Abstract
We describe the first reported case of a spontaneous septic arthritis caused by Burkholderia cepacia, the organism responsible for onion skin rot. The source of infection was most likely from hematogenous spread, as the patient's blood cultures were positive for B. cepacia. Treatment involved arthroscopic irrigation and drainage of the affected shoulder. Despite post-operative resolution of this immunocompromised patient's shoulder symptoms, he was unable to survive the B. cepacia bacteremia. Our report not only describes the case but also reviews the difficulty in treating B. cepacia infections.
INTRODUCTION
Burkholderia cepacia (B. cepacia), formerly known as Pseudomonas cepacia, is an aerobic, catalase-positive, gram-negative rod that was first isolated in 1950 as the responsible agricultural pest for onion skin rot.1 Since that time, the bacteria has been identified as an opportunistic pathogen, commonly colonizing cystic fibrosis patients. In these patients, colonization with B. cepacia leads to worsening lung function and an increased mortality rate.2 Highly transmissible strains have caused infections between cystic fibrosis patients and asymptomatic non-cystic fibrosis carriers, as well as patients with chronic granulomatous disease.3,4
B. cepacia is an extremely resilient species which can survive harsh environmental conditions with minimal nutritional needs.5 The bacterium's large genome, twice the size of Escherichia coli (E. coli), allows for this tremendous adaptability and inherent resistance to multiple antibiotics.6 This article describes the first reported case of spontaneous septic arthritis with B. cepacia from hematogenous spread.
CASE REPORT
We present the case of a 65-year-old white man with a history significant for recurrent angioimmunoblastic T-cell lymphoma and an allogenic stem cell transplant complicated by graft versus host disease. He was maintained on the immunosuppressive medications mycophenolate mofetil and tacrolimus following his transplant. In the three months preceding his presentation for septic arthritis, the patient had been admitted on four occasions for B. cepacia bacteremia. No source of infection had been isolated despite an extensive infectious disease work-up. For this particular hospital stay, he was admitted with a diagnosis of hyperkalemia. On hospital day two, the orthopaedic surgery service was consulted for increasing left shoulder pain and limited range of motion.
There was no prior history of trauma, similar symptoms, injections, or surgery to the left shoulder. The patient stated that his shoulder pain was localized, had an insidious onset, and was not accompanied by any other symptoms. Pertinent physical exam findings demonstrated a temperature of 99.7° F and no palpable effusion, erythema, or warmth. There was pain with passive range of motion of the shoulder including abduction, forward flexion, and rotation. Active range of motion was 30 degrees of forward flexion, 90 degrees of abduction, 45 degrees of external rotation, and internal rotation to the lumbar spine. Neurovascular examination of the extremity was normal.
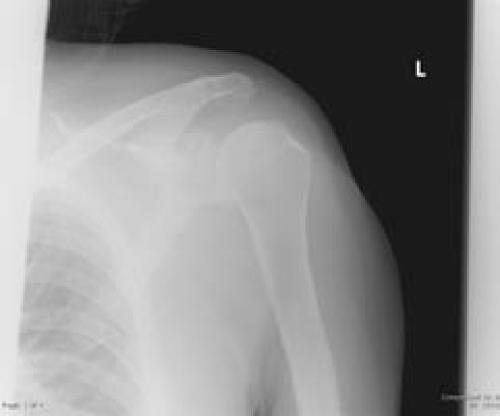
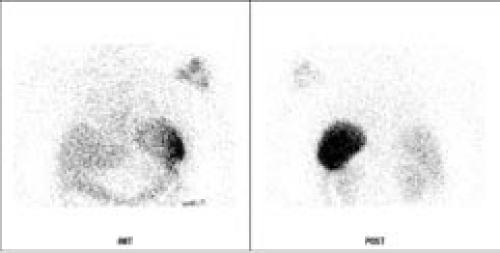
Initial laboratory results included a correcting hyperkalemia and a leukocyte cell count of 4,000 cells per microliter, an erythrocyte sedimentation rate of 84, and a C-reactive protein of 7.7. Radiographs of the left shoulder only showed degenerative joint disease in the glenohumeral joint (Figure 1). A tagged white blood cell scan obtained as a part of the patient's general infectious disease work-up demonstrated marked uptake in the left shoulder (Figure 2). Arthrocentesis of the left shoulder revealed frankly purulent joint fluid with a nucleated cell count of 121,000 cells per microliter.
