Abstract
Angiogenesis, the sprouting of new capillaries from existing blood vessels, is crucial for normal fracture healing. Angiogenesis is a complex process involving a variety of growth factors and several cell types. The mechanism regulating angiogenesis during fracture repair is not well understood, and the relationships between angiogenesis, chondrogenesis, and osteogenesis are also undefined. In vivo animal models have been useful for determining angiogenic mechanisms. In particular, a murine model has been developed that offers the advantages of easy animal handling, low cost, reliable healing, and the availability of molecular and genetic techniques for research. However, the small size of mice provides challenges, including the inability to assess vascularization using techniques that have been employed in larger animals. Therefore, we developed and optimized techniques specifically for studying angiogenesis during mouse fracture repair. These techniques include blood vessel casting, micro-computed tomography (micro- CT), immunohistochemistry, in situ hybridization, and genetic labeling of endothelial cells. Blood vessel casting and micro-CT are useful for visualization of small blood vessels. Immunohistochemistry using anti-PECAM (platelet endothelial cell adhesion molecule) or CD34 antibodies and genetic approaches using Tie2-cre transgenic mice can be used to label endothelial cells, visualize blood vessels including capillaries, and provide structural information about the vascularization of the fracture callous. Lastly, expression patterns of important growth factors regulating angiogenesis could be assessed by molecular approaches such as in situ hybridization.
INTRODUCTION
Bone fractures are usually accompanied by injuries to the vasculature. A hematoma forms around the fracture site, and an inflammatory response is initiated. This initial reaction stimulates angiogenesis, and the blood supply to the injured bone returns. Previous clinical observations and experimental studies have determined that angiogenesis is necessary for normal fracture repair. Inhibition of new blood vessel formation induced by the administration of TNP-470 was shown to prevent fracture healing in rats.1 Conversely, exogenous application of vascular endothelial growth factor (VEGF), a potent pro-angiogenic agent, significantly accelerates fracture healing.2,3,4,5
The importance of the blood supply to fracture healing has been long recognized. However, the cellular and molecular mechanisms that govern angiogenesis and the role that the blood supply plays during fracture healing remain largely unknown. Regulation of angiogenesis during endochondral ossification of the fracture callus involves complex signaling processes, including that via VEGF,6,7 but the mechanisms regulating vascular repair during the early stages of healing are unknown. The blood supply certainly provides essential nutrients to cells, but whether and through what mechanism vascularity influences events that occur during fracture repair is not clear. For instance, in vitro chondrocyte differentiation can be enhanced by low oxygen tension (hypoxia); 8,9 however, this phenomenon was not confirmed in an animal fracture model that had impaired vascular regeneration.1 Additionally, the blood supply could be a route for migration of systemic stem cells to sites of bone injury. In both of these cases the extent of the vascular injury and the rate of vascular repair could significantly influence cell differentiation during skeletal repair. To address the extent to which angiogenesis impacts cell fate decisions during fracture repair, a thorough assessment of angiogenesis during healing is required.
Rodent models are excellent tools to study bone biology and skeletal repair for a variety of reasons. Mouse models in particular are desirable due to the availability of a plethora of genetically engineered strains and the abundance and availability of reagents for cellular and molecular analyses. Our laboratory has developed several mouse models of tibia fractures that have provided valuable information regarding mechanical, cellular, and molecular mechanisms that control bone regeneration.10,11,12,13 However, due to the small size of mice, assessing vascularization during fracture healing using techniques developed for larger animals has proven difficult. The goal of this work was to assess a variety of methods to analyze structural and functional aspects of angiogenesis during fracture healing. We have developed and/or optimized a variety of techniques to analyze the structural, functional, cellular, and molecular features of angiogenesis during healing of non-stabilized fractures of mouse tibias.
MATERIALS AND METHODS
Animals
Ten to 14 week old male wild type (129J/B6, The Jackson Laboratory, Bar Harbor, Maine, USA) and transgenic (Tie2-cre,14 Rosa26R) mice were used in this study. All animal procedures were approved by the UCSF Institutional Animal Care and Use Committee and conformed to state and federal regulations for use of vertebrate animals in research.
Creating tibia fractures
Animals were anesthetized with 2% Avertin (2,2,2 tribromoethanol, Sigma-Aldrich), and then a closed transverse fracture was created by three-point-bending of the mid-diaphysis of the right tibia.10,11 These fractures were not stabilized, and animals were allowed to move freely after recovering from anesthesia. Buprenex was administered subcutaneously to relieve post-surgical pain.
