Coomassie blue.
The fusion proteins, without exception, were separated into two non-overlapping peaks upon Superdex-200 gel filtration. The first peak, eluted close to the exclusion limit of the gel matrix (600 kDa), contained protein aggregates, whereas the second peak corresponded to a dimer-size fusion protein. Analytical ultracentrifugation revealed that the latter fraction is indeed a monomer but anomalously migrated as a higher mol. wt. species [14]. Fusion to the rod-shaped Hoc apparently results in a large shape-asymmetry and high Stokes radius, leading to anomalous elution of the fusion proteins under size exclusion conditions.
3.2 Anthrax toxin-Hoc fusion proteins fold into a functionally competent conformation
All the fusion proteins migrated as a single sharp band following denaturing (SDS) as well as non-denaturing (native) PAGE (Fig. 2, panels A and B, respectively). This indicated that the E. coli expressed proteins, like their native counterparts purified from B. anthracis, appear to be correctly folded. Had they been misfolded or aggregated, they would have migrated as a broad smear under non-denaturing conditions due to non-compact and/or heterogeneous conformations. This point was further corroborated by numerous functional analyses. For instance, like the native PA, PA-Hoc was cleaved by trypsin to PA63-Hoc at the same trypsin:protein ratio as was needed to cleave native PA (Fig. 2, panel C, lanes 3 and 4), and it oligomerized into heptamers and formed higher order complexes with LF or EF (Fig. 2, panel C, lanes 6, 8, 11 and 13; the high mol. wt. band(s) correspond to complexes). Similarly, the LF-Hoc, LFn-Hoc, and EF-Hoc, like their native counterparts, interacted with PA63 as well as PA63-Hoc, forming the respective complexes (Fig. 2, panel C, lanes 7, 9, 12 and 14; LFn data not shown). The Hoc-PA4 fusion lacking PA domains 1-3 was neither cleaved with trypsin nor formed complexes with LF or EF (data not shown). LFc-Hoc was also unable to form complexes with nPA63 as it lacked the N-terminal interacting domain (data not shown).
3.3 In vitro assembly on phage T4 of PA fused to the N- and C-termini of Hoc
Although both the N- and C-termini of Hoc could be used to fuse short peptides [13,33], it is unknown whether the same holds true for a large full-length protein such as the 83 kDa PA. In vitro display showed that both the N-terminal and C-terminal Hoc fusions can be displayed on the capsid (Fig. 3A); however, Hoc-PA, the C-terminal fusion, showed reduced assembly (Fig. 3A, compare lane P of Hoc-PA with PA-Hoc). Only about a third of the capsid binding sites are filled with Hoc-PA even when an excess of Hoc-PA (Hoc-PA:binding site ratio of 50:1) was used in the binding reaction. In contrast, the N-terminally fused PA-Hoc bound to saturating capacity at a ratio of 30:1 (Fig. 3A, lane P of PA-Hoc and data not shown; see Shivachandra et al. [14]). Quantitative data from saturation binding curve and Scatchard plots showed that the apparent binding constant, Kd, for Hoc-PA was 34 μM whereas for PA-Hoc and native Hoc (his-tagged recombinant), it was 69 nM and 85 nM respectively, indicating that the C-terminal fusion exhibited about 500-fold lower affinity than the N-terminal fusion (Fig. 3, panel B). This is consistent with the recent mutagenesis data, which mapped the capsid binding site to the C-terminal domain of Hoc [Sathaliyawala and Rao, unpublished data]. Fusion to the C-terminus likely distorted the binding site, lowering its affinity to the capsid.
Fig. 3.

In vitro assembly of PA-Hoc and Hoc-PA on hoc-soc- T4 phage. PA fused either to the N-terminus or the C-terminus of Hoc was assembled in vitro as described in Materials and Methods. The supernatant and pellet samples following incubation of PA-Hoc and Hoc-PA with hoc-soc- phage were electrophoresed on a 10% SDS-PAG and stained with Coomassie blue. A: lane M, mol. wt. standards, lanes S, supernatants showing unbound protein; lanes P, phage pellets showing bound protein. The arrow points to the position of the fusion proteins. B: Binding parameters of PA-Hoc, Hoc-PA and native Hoc, as determined by saturation binding analyses ([14]; see Fig. 5).