Figure 1.
The patient's left shoulder AP radiograph showed generalized osteoarthritis.
Figure 2. 24 HR WBC.
A tagged white-blood-cell scan demonstrated increased uptake in the patient's left shoulder.
The patient was taken to the operating room for arthroscopic irrigation and debridement of the left shoulder. A temporary drain was placed in the left glenohumeral joint and was removed post-operatively, after 48 hours of minimal drain output. Admission blood cultures eventually grew B. cepacia, as did the final cultures from the shoulder. Both sets of positive cultures demonstrated identical antibiotic sensitivities. Following the recommendations of infectious disease consultants, the patient was treated with ceftriaxone.
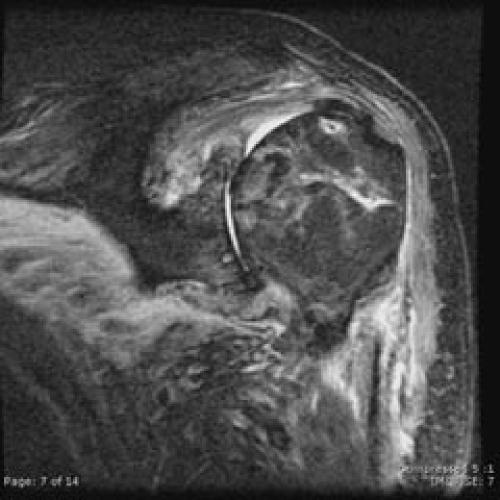
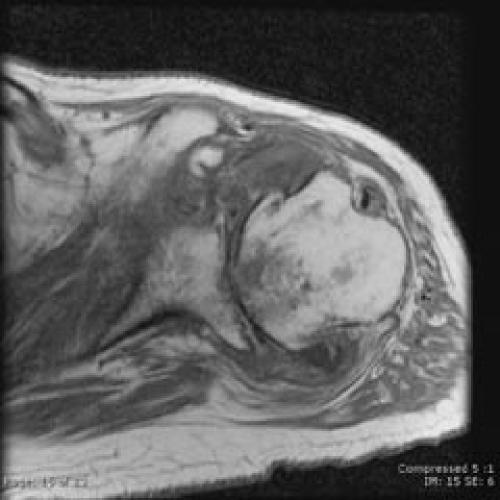
Despite surgical management of the septic shoulder (with relief of symptoms) and culture-sensitivity based intravenous antibiotic therapy, the patient continued to have persistent B. cepacia bacteremia. He was re-admitted to the oncology service twice after discharge following his shoulder infection. Diagnostic investigations including multiple transesophageal echocardiograms, colonoscopy, lymph node biopsy, and CT scans of the thorax, abdomen, and pelvis failed to reveal a source for infection. In spite of a benign shoulder exam, an MRI of the shoulder was performed two months after surgery. This study showed inflammatory changes and avascular necrosis of the humeral head, but no evidence of recurrent fluid collection or osteomyelitis (Figures 3 and 4).
Figure 3.
An MRI obtained two months after surgical management, despite resolution of shoulder symptoms, reveals chronic inflammation and avascular necrosis of the humeral head on this coronal oblique T2 image.
Figure 4.
Axial T1 image from same shoulder MRI highlights the degenerative joint destruction and the avascular necrosis of the humeral head.
Despite careful medical management and multiple consulting services, the patient eventually succumbed to the B. cepacia bacteremia and died. An autopsy was performed and attributed his death to persistent bacteremia with multi-system organ failure secondary to lymphoma. No other obvious sites of infection or abscess were discovered at autopsy. Interestingly, the patient lived on a farm with a well water supply. While this water supply tested negative for B. cepacia on two separate occasions, it was still presumed that the patient's habitat was somehow the source for his persistent infection with B. cepacia.