Blood vessel casting and micro-CT scanning
At 14 days post-fracture, animals were euthanized with an overdose of 2% Avertin, and the femoral vessels and the heart were exposed. The right atrium was opened. The entire vascular system was then flushed by injecting heparinized saline (100U/ml) into the left ventricle until the femoral vessels turned white and the saline flowing out of the right atrium became clear. Microfil (Flow Tech. MV-diluent:MV-compound (5:1) and MV-curing agent (10% of the total volume)) was prepared immediately before injection. The entire vascular system was then perfused by intracardiac injection (left ventricle) of 3-5 ml of the Microfil. After perfusion, animals were placed at 4°C for 2 hours or overnight in order for the compound to polymerize. The fractured tibiae were then collected, fixed, dehydrated through a series of ethanol washes, and cleared in methyl salicylate. The vasculature was then evaluated using a Leica MZFLII dissecting microscope. One of the samples was rehydrated and examined by x-ray radiography and micro-CT (micro-computed tomography) scanning (Scanco, 20 µm) system.
For micro-CT scanning, the vasculature was perfused with a barium sulfate suspension (Liquid Barosperse, Lafayette Pharmaceuticals, Inc. Yorba Linda, CA) following the same technique described above. The fractured leg was ligated at the mid-femur level and cut proximal to the ligation to minimize the barium leakage from open blood vessels. The entire leg was fixed in 4% Paraformaldehyde (PFA; 4°C overnight), decalcified in 19% EDTA for 3-5 days, and then examined by x-ray radiography and micro-CT scanning.
Processing tissue for histology
Ten 129J/B6 mice were sacrificed at 14 days after fracture and the fractured legs were collected, fixed in 4% PFA (4°C overnight), and decalcified in 19% EDTA for 10-14 days (4°C). Decalcification was confirmed by x-ray radiography. Decalcified tissues from five mice were dehydrated in a graded series of ethanol washes, cleared with xylene and then embedded in paraffin. Sections (10 µm) were prepared with a Leica microtome and mounted on glass slides. Tissue from the other five mice were infused with 30% sucrose in PBS (overnight) and then embedded in O.C.T. compound (Tissue-Tek, Sakura Finetek U.S.A., Inc, Torrance, CA) on dry ice. Sections of 20 µm were prepared using a cryostat. Frozen sections were kept in a sealed box at -20°C until analysis.
Immunohistochemistry
Paraffin sections were dewaxed with HemoD (histoclear, Fisher Scientific), rehydrated through a graded series of ethanol washes, and maintained in phosphate buffered saline (pH 7.4; PBS). Frozen sections were air dried for two hours and then placed in PBS. Sections were treated with 0.3% H2O2 in methanol for 1 hour to quench endogenous peroxidase activity. Blocking solution (5% powder milk, 0.1% ovalbumin, and 5% goat serum in PBS) was applied for 10-30 minutes to reduce non-specific binding of the antibodies. Antigen retrieval of freshly prepared paraffin sections (<1 week old) was performed by incubation in 0.1% trypsin in PBS for 15 minutes at room temperature. To unmask antigens from old paraffin tissues (stained more than 3 to 4 months after section), different protocols were tested: 0.1% trypsin for 15 or 30 minutes, Ficin solution (Zymed) for 10 minutes, or heat mediated antigen retrieval (citrate buffer epitope retrieval, 100°C for 20 minutes). Ficin solution (5 minutes at room temperature) was used to recover antigens on frozen sections. All sections were incubated (overnight, 4°C) with an anti-mouse PECAM (Platelet endothelial cell adhesion molecule-1, also called CD31) antibody (IgG, Pharmingen, cat#: 553370, diluted 1:500 in 5% goat serum) or an anti-mouse CD34 antibody (IgG, Abcam, cat#: ab8158, diluted 1:25 in 5% goat serum) that was generated in rats. A biotinylated goat anti-rat IgG (Pharmingen, cat#: 554014) was used (1 hour at room temperature, diluted 1:100 in 5% goat serum) as the second antibody to detect each of the primary antibodies. Streptavidin-horseradish peroxidase (diluted 1:100, Amersham, cat#: RPN1231) was then applied and the sections were incubated at room temperature for 1 hour. The immune complexes were visualized using diaminobenzidine (DAB) as the substrate.11,15
In situ hybridization
In situ hybridization was performed as previously described. 11 Briefly, a subclone of cDNA corresponding to the murine VEGF gene was linearized and used to generate an antisense 35S-UTP-labelled riboprobe. Paraffin sections were hybridized with probes overnight at 48°C and post-washed at 53°C with increasing stringency. After drying, slides were coated with emulsion and exposed for 7-14 days. After developing, sections were stained with Hoechst dye to visualize the nuclei. A dark-field image of the exposed silver grains was superimposed on the fluorescent image of the nuclei in Adobe Photoshop CS.