3.4 Specificity of in vitro assembly
The in vitro system efficiently assembled the 126-134 kDa PA-Hoc, LF-Hoc and EF-Hoc, demonstrating the broad applicability of the approach (Fig. 4, panel A). Assembly was highly specific. When PA-Hoc, LF-Hoc, or EF-Hoc were incubated in the presence of an excess of the respective free native toxin (PA, LF, or EF) and a non-specific protein, BSA (Fig. 4, panel A, lanes S), only the Hoc-fused toxin selectively bound to the phage (Fig. 4, panel A, lanes P). This was further confirmed by immunoblotting of the samples with the respective toxin-specific antisera, which showed no non-specific binding of the native toxin (Fig. 4, panel B).
Fig. 4.
Specificity of anthrax toxin display. PA-Hoc, LF-Hoc, and EF-Hoc were assembled in the presence of the respective free antigen and BSA as per the standard procedure described in Materials and Methods. A: lane M, mol. wt. standards; lane T4, hoc-soc- T4 phage control; lanes S, supernatant (unbound protein); lanes P, phage pellet (bound protein). The arrow points to the position of the fusion proteins. B: the corresponding lanes from panel A were blotted and immuno-stained with the respective anti-toxin polyclonal rabbit antibodies.
3.5 Different antigens assemble on phage with similar affinities
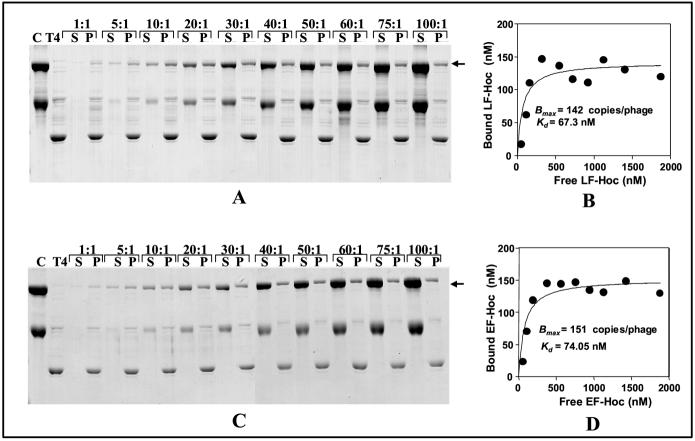
The saturation curves for LF-Hoc and EF-Hoc binding showed patterns that are similar to that of native Hoc and PA-Hoc (see Fig. 3 and data not shown; [14]). With increasing addition of the fusion protein (plus BSA), increased and specific binding was observed, reaching a plateau at an antigen:binding site ratio of 20/30:1 (Fig. 5, panels A and C). The maximum copy number of the fusion protein per phage particle (Bmax) was in the range of 142-151 (Fig. 5, panels B and D), suggesting that the capsid binding sites were fully saturated with the fused antigen. The apparent binding constant (Kd) for different fusion proteins (67-74 nM) was very similar to that of native Hoc (85 nM), suggesting that the high affinity interaction between Hoc and gp23* hexon was not altered by antigen(s) fusion to the N-terminus.
Fig. 5.
Quantification of LF-Hoc and EF-Hoc assembly on hoc-soc- phage. A constant amount of hoc-soc- phage (∼2 x1010 particles) and increasing amounts of LF-Hoc (A) or EF-Hoc (C) plus BSA were used for in vitro assembly. The ratios of LF-Hoc or EF-Hoc:capsid binding sites are shown at the top of the lanes. The supernatant (unbound protein, lanes S) and phage pellet (bound protein, lanes P) were analyzed by 10% SDS-PAGE and Coomassie blue staining. Lanes C, starting protein controls (in panel A, LF-Hoc and BSA; in panel C, EF-Hoc and BSA); Lanes T4, hoc-soc- phage control. The bound protein bands (position marked with an arrow) were quantified by laser densitometry. Panels B and D show saturation binding curves for LF-Hoc and EF-Hoc binding, respectively. The binding parameters, Bmax and Kd, as calculated from these data are given in the insets. See Materials and Methods for methodological details.