DISCUSSION
Burkholderia cepacia is a hardy bacterium that can survive in otherwise inhospitable environments. Studies have demonstrated that it is capable of living for over a year in a 10% iodine solution and can use penicillin as its only energy source.7,8 Transmission of the species occurs through physical contact with patients or aerosolized droplets.9 Nosocomial infections have involved direct patient inoculation with contaminated solutions with resulting B. cepacia bacteremia.10 Once acquired, the species is very difficult to eradicate. Even if patients receive aggressive treatment with culture-sensitive antibiotics, there is typically minimal clinical improvement. For example, cystic fibrosis patients rarely have reduction in the number of bacteria in sputum samples.11 Only two immunocompetent patients have been reported to succumb to fatal B. cepacia infections.12,13
Burkholderia cepacia possesses multiple virulence factors within its genome, particularly for antimicrobial resistance.6 The bacteria's genomic material has transposable elements and is divided into one to four circular replicons. This arrangement has a high likelihood for recombination events and increased genetic diversity.14 Structural features that contribute to B. cepacia's multi-drug resistance are β-lactamase proteins, antibiotic efflux pumps, and an outer membrane ten times less permeable than that of E. coli.14,15 The bacteria also produce acyl-homoserine lactones, which are small signaling proteins that diffuse to neighboring cells and affect gene transcription. These signaling proteins allow the bacteria to rapidly respond to environmental changes, increasing their virulence.6
There have only been two previous reports in the English literature of Burkholderia cepacia isolated from joint cultures. Both were from direct inoculation following a joint injection with B. cepacia-contaminated steroid solution from multi-use vials.16,17 The first case described was in a 58-year-old female with an ankle septic arthritis following an injection with contaminated methylprednisolone. 16 The septic joint responded to intravenous gentamicin and serial aspirations. After 17 days of treatment, the patient was walking and recovered uneventfully. Matteson described a second case in a 72-year-old female with a septic knee one week after an intra-articular injection of steroid.17 The bacteria proved exceptionally difficult to eradicate from the joint despite multiple aspirations and operative debridements over a 60-day period.
The patient in our report had no prior injections or surgeries on the infected left shoulder joint. To our knowledge, this case is the first reported in the English literature of a spontaneous B. cepacia septic arthritis. With other pathogenic bacteria, the most common etiology of septic arthritis is hematological seeding of the synovial joint membrane.18 Considering the positive blood cultures in our patient, the most likely etiology for his septic arthritis is hematologic spread. No source was ever found to account for the patient's persistent bacteremia. Nevertheless, our infectious disease service recommended antibiotic treatment for non-vegetative endocarditis after resolution of his septic shoulder, as others have reported B. cepacia endocarditis.19 A theoretical consideration for an additional endovascular source involves the spleen. For instance, in a murine model, B. cepacia has been isolated in the spleen 35 days after initial infection.20
Patients with chronic diseases and on immunosuppressive therapy have long been known to have an increased risk for septic arthritis. These patients represent a diagnostic and treatment challenge for orthopaedic surgeons. A high index of suspicion is required for diagnosing septic arthritis in the immunocompromised patient, since they may have only mild symptoms or an atypical presentation. Fever and an elevated leukocyte count may not be present and symptoms may only include pain or decreased range of motion. While most cases of septic arthritis are caused by gram positive organisms, immunocompromised patients are also at increased risk for infection with gram negative bacteria.18 Other rarer causes of septic arthritis, such as mycobacterium and fungi, must also be considered.
SUMMARY
We present the first case of a hematogenously spread septic arthritis caused by Burkholderia cepacia. This hardy bacterium is very difficult to eradicate and will typically colonize infected patients. Orthopaedic surgeons should be suspicious for this potentially fatal pathogen in the care of immunocompromised patients and patients with cystic fibrosis or chronic granulomatous disease. Finally, consultation with an infectious disease service should be considered, as B. cepacia is naturally resistant to multiple antibiotics. Patients may require complex multi-antibiotic regimens with regular follow up.