Detecting beta-galactosidase in Tie2-cre/R26R mice
Transgenic mice that express Cre-recombinase under the control of the Tie2 promoter (Tie2-cre) were mated to the reporter mouse strain Rosa26R, generating Tie2-cre/R26R mice that exhibit beta-galactosidase activity in cells of Tie2 lineage. The expression of beta-galactosidase was detected using a standard X-gal reaction. Fractures were created as described above in the mid-diaphysis of the right tibia of 3 mice and the animals were euthanized 7 days after injury. Tissue was fixed in 0.4% PFA/PBS with 5mM EDTA and 2mM MgCl2 overnight at 4°C, decalcified in 19% EDTA for 10-14 days, and embedded in O.C.T. Compound. Frozen sections (10 µm) were prepared, air dried for 2 hours, fixed in 0.2% Glutaraldehyde/PBS containing 5mM EDTA and 2 mM MgCl2 for 30 minutes (room temperature), and washed with wash buffer (PBS with 0.1% Tween20 and 2 mM MgCl2). The tissues were then incubated overnight at 37°C in X-gal staining solution (wash buffer with 1mg/ml X-Gal, 5mM Potassium Ferrocyanide, 5 mM Potassium Ferricyanide, and 20 mM Tris) in order to stain endothelial cells blue.
RESULTS
Visualizing the circulatory system of the mouse hind limb
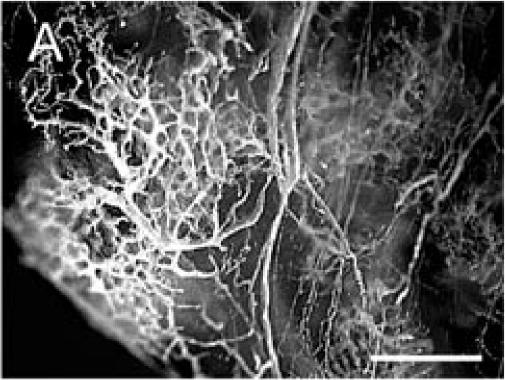
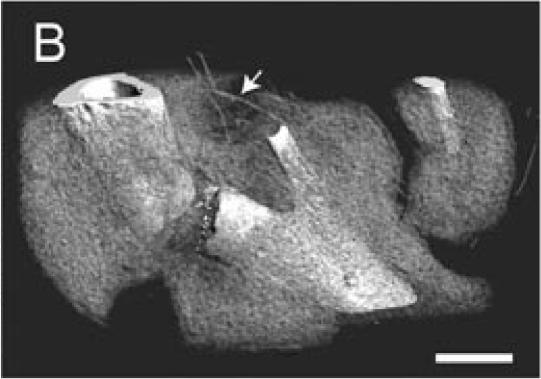
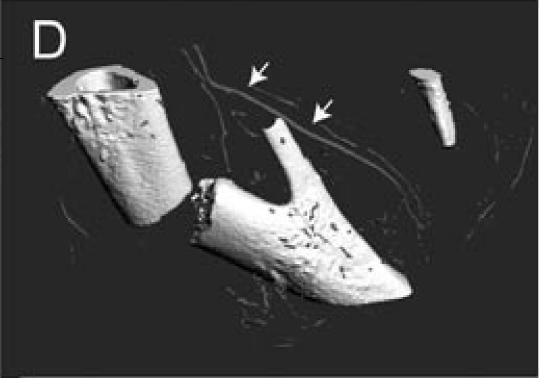
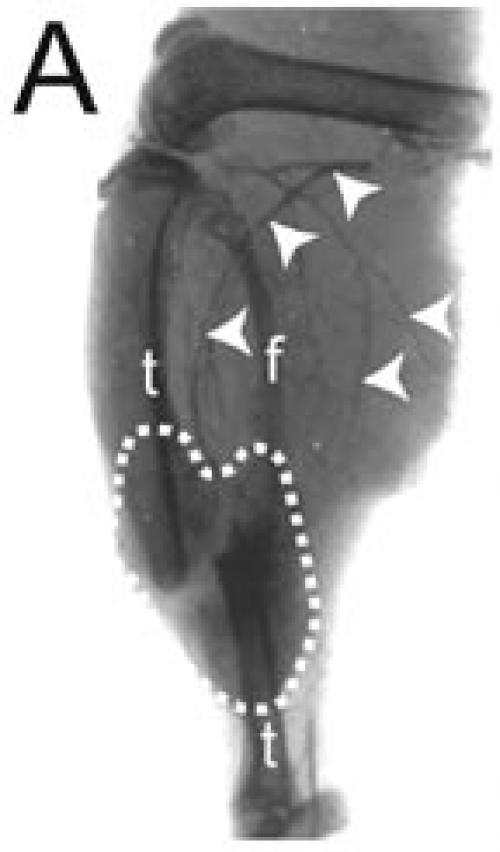
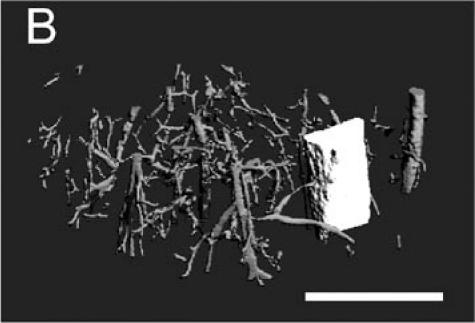
The Microfil compound was successfully used to perfuse the whole circulatory system. This technique allowed visualization of the blood vessels in the leg and around the fracture site using a dissecting microscope (Figure 1A). Micro-CT scanning was then performed on one of the injected samples. When scanned at a resolution of 20 µm, the bone and mineralized portion of the callus were clearly visible, but only big blood vessels were observed (Figures 1B-D). The smaller blood vessels were successfully imaged by micro-CT scanning using a suspension of barium sulfate as a contrast agent (Figure 2A). Partial decalcification enhanced the contrast between blood vessels and the surrounding tissues without affecting the radio-opacity of the intravascular barium sulfate. After reconstructing the micro-CT images, many blood vessels, both large and small, could be observed (Figure 2B).