3.6 Assembly of anthrax toxin domains
For developing the next generation anthrax vaccine, and vaccine development in general, it would be necessary to evaluate the protection offered by domains rather than full-length proteins. This is because, in some cases, the domains may expose the neutralizing epitopes “hidden” in the full-length protein, leading to more efficient B-cell recognition and elicitation of stronger neutralization responses.
Three structurally stable anthrax toxin domains are known to be critical for toxin function: i) receptor interacting PA4 domain, ii) PA-interacting LFn domain, and iii) catalytically active LFc domain [26]. To better expose the functionally critical epitopes, the LFn and LFc domains were fused to the N-terminus of Hoc, whereas the PA domain-4 was fused to the C-terminus of Hoc (see Fig. 1). The endpoints of the domains were designed so that they fall in the potentially more flexible regions between the domains, as defined by the solved atomic structures [34,35]. The N-terminally fused LFn-Hoc and LFc-Hoc assembled on T4 phage to saturation (Fig. 6, lanes P under LFn-Hoc and LFc-Hoc), whereas, the C-terminally fused Hoc-PA4 assembly, as expected, was inefficient (Fig. 6, lane P under Hoc-PA4). The Bmax and Kd values of LFn-Hoc and LFc-Hoc binding were very similar to that of native Hoc and other N-terminally fused antigens, whereas those of Hoc-PA4 were similar to the C-terminally fused Hoc-PA.
Fig. 6.
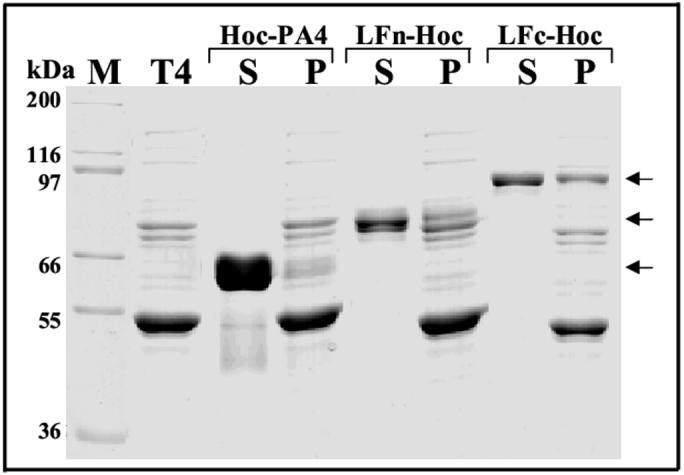
Display of the functional domains of anthrax toxins. In vitro assembly reactions were done with toxin domains fused to Hoc and hoc-soc- phage (∼3×1010 particles) as described in Materials and Methods. Lane M, mol. wt. standards; Lane T4, hoc-soc- phage control; Lanes S, unbound protein in the supernatant. Lanes P, phage pellet containing the bound protein. The arrows indicate the band positions of the displayed domain-Hoc fusion proteins.
3.7 Assembly of multiple antigens
The possibility of displaying all three anthrax toxins on T4 phage was tested by adding PA-Hoc, LF-Hoc, and EF-Hoc along with their native proteins (PA, LF and EF) and BSA to the same binding reaction at an antigen:binding site ratio of 30:1. The data showed that all three toxin-Hoc antigens were assembled in approximately equivalent amounts. However, since the mol. wts. of the antigens are very close to each other (126-134 kDa), the three bands could not be discernable by SDS-PAGE (Fig. 7, panel A, lane P) but were clearly identified by immunoblotting (Fig. 7, panel B).
Fig. 7.