References
- 1.Burkholder W. Sour skin, a bacterial rot of onion bulbs. Phytopathology. 1950;40:115–118. [Google Scholar]
- 2.Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 3.Holmes A, Nolan R, Taylor R, et al. An epidemic of Burkholderia cepacia transmitted between patients with and without cystic fibrosis. J Infect Dis. 1999;179(5):1197–1205. doi: 10.1086/314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neil KM, Herman JH, Modlin JF, et al. Pseudomonas cepacia: an emerging pathogen in chronic granulomatous disease. J Pediatr. 1986;108(6):940–942. doi: 10.1016/s0022-3476(86)80934-9. [DOI] [PubMed] [Google Scholar]
- 5.Beringer PM, Appleman MD. Unusual respiratory bacterial flora in cystic fibrosis: microbiologic and clinical features. Curr Opin Pulm Med. 2000;6(6):545–550. doi: 10.1097/00063198-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3(2):144. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RL, Vess RW, Panlilio AL, et al. Prolonged survival of Pseudomonas cepacia in commercially manufactured povidone-iodine. Appl Environ Microbiol. 1990;56(11):3598–3600. doi: 10.1128/aem.56.11.3598-3600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckman W, Lessie TG. Response of Pseudomonas cepacia to beta-lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1970;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168(8):918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 10.Flaherty JP, Garcia-Houchins S, Chudy R, et al. An outbreak of gram-negative bacteremia traced to contaminated O-rings in reprocessed dialyzers. Ann Intern Med. 1993;119(11):1072–1078. doi: 10.7326/0003-4819-119-11-199312010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Jones AM, Dodd ME, Webb AK. Burkholderia cepacia: current clinical issues, environmental controversies and ethical dilemmas. Eur Respir J. 2001;17(2):295–301. doi: 10.1183/09031936.01.17202950. [DOI] [PubMed] [Google Scholar]
- 12.Hobson R, Gould I, Govan J. Burkholderia (Pseudomonas) cepacia as a cause of brain abscesses secondary to chronic suppurative otitis media. Eur J Clin Microbiol Infect Dis. 1995;14(10):908–911. doi: 10.1007/BF01691499. [DOI] [PubMed] [Google Scholar]
- 13.Wong S-N, Tam AY-C, Yung RW-H, et al. Pseudomonas septicaemia in apparently healthy children. Acta Paediatr Scand. 1991;80(5):515–520. doi: 10.1111/j.1651-2227.1991.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 14.Govan JR, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia . Microbiol Rev. 1996;60(3):539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LiPuma JJ. Burkholderia cepacia: management issues and new insights. Clin Chest Med. 1998;19(3):473–486. doi: 10.1016/s0272-5231(05)70094-0. [DOI] [PubMed] [Google Scholar]
- 16.Kothari T, Reyes MP, Brooks N. Pseudomonas cepacia septic arthritis due to intra-articular injections of methylprednisolone. CMAJ. 1977;116(111):1230. [PMC free article] [PubMed] [Google Scholar]
- 17.Matteson EL, McCune WJ. Septic arthritis caused by treatment-resistant Pseudomonas cepacia . Ann Rheum Dis. 1990;49(4):258–259. doi: 10.1136/ard.49.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15(4):527–544. doi: 10.1128/CMR.15.4.527-544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahal JJ, Jr, Simberkoff MS, Hyams PJ. Pseudomonas cepacia tricuspid endocarditis: treatment with trimethoprim, sulfonamide, and polymyxin B. J Infect Dis. 1973;128(Suppl):762–767. doi: 10.1093/infdis/128.supplement_3.s762. [DOI] [PubMed] [Google Scholar]
- 20.Speert DP, Steen B, Halsey K, et al. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect Immun. 1999;67(8):4027–4032. doi: 10.1128/iai.67.8.4027-4032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]