Figure 1.
Microfil blood vessel casting and micro-CT imaging. (A) Microfil casting demonstrated a network of blood vessels around the callus of a day 14 non-stabilized mouse tibia fracture. The same sample was scanned at 20 µm under micro-CT, and 3-dimensional images were reconstructed to show (B) the site of fracture, the mineralized callus, and blood vessels (arrow); (C) the mineralized callus only; and (D) the site of fracture and blood vessels (arrows). Scale bar = 1mm.
Figure 2.
Blood vessels perfused by intracardiac barium suspension injection. (A) X-ray radiography was used to image the fracture, fracture callus (outlined), and the filled blood vessels (arrowheads) of a day 14 non-stabilized mouse tibia fracture. (B) A 3-dimensional image of blood vessels around the fractured bone was created after micro-CT scanning. t = tibia, f = fibula. Scale bar: B = 1mm.
Identification of endothelial cells
In order to identify endothelial cells in tissue section, two distinct approaches were used. In the first approach, endothelial-specific gene products (PECAM and CD34) were visualized using immunohistochemistry on paraffin and frozen sections. In the second approach genetically labeled endothelial cells (Tie2-cre/R26R) were visualized in situ during fracture healing.
PECAM:
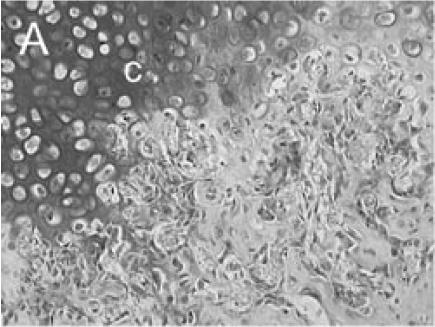
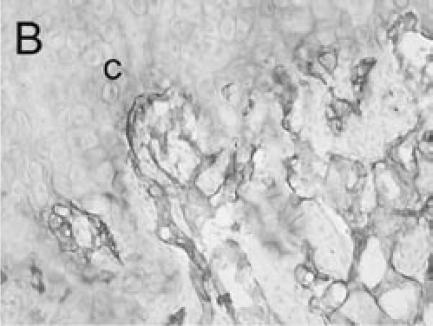
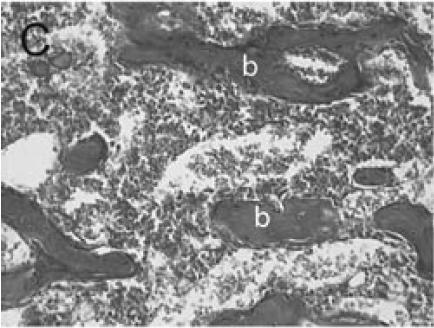
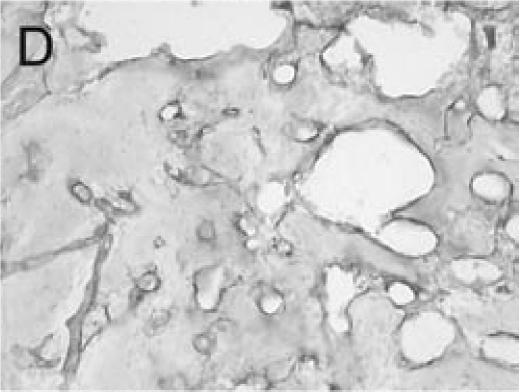
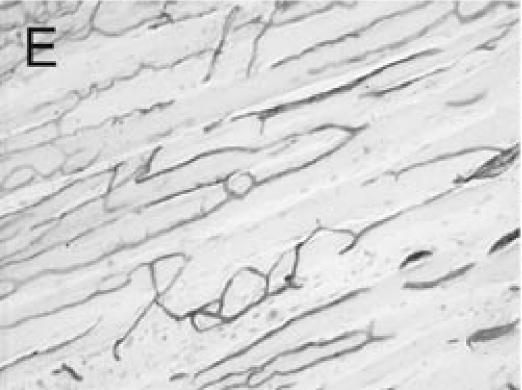
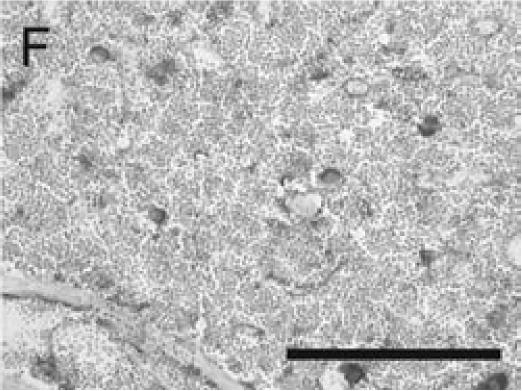