Multicomponent anthrax toxin display. The in vitro assembly was performed using ∼3×1010 hoc-soc- phage and 2 to 5 five anthrax toxin-Hoc fusion proteins in the same reaction mixture, and the bound protein was quantified (see Materials and Methods for details). A: Binding of PA-Hoc, LF-Hoc, and EF-Hoc in the presence of the respective free antigens and BSA. Lanes: M, mol. wt. standards; T4, hoc-soc- phage control; S, unbound protein in the supernatant; P, phage-bound PA-Hoc, LF-Hoc and EF-Hoc. B: lane P of panel A was subjected to Western blotting with anti-PA, anti-LF, and anti-EF polyclonal antibodies. C: Multicomponent display. Lanes: S and P correspond to the supernatant and phage pellets; 2, binding of PA-Hoc + LF-Hoc; 3, binding of PA-Hoc + LFn-Hoc; 4, binding of LF-Hoc + LFn-Hoc; 5, binding of PA-Hoc + LFn-Hoc + LFc-Hoc; 6, binding of LF-Hoc + LFn-Hoc + LFc-Hoc; 7, binding of LF-Hoc + LFn-Hoc + LFc-Hoc + EF-Hoc; 8, binding of PA-Hoc + LF-Hoc + LFn-Hoc + LFc-Hoc + EF-Hoc. The number of antigens used is indicated at the top of the figure and the positions of the bound protein bands are indicated with dots.
A series of experiments were then performed by adding a cocktail of multiple antigens, both full-length proteins and domains, to the binding reaction. These include: two antigens (PA-Hoc and LF-Hoc; PA-Hoc and LFn-Hoc; LF-Hoc and LFn-Hoc; Fig. 7, panel C lanes under 2, 3 and 4, respectively), three antigens (PA-Hoc, LFn-Hoc and LFc-Hoc; LF-Hoc, LFn-Hoc and LFc-Hoc, lanes under 5 and 6, respectively), four antigens (LF-Hoc, LFn-Hoc, LFc-Hoc and EF-Hoc, lanes under 7), and five antigens (PA-Hoc, LF-Hoc, LFn-Hoc, LFc-Hoc and EF-Hoc, lanes under 8). The data show that combinations of multiple Hoc-fused antigens and domains efficiently assembled on the capsid surface (note that the positions of the displayed antigen bands are marked with a dot in Fig. 7A and C).
3.8 Control of the copy number of displayed antigens
Manipulation of the copy number of the displayed antigens would be necessary for designing customized vaccine formulations. Two sets of antigens that differ significantly in mol. wt., PA-Hoc (126 kDa) and LFn-Hoc (76 kDa) or LFc-Hoc (103 kDa), were used at varying concentrations and the copy number of the bound antigens was determined. The data showed that when the antigens were present at equimolar ratios (antigen to binding site ratio of 30:1), the copy number of each displayed antigen was reduced by half (Fig. 8). At other ratios, the copy number of the bound antigens was proportionally distributed. For instance, the copy number of PA-Hoc at 148-153 when no LFn-Hoc or LFc-Hoc was present was progressively reduced with increasing concentration of LFn-Hoc or LFc-Hoc. However, the total copy number of the bound antigens remained constant, close to the expected 155 per capsid. Thus the binding process exhibits a direct relationship between the molar ratio of the antigens used and the copy number of the bound antigen that is independent of the molecular size. This is consistent with the quantitative data given above, which showed that the antigens and domains of various mol. wts. bound to the capsid with similar Bmax (approximately 155) and Kd (65-70 nM) values.
Fig. 8.
Control of the copy number of bound antigens. A constant number of hoc-soc- T4 phage (∼3×1010 particles) were incubated with varying amounts of PA-Hoc and LFn-Hoc (A), or PA-Hoc and LFc-Hoc (C). The total antigen:binding sites ratio was kept constant at 30:1. In vitro assembly was performed as described in Materials and Methods. The supernatant (lanes S) and pellet (lanes P) were analyzed by 10% SDS-PAGE and stained with Coomassie blue. Lane T4 shows the hoc-soc- phage control. The positions of PA-Hoc (big arrow) and LFn-Hoc or LFc-Hoc (small arrow) are marked. B and D: Histograms depicting the copy number of each antigen at varying amounts of the fusion proteins used for binding (shown in the table below).
3.9 Immunogenicity of multicomponent phage T4
The main objective of immunogenicity experiments is to determine whether the multicomponent T4 phage stimulates immune responses against all the displayed antigens in the absence of any externally added adjuvant. Mice were immunized either with a single antigen-T4 (PA-Hoc-T4) or triple antigen-T4 (PA/LF/EF-Hoc-T4) and the amount of each antigen(s) was kept low (1.25 μg). The data showed that the triple antigen-T4 particles elicited strong antigen-specific antibody titers against all three antigens as well as lethal toxin neutralization titers (Fig. 9). The antibody end point antibody titers for PA (white bars), LF (black bars), and EF (grey bars), were about 3.4 × 106, 9.8 × 105, and 1.4 × 105, respectively (p<0.0001), whereas the ED50 titers were about 12,000. Thus, PA is a more potent immunogen than LF or EF, eliciting 3.4-fold and 24-fold greater antibody titers, respectively. The triple antigen-T4 immunized mice elicited 3-fold higher PA-specific IgG antibody titers than the single antigen-T4 immunized mice (p<0.00001) indicating that the immunogenicity of PA was enhanced in the multicomponent delivery. The ED50 titers for the triple antigen-T4 immunized mice were about 12,000 (Fig. 9, hatched bars) and like the PA-specific antibody titers, were about 3-fold higher than the single antigen-T4 (p<0.001).
Fig. 9.
Immunogenicity of T4-displayed PA, LF and EF. Individual serum samples (10 CBA/J mice/group) from mice immunized intramuscularly with PA-Hoc-T4 or PA/LF/EF-Hoc-T4 were analyzed by ELISA 6 weeks post-immunization for PA-specific (white bars), LF-specific (black bars) or EF-specific (grey) IgG antibodies. The ED50 neutralization titers (hatched bars) were determined by macrophage toxicity assays. See Materials and Methods for details. PA-specific IgG endpoint titers are significantly higher in mice immunized with PA/LF/EF-Hoc-T4 than with PA-Hoc-T4 alone (p<0.000001). Similarly, ED50 neutralization titers are significantly higher in the triple antigen-T4 (p<0.0001). P values were calculated using an unpaired T-test. For PA, LF and EF, bars represent arithmetic mean endpoint titers ± standard error. The endpoint titer is defined as the highest dilution that yielded an optical density reading greater than or equal to twice the background value. The titers were calculated after subtracting the mean absorbance of triplicate wells lacking antigen from wells containing antigen. For lethal toxin neutralizing titers (ED50), bars represent 4 parameter arithmetic mean ED50 titers of individual mice ± standard error.
4. Discussion
In this study, we report the development of a phage T4 multi-component antigen display and delivery system by combining the unique features of T4 capsid and the recently developed in vitro display. The data show that combinations of anthrax toxins, PA, LF, and EF, and their domains, can be efficiently displayed. The displayed antigens are immunogenic in mice. This system offers insights on how T4 can be engineered to construct future generation vaccines.
Seven anthrax antigens and domains ranging from 30-90 kDa were fused to the N-terminus of Hoc without disrupting the folding and structure of either the antigen or the Hoc protein. The Hoc fusion proteins so constructed could interact with the respective partner on one hand and the capsid on the other. Several fundamental features of Hoc-capsid binding led to efficient multicomponent display. Among these are: i) specificity - binding to T4 was exquisitely specific; no significant binding of either the free antigen or a nonspecific protein such as BSA was detectable; ii) size independence - anthrax toxins of up to 90 kDa molecular mass could be efficiently anchored, saturating the 155 capsid binding sites; and iii) high binding affinity - Kd for binding of various antigens was about 70 mM, the same as that for native Hoc binding.