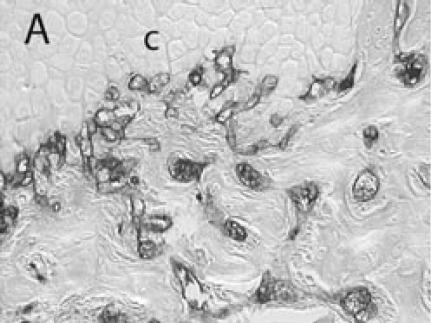
Immuno-detection of PECAM allowed visualization of blood vessels and endothelial cells in and around the fracture callus (Figure 3). Endothelial cells were present at sites of vascular invasion during endochondral ossification (Figures 3A, B), throughout newly formed bone (Figures 3C, D), and in muscle (Figure 3E) and bone marrow (Figure 3F). In addition to endothelial cells, other cell types in bone marrow, primarily monocytes, were also recognized by the anti-PECAM antibody (Figure 3F). Consistent PECAM immunostaining was achieved on fresh cut sections with 0.1% trypsin digestion (Figure 4A). However, efforts to unmask PECAM antigen using multiple antigen retrieval techniques on stored paraffin sections were not successful, providing weak and inconsistent staining (Figure 4B).
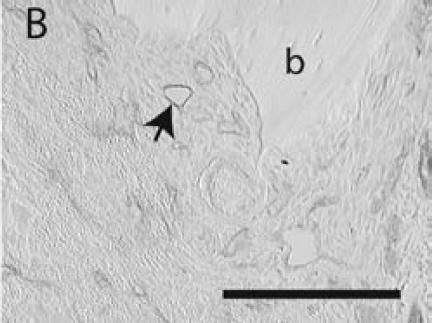
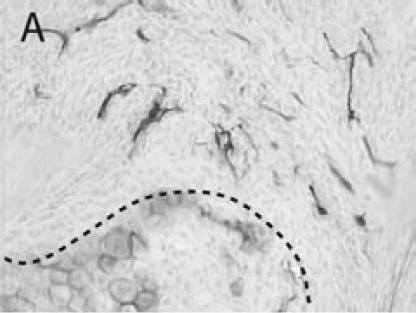
Figure 3.
PECAM immunohistochemistry on frozen section (A) Safranin O/Fast Green staining shows that cartilage (c) is being replaced by new bone in the callus of day 14 non-stabilized mouse tibia fractures. (B) PECAM immunostaining shows blood vessels (black) invading cartilage (c). (C) Trichrome staining shows the newly formed trabeculae (b). (D) PECAM positive cells formed tubes within lumens between trabeculae. (E) A large number of blood vessels in muscle were labeled after PECAM immunostaining. (F) Some monocytes in bone marrow were also PECAM-positive. Scale bar = 200 µm.
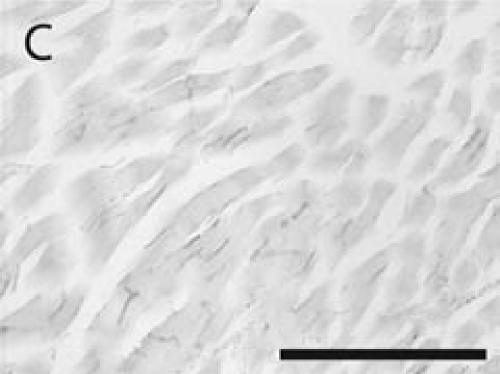
Figure 4.