The copy number of displayed antigens could be controlled by manipulating the binding conditions. When equimolar concentrations of anthrax proteins were used, the copy number of each protein per particle was reduced equivalently. The total number of bound antigens however remained constant, at the expected 155 per particle. When different concentrations of the antigens were used, the copy number of the respective antigen varied proportionally. These data are consistent with the hypothesis that the antigens filled the binding sites in a random fashion and no evidence of cooperativity was observed in the saturation binding experiments. Thus each binding site behaves essentially as an independent ligand-receptor interaction site, with 155 such interactions occurring on a single phage particle. This is consistent with the fact that adjacent Hoc monomers are about 140Å apart, providing adequate three dimensional space for independent attachment of ∼90 kDa antigen mass fused to Hoc.
Immunogenicity data showed that phage T4 with three displayed antigens stimulated specific antibody responses against all three antigens, in the absence of any externally added adjuvant. The PA-specific end point antibody titer of 3.4 million was ∼3 and 24-fold higher than that of LF and EF, respectively. This is not surprising since PA is known to be a potent immunogen [28]. So is the N-terminal domain of LF [36]. Thus, it is clear that a single T4 preparation can serve as a vehicle to deliver multiple antigens. Since the antibody responses towards the T4-displayed antigens are known to be significantly stronger than that of their soluble counterparts [12-14], this strategy could lead to the construction of effective multicomponent vaccine formulations.
Interestingly, co-display and delivery of PA, LF, and EF, enhanced the PA-specific antibody titers by about 3-fold. The same was observed with respect to the lethal toxin neutralization titers, although part of this enhancement was likely due to the presence of LF, which does induce neutralization titers on its own. The enhanced immune responses was consistently observed with all the animals in the group. This could be due to efficient uptake of the particulate T4 by the antigen presenting cells such as dendritic cells and macrophages because the triple antigen-T4 consists of three different antigens as well as three times more total antigen molecules than the single antigen-T4. The same could hold true with respect to recognition and processing of the displayed particles by the B-cells. It is however unlikely that the observed enhancement is specific for the tripartite anthrax toxin components since no interactions among the T4-attached components is expected prior to antigen presentation. This could be an additional useful feature of the multicomponent display and delivery, which needs to be further investigated.
The ability of a single phage T4 particle to deliver multiple antigens will have novel applications in vaccine biotechnology. Combined with Soc display, the multiple antigen display can be extended to 965 copies per particle. Recent data show that antigens fused to Soc can be efficiently displayed on T4 [Li et al., unpublished results]. Display can be amplified by adding either short epitopes or large complexes through interaction with the first “layer” of displayed antigens [37]. Further enhancement of immune responses can be achieved through display of targeting molecule(s) and/or incorporating expressible genes into phage T4 genome. Thus the T4 multicomponent system offers novel avenues to generate customized vaccine candidates by incorporating all the critical components of a virulent entity such as the tripartite anthrax toxin or the whole virus such as HIV. Various recombinant combinations described here are being evaluated with the goal of developing a next generation multicomponent anthrax vaccine.
Acknowledgments
We thank Ms. Elaine Morrison for her expert assistance with the immunization experiments. This work was supported by the grant AI056443 from the National Institute of Allergy and Infectious Diseases (NIAID), and in part by the Intramural Research Program of the NIAID, NIH. The research was conducted in compliance with the Animal Welfare Act and other Federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition.
Footnotes
The terms “assembly”, “binding”, and “display” are interchangeably used to refer the binding of antigen-Hoc fusion protein molecules on the T4 capsid.
In this study, LF and EF refer to the null mutants of LF and EF, LFE687C and EFK346R. These mutants, which exhibit no detectable MAPKK protease and adenyl cyclase activities respectively [26], were used to eliminate any potential toxicity of anthrax toxin complexes. The mutants however retained their binding functions and immunogenicity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. Government.
References
- [1].Clark TG, Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev Biol. 2005;121:153–63. [PubMed] [Google Scholar]
- [2].O’Hagan DT. Recent developments in vaccine delivery systems. Current drug targets-infectious disorders. 2001;1:273–86. doi: 10.2174/1568005014606008. [DOI] [PubMed] [Google Scholar]
- [3].Fokine A, Chipman PR, Leiman PG, Mesyanzhinov VV, Rao VB, Rossmann MG. Molecular architecture of the prolate head of bacteriophage T4. Proc Natl Acad Sci USA. 2004;101:6003–8. doi: 10.1073/pnas.0400444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ishii T, Yanagida M. Molecular organization of the shell of the Teven bacteriophage head. J Mol Biol. 1975;97:655–60. doi: 10.1016/s0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- [5].Ishii T, Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977;109:487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- [6].Black LW, Showe MK, Steven AC. Morphogenesis of the T4 head. In: Karam JD, editor. Molecular Biology of Bacteriophage T4. Washington DC; ASM press: 1994. pp. 218–58. [Google Scholar]
- [7].Iwasaki K, Trus BL, Wingfield PT, Cheng N, Campusano G, Rao VB, et al. Molecular architecture of bacteriophage T4 capsid: vertex structure and bimodal binding of the stabilizing accessory protein. Soc. Virology. 2000;271:321–33. doi: 10.1006/viro.2000.0321. [DOI] [PubMed] [Google Scholar]
- [8].Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- [9].Maruyama IN, Maruyama HI, Brenner S. Lambda foo: A lambda phage vector for the expression of foreign proteins. Proc Natl Acad Sci USA. 1994;91:8273–7. doi: 10.1073/pnas.91.17.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sternberg N, Hoess RH. Display of peptides and proteins on the surface of bacteriophage lambda. Proc Natl Acad Sci USA. 1995;92:1609–13. doi: 10.1073/pnas.92.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosenberg A, Griffin K, Studier W, McCormick M, Berg J, Novy R, et al. T7 select phage display system: A powerful new protein display system based on bacteriophage T7. Innovations Novagen Newslett. 1996;6:1–6. [Google Scholar]
- [12].Ren ZJ, Lewis GK, Wingfield PT, Locke EG, Steven AC, Black LW. Phage display of intact domains at high copy number: a system based on SOC, the small outer capsid protein of bacteriophage T4. Protein Sci. 1996;5:1833–43. doi: 10.1002/pro.5560050909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jiang J, Abu-Shilbayeh L, Rao VB. Display of a PorA peptide from Neisseria meningitidis on the bacteriophage T4 capsid surface. Infect Immun. 1997;65:4770–7. doi: 10.1128/iai.65.11.4770-4777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shivachandra SB, Rao M, Janosi L, Sathaliyawala T, Matyas GR, Alving CR, et al. In vitro binding of anthrax protective antigen on Bacteriophage T4 capsid surface through Hoc-capsid interactions: a strategy for efficient display of large full length proteins. Virology. 2006;345:190–8. doi: 10.1016/j.virol.2005.10.037. [DOI] [PubMed] [Google Scholar]
- [15].Sathaliyawala T, Rao M, Maclean DM, Birx DL, Alving CR, Rao VB. Assembly of HIV antigens on bacteriophage T4: a novel in vitro approach to construct multicomponent HIV Vaccines. J Virol. 2006 doi: 10.1128/JVI.00235-06. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Friedlander AM, Welkos SL, Ivins BE. Anthrax vaccines. Curr Top Micorbiol Immunol. 2002;271:33–60. doi: 10.1007/978-3-662-05767-4_3. [DOI] [PubMed] [Google Scholar]
- [17].Wang JY, Roehrl MH. Anthrax vaccine design: strategies to achieve comprehensive protection against spore, bacillus, and toxin. Med Immunol. 2005;4:4. doi: 10.1186/1476-9433-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun. 2001;69:4509–15. doi: 10.1128/IAI.69.7.4509-4515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zeng M, Xu Q, Hesek ED, Pichichero ME. N-fragment of edema factor as a candidate antigen for immunization against anthrax. Vaccine. 2006;24:662–70. doi: 10.1016/j.vaccine.2005.08.056. [DOI] [PubMed] [Google Scholar]