PECAM immunohistochemistry on paraffin section (A) Strong PECAM staining was achieved on freshly cut paraffin sections through a day 14 non-stabilized fracture. (B) PECAM yielded weak (arrow) and inconsistent staining on old paraffin sections of a day 5 non-stabilized fracture. Scale bar = 200 µm.
CD34:
The CD34 antibody tested in this study also worked on frozen but not paraffin sections. Endothelial cells in the fracture callus (Figure 5A) and new bone (Figure 5B) were clearly recognized by this antibody. However, detection of endothelial cells in muscle was inadequate, and only a relatively weak immuno-reaction was observed (Figure 5C).
Figure 5.
CD34 immunohistochemistry on frozen section (A) CD34 immunostaining was detected in blood vessels around a cartilage island (outlined) and (B) in newly formed bone of a day 14 non-stabilized mouse tibia fracture. (C) Blood vessels in muscle were weakly stained. Scale bar = 200 µm.
Tie2-cre/R26R:
Endothelial cells were clearly and unequivocally observable adjacent to the fracture callus (Figure 6). The X-gal reaction renders the labeled endothelial cells blue due to Cre-mediated recombination to activate expression of latent β-galactosidase. In addition to the large number of blood vessels near the fracture callus and in the surrounding tissues, a large number of bone marrow cells were also blue as a result of X-gal staining (data not shown).
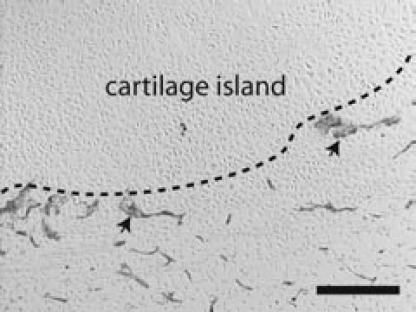
Figure 6.
Visualization of blood vessels in Tie2-cre/R26R mice Beta-galactosidase activity was assessed by a standard X-gal reaction on frozen section of a day 7 fracture. Blood vessels (arrows) around a cartilage island (outlined) were stained blue. Scale bar = 200 µm.
Analysis of an angiogenic factor
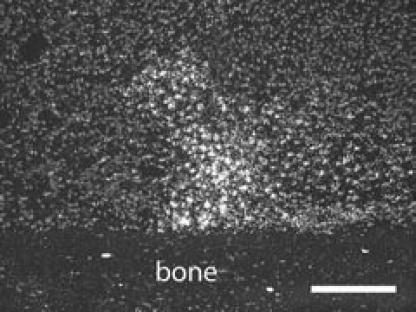
In situ hybridization for VEGF transcripts was successfully performed and revealed VEGF expression in hypertrophic chondrocytes that comprise the cartilaginous scaffold of the fracture callus (Figure 7).
Figure 7.
VEGF expression in fracture callus. VEGF transcripts were detected by in situ hybridization in a day 7 fracture. Hypertrophic chondrocytes expressed high level of VEGF. Scale bar = 200 µm
DISCUSSION
In order to better understand angiogenesis during fracture healing, we optimized several techniques to assess different aspects of vasculature using a murine tibia fracture model. These techniques allow visualization of the macro-, micro-, and molecular organization of the circulatory system. Casting of the entire circulatory system in mice allows investigators to assess the extent of vascular damage in a manner similar to angiography in patients. Additionally, this approach allows researchers to assess only the patent circulatory system and provides a method to determine the volume of the blood vessels in a given region. Identification of endothelial cells in tissue sections provides a means to begin to understand the histological components required for blood vessel formation and reconstitution of an intact vascular supply. Lastly, determination of the temporal and spatial patterns of expression of various angiogenic molecules during fracture repair will enhance the understanding of how vascular repair is regulated.
Assessing vasculature at a macroscopic level
The technique of vascular perfusion has been used to visualize the circulatory system for a long period of time and has gained wide clinical use. Adaptation of this technique to rodent models allows the vasculature to be examined at a macroscopic level during fracture repair. Perfusion of the vessels with Microfil and subsequent evaluation by micro-CT is an easy and efficient way to visualize the circulatory system. Microfil casting requires no special equipment, is cheap, and provides decent perfusion of large blood vessels. In the field of angiogenesis research, micro-CT is superior to conventional angiography or blood vessel casting. Three-dimensional images can be generated from micro-CT scans, which can be used to determine the volume of the perfused vasculature in order to provide a quantitative assessment of vascularization.