- [20].Welkos SL, Lowe JR, Eden-McCutchan F, Vodkin M, Leppla SH, Schmidt J. Sequence and analysis of the DNA encoding protective antigen of Bacillus anthracis. Gene. 1988;69:287–300. doi: 10.1016/0378-1119(88)90439-8. [DOI] [PubMed] [Google Scholar]
- [21].Robertson DL, Leppla SH. Molecular cloning and expression in Escherichia coli of the lethal factor gene of Bacillus anthracis. Gene. 1986;44:71–8. doi: 10.1016/0378-1119(86)90044-2. [DOI] [PubMed] [Google Scholar]
- [22].Tippetts MT, Robertson DL. Molecular cloning and expression of the Bacillus anthracis edema factor toxin gene: a calmodulin-dependent adenylate cyclase. J Bacteriol. 1988;170:2263–6. doi: 10.1128/jb.170.5.2263-2266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Studier FW, Rosenberg AH, Dunn JJ, Dubendorf JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- [24].Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–8. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- [25].Rao VB, Mitchell MS. The N-terminal ATPase site in the large terminase protein gp17 is critically required for DNA packaging in bacteriophage T4. J Mol Biol. 2001;314:401–11. doi: 10.1006/jmbi.2001.5169. [DOI] [PubMed] [Google Scholar]
- [26].Leppla SH. Bacillus anthracis toxins. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Academic Press; Burlington, MA: 2006. pp. 323–47. [Google Scholar]
- [27].Park S, Leppla SH. Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif. 2000;18:293–302. doi: 10.1006/prep.2000.1208. [DOI] [PubMed] [Google Scholar]
- [28].Ramirez DM, Leppla SH, Schneerson R, Shiloach J. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J Ind Microbiol Biotechnol. 2002;28:232–8. doi: 10.1038/sj/jim/7000239. [DOI] [PubMed] [Google Scholar]
- [29].Doermann AH, Eiserling FA, Boehner L. Genetic control of capsid length in bacteriophage T4. Isolation and preliminary description of four new mutants. J Virol. 1973;12:374–85. doi: 10.1128/jvi.12.2.374-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sambrook J, Russel D. Molecular cloning- a laboratory manual. 3rd ed. Cold Spring Harbour Press, Cold Spring Harbour; NY, USA: 2001. [Google Scholar]
- [31].Peachman KK, Rao M, Alving CR, Burge R, Leppla SH, Rao VB, et al. Correlation between lethal toxin-neutralizing antibody titers and protection from intranasal challenge with Bacillus anthracis Ames strain spores in miceafter transcutaneous immunization with recombinant anthrax protective antigen. Infect Immun. 2006;74:794–7. doi: 10.1128/IAI.74.1.794-797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hering D, Thompson W, Hewetson J, Little S, Norris S, Pace-Templeton J. Validation of the anthrax lethal toxin neutralization assay. Biologicals. 2004;32:17–27. doi: 10.1016/j.biologicals.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [33].Ren Z, Black LW. Phage T4 Soc and Hoc display of biologically active, full-length proteins on the viral capsid. Gene. 1998;215:439–44. doi: 10.1016/s0378-1119(98)00298-4. [DOI] [PubMed] [Google Scholar]
- [34].Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- [35].Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, Lacy DB, Collier RJ, Park S, Leppla SH, Hanna P, Liddington RC. Crystal structure of the anthrax lethal factor. Nature. 2001;414:229–33. doi: 10.1038/n35101998. [DOI] [PubMed] [Google Scholar]
- [36].Kushner N, Zhang D, Touzjian N, Essex M, Lieberman J, Lu Y. A fragment of anthrax lethal factor delivers proteins to the cytosol without requiring protective antigen. Proc Natl Acad Sci USA. 2003;100:6652–57. doi: 10.1073/pnas.1131930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li Q, Shivachandra SB, Leppla SH, Rao VB. Bacteriophage T4 capsid: A unique platform for efficient surface assembly of macromolecular complexes. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.08.049. (in press). [DOI] [PubMed] [Google Scholar]