Micro-CT scanning does have drawbacks. First, it requires an investment in equipment and trained personnel to undertake the required analyses. In addition, it is challenging to achieve high quality images of blood vessels by micro-CT scanning. Results could be affected by the quality of blood vessel perfusion, unknown characteristics of the fracture callus, and the resolution used for scanning. For instance, the absence of blood vessels in certain areas or the appearance of "dead ends" may reflect physiologically relevant processes: the dead ends could be areas where blood vessels are damaged, sealed, or undergoing active angiogenesis. Alternatively, these regions could have simply not been filled due to fluctuations in perfusion pressures or other unknown variables. Similarly, mineralization of the fracture callus may render the contrast dye ineffective. The data presented in this work illustrate that perfusion with a suspension of barium sulfate followed by partial decalcification of tissues may be used to achieve high quality imaging. Lastly, while the resolution of micro-CT imaging has improved dramatically, adequate visualization of micro-vessels may still be limited. Unfortunately, these vessels are probably more important for vascular function than the larger vessels, since the microcirculation provides a larger surface area for gas and nutrient exchange.
Assessing angiogenesis on tissue sections
Small blood vessels are composed of endothelial cells, a basement membrane, and pericytes. Markers expressed by endothelial cells or pericytes are commonly used to label blood vessels by immunohistochemical approaches. In this study, we tested two markers of endothelial cells: PECAM11 and CD34. PECAM is a 130 kd transmembrane glycoprotein belonging to the immunoglobulin superfamily of cell adhesion molecules. This protein functions as an adhesive molecule and a mediator of signal transduction through modulation of integrin function in order to regulate vascular integrity and cell survival.16 PECAM protein is found at the cell-cell borders of neighboring endothelial cells and is also expressed by platelets, monocytes, neutrophils, or some T cells. CD34 is another transmembrane glycoprotein present on endothelial cells, leukemic cells, and some progenitor cells. The function of the CD34 protein remains unknown. Nonetheless, detection of these two molecules provides an easy way to identify blood vessels and endothelial cells in tissues adjacent to the fracture site.
In general, immunohistochemistry to detect PECAM on frozen sections was much more effective and consistent compared to paraffin embedded tissues. On freshly cut paraffin sections less than 1 week old, antigen retrieval was effective at unmasking the epitope recognized by the antibody. However, if sections were stored, then results of immunohistochemical detection of PECAM became inconsistent. The reason for this is unclear but could involve either degradation of the protein upon storage or alteration of the protein that makes it resistant to unmasking via antigen retrieval methods.
A novel approach to the study of angiogenesis utilizes a creative molecular technique that indelibly marks specific cell types and thus allows a lineage analysis in embryonic and adult mice. This approach uses the Cre/loxP system and a tissue specific promoter to stimulate expression of a transgene in specific cells. Cre is a bacteriophage P1-derived recombinase that efficiently excises DNA that is flanked by two repeated loxP recognition sites. The DNA is then recombined, and the result is removal of the piece of DNA flanked by the two loxP sites (usually referred to as "floxed"). The ease of creating DNA constructs containing a specific promoter that drives expression of Cre recombinase has made use of the Cre/loxP system to mediate site-specific DNA recombination versatile.17,18
The Tie2-cre mice14 provide a mechanism of identifying endothelial cells that does not require the use of immune reagents or other stains that often give inadequate results. In adult mice, an endothelial cell specific enhancer has been co-opted to drive Cre-expression. Hence, wherever the Tie2 enhancer is activated, Cre is also expressed. Since this enhancer functions exclusively in endothelial cells and their precursors, Cre recombinase is expressed only in derivatives of the endothelial cells and their precursors.14 The Tie2-cre mouse was mated to the Rosa26R mouse, which is widely used to analyze expression of Cre recombinase. β-galactosidase is separated from a ubiquitous promoter by a large region of floxed DNA. Upon Cre-mediated recombination, the β-galactosidase gets "placed" adjacent to the promoter and becomes activated. Thus, endothelial cells express β-galactosidase and can be tracked during fracture repair.
The data presented demonstrate that PECAM immunostaining and genetic labeling of endothelial cells using the Tie2-cre/R26R system are reliable techniques to visualize blood vessels in mouse fracture calluses at the microscopic level. One advantage of these techniques is that the tissues surrounding the blood vessels could easily be evaluated by histological or molecular (i.e., in situ hybridization) approaches, thus allowing analyses of the interaction between vascularization and tissue formation (such as chondrogenesis or osteogenesis) during fracture healing. Another advantage is that qualitative analysis can be achieved on these PECAM or X-gal stained sections by stereology or other histomorphometric methods to generate important parameters of vascular structure, such as vascular density and vascular bifurcation ratio.19, 20,21 However, one must keep in mind that neither PECAM immunostaining nor genetic labeling of endothelial cells using the Tie2-cre/R26R system is specific for new blood vessels. Markers specifically expressed by new blood vessels have been extensively explored during recent years. Among the most promising markers are the extra-B domain of fibronectin,22 prostate-specific membrane antigen (PSMA),23,24,25,26,27 and antibody E4G10,28 which are reported to be expressed by tumor blood vessels but not vessels in surrounding normal tissues.
Lastly, commonly employed techniques such as in situ hybridization work well to assess expression patterns of key angiogenic genes during fracture healing. These studies are necessary in order to identify key molecular and cellular regulators of angiogenesis during fracture repair. Future work is related to utilizing these methodologies to define in detail the molecular and cellular events that regulate angiogenesis during fracture healing and to assess the role of the vasculature during bone repair.
ACKNOWLEDGMENTS
This work is funded by NIH-NIAMS (KO8-AR002164 and RO1-AR053645-01to T.M.) and the Zimmer Corporation through a grant provided by the Orthopaedic Trauma Association (Basic Research Grant to R.M.). Tie2-cre and Rosa26R mice were provided by Dr. R. Wang. We would like to thank Dr. P. Choi, Dr. S. Majumdar, and Andrew Burghardt for technical support.
References
- 1.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt H, Ding M, Lind M, Hansen ES, Christensen KS, Hvid I. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Joint Surg Br. 2005;87:1434–1438. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 3.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarkka T, Sipola A, Jamsa T, Soini Y, Yla-Herttuala S, Tuukkanen J, Hautala T. Adenoviral VEGF-a gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–566. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 5.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mechanisms of Development. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 8.Bassett CA, Herrmann I. Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature. 1961;190:460–461. doi: 10.1038/190460a0. [DOI] [PubMed] [Google Scholar]
- 9.Grimshaw MJ, Mason RM. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage. 2000;8:386–392. doi: 10.1053/joca.1999.0314. [DOI] [PubMed] [Google Scholar]
- 10.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le AX, Miclau T, Hu D, Helms JA. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19:78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 13.Choi P, Ogilvie C, Thompson Z, Miclau T, Helms JA. Cellular and molecular characterization of a murine non-union model. J Orthop Res. 2004;22 :1100–1107. doi: 10.1016/j.orthres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Braren R, Hu H, Kim YH, Beggs HE, Reichardt LF, Wang R. Endothelial Fak is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172:151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu C, Huang S, Miclau T, Helms JA, Colnot C. MEPE is expressed during skeletal development and regeneration. Histochem Cell Biol. 2004;121:493–499. doi: 10.1007/s00418-004-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between LoxP sites. J Mol Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg N, Hamilton D, Hoess R. Bacteriophage P1 site-specific recombination. II. Recombination between LoxP and the bacterial chromosome. J Mol Biol. 1981;150:487–507. doi: 10.1016/0022-2836(81)90376-4. [DOI] [PubMed] [Google Scholar]
- 19.Fonta C, Imbert M. Vascularization in the primate visual cortex during development. Cereb Cortex. 2002;12:199–211. doi: 10.1093/cercor/12.2.199. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi K, Ohshima N. Angiogenic vascular growth in the rat peritoneal disseminated tumor model. Microvasc Res. 1996;51:15–28. doi: 10.1006/mvre.1996.0003. [DOI] [PubMed] [Google Scholar]
- 21.Dunlop SA, Moore SR, Beazley LD. Changing patterns of vasculature in the developing amphibian retina. J Exp Biol. 1997;200:2479–2492. doi: 10.1242/jeb.200.18.2479. [DOI] [PubMed] [Google Scholar]
- 22.Birchler MT, Milisavlijevic D, Pfaltz M, Neri D, Odermatt B, Schmid S, Stoeckli SJ. Expression of the extra domain B of fibronectin, a marker of angiogenesis, in head and neck tumors. Laryngoscope. 2003;113:1231–1237. doi: 10.1097/00005537-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Tsui P, Rubenstein M, Guinan P. Correlation between PSMA and VEGF expression as markers for LNCAP tumor angiogenesis. J Biomed Biotechnol. 2005;2005:287–290. doi: 10.1155/JBB.2005.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, Knudsen B, Bander NH. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 25.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-Specific Membrane Antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 26.Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-Prostate- Specific Membrane Antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 27.Chang SS, Reuter VE, Heston WD, Gaudin PB. Metastatic renal cell carcinoma neovasculature expresses Prostate-Specific Membrane Antigen. Urology. 2001;57:801–805. doi: 10.1016/s0090-4295(00)01094-3. [DOI] [PubMed] [Google Scholar]
- 28.May C, Doody JF, Abdullah R, Balderes P, Xu X, Chen CP, Zhu Z, Shapiro L, Kussie P, Hicklin DJ, Liao F, Bohlen P. Identification of a transiently exposed Ve-Cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood. 2005;105:4337–4344. doi: 10.1182/blood-2005-01-0010. [DOI] [PubMed] [Google Scholar]