Abstract
Giant cell tumor is an aggressive benign neoplasm of bone. A number of adjuvant agents have been used to supplement intralesional curettage to reduce the otherwise high local recurrence rate. High concentration ethanol is more readily available and less toxic to use than some common alternatives. No report on its use in a group of patients with giant cell tumor is available. Records were retrospectively reviewed for all giant cell tumors treated by intralesional curettage and high concentration ethanol irrigation as the only chemical adjuvant. Twenty-five primary excisional curettages and 12 repeat curettages for giant cell tumors of bone were performed in 31 patients. Patients were followed for a mean of three years and 10 months. There were five recurrences after primary excision procedures, and three after repeat excisions. Only use of a high-speed burr and lower Campanacci staging correlated with reduced recurrence rate, and these were not statistically significant. Most defects were filled with allograft or calcium sulfate. In the 11 patients treated primarily with curettage using a high-speed burr and adjuvant ethanol with minimum two-year follow-up, only one stage 3 lesion in a distal radius recurred. Multiple washes with high concentration ethanol, when used in conjunction with aggressive curettage including high-speed burring, is an effective and safe adjuvant. The necessity of any chemical adjuvant after appropriately aggressive curettage and burring can only be definitively demonstrated with a prospective, randomized, multi-center trial. Until such evidence becomes available, the use of adjuvant ethanol offers a compromise between higher toxicity adjuvants and no chemical adjuvant at all.
INTRODUCTION
Jaffe and colleagues offered the first thorough characterization of giant cell tumor (GCT) of bone in 1940.1 Since then, large series of bone tumors have found GCTs to represent approximately 20 percent of all benign bone tumors and five percent of all osseous neoplasms.2
Giant cell tumor is a locally aggressive but usually benign neoplastic disease of bone. In the appendicular skeleton, it typically arises eccentrically in the metaphysis, but usually extends into the epiphysis, often involving the subchondral bone.
Because of its periarticular location, resection for wide oncologic margins would require complex joint reconstructions and incur significant morbidity with regard to joint function in the long term. Intralesional curettage through a broad cortical window therefore remains the treatment of choice for most GCTs of bone in most treatment centers.
Early reports of curettage alone noted high rates of local recurrence.3–7 This prompted the use of a variety of local adjuvants, most commonly including phenol and liquid nitrogen cryotherapy. Concomitant to the use of these adjuvants are complexities and complications that some surgeons find undesirable.
For the last few years, three to four 60-second washes with 95 percent ethanol have been used at the University of Iowa as local adjuvant treatment after aggressive curettage of giant cell tumors in the appendicular skeleton. We retrospectively review this experience.
METHODS
With the permission of the Institutional Review Board, electronic pathology records were searched to identify all giant cell tumors of bone treated at the University of Iowa Hospitals and Clinics over the last 20 years. Extant medical records were reviewed. Patients were excluded if the tumor was located in the axial skeleton or if adjuvant ethanol was not used during intralesional curettage. For the included patients, basic demographic data were recorded in addition to lesion location, Campanacci staging,8 use of a high-speed burr, defect-filling material selected, perioperative complications, and details of longer- term follow-up such as recurrence and metastasis. Patients were not excluded for follow-up of less than two years, so as not to bias the study group.
With recurrence as the primary outcome, survival curves were independently generated for both primary excisions and recurrence excisions. Fisher's exact test was used to test categorical variables such as use of a high-speed burr, defect-filling agent used, and Campanacci staging for their relationships with rate of recurrence.
RESULTS
The electronic pathology records identified 87 tissue reports containing "giant cell" and "bone" since 1985. Of these, 26 were other bone lesions containing giant cells, such as aneurysmal bone cysts and giant-cell rich osteosarcomas. Sixty-one records showed giant cell tumors of bone, prompting review of additional medical records. With additional medical record information, two were incisional biopsies, one was a lung wedge resection of a benign metastasis from a GCT of bone, 12 were GCTs of the axial skeleton, six were GCT resection specimens from the appendicular skeleton, and 40 were excisional curettage specimens from appendicular skeleton GCTs. The six resections had been performed for three highly aggressive GCTs with widely displaced intra-articular fractures, two typical GCTs in expendable bones, and one highly aggressive, multiply recurrent GCT of the proximal tibia. Of the 40 excisional curettages, three did not use ethanol as an adjuvant.
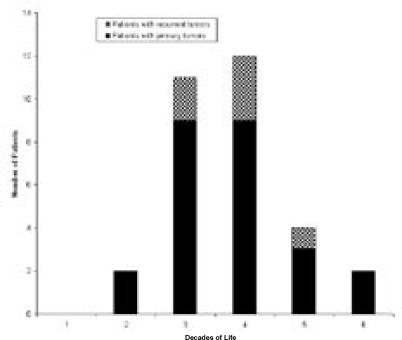
The final study group included 37 excisional curettages in 31 patients. Twenty-five patients presented primarily and six presented with a recurrent GCT after previous curettage by another surgeon. Among the patients receiving primary excisional curettage, 16 were female and nine male. The average age at surgery was 31.6 years (range 19 to 58 years) (Figure 1). Among patients presenting with recurrent lesions, four were male and two female, with an average age of 32 years, (range 27 to 42 years). Patients were followed for a mean of three years and ten months.
Figure 1.
Distribution of ages of presentation among patients with giant cell tumor of bone treated by intralesional excisional curettage, adjuvant ethanol irrigation, and defect filling.
One of the GCTs in the primary group was Campanacci stage 1, 11 were stage 2, and 10 were stage 3. Three others had associated fractures with significant displacement. For 12 of the primary excisions, a high-speed burr was used after curettage prior to ethanol irrigation. For 13 primary excisions, no burr was used. All defects were filled after lesion ablation, one with autograft, nine with allograft, nine with calcium sulfate putty or pellets, three with a mixture of allograft and calcium sulfate, and three with polymethylmethacrylate cement.
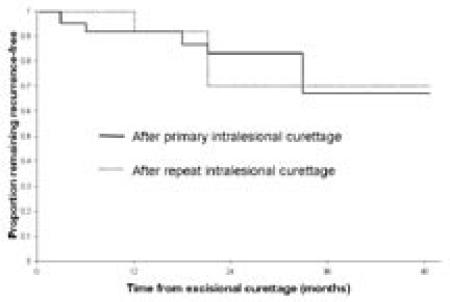
Following primary excisional curettage, five GCTs recurred (Figures 2 and 3). One tumor recurred after use of a high-speed burr and acrylic cement in addition to adjuvant ethanol. The other four recurrences followed curettage with ethanol irrigation but without the aid of a high-speed burr. No wound problems or post-operative fractures were noted following primary excisions. Two patients without recurrence had further surgery, one to replace acrylic cement with allograft and another to fill with acrylic cement an area where allograft had poorly incorporated.
Figure 2. Time to Recurrence of GCT After Curettage.
Kaplan-Meier plot of time to recurrence following primary intralesional excisional curettage with adjuvant ethanol for giant cell tumor of bone, and following repeat intralesional excisional curettage with adjuvant ethanol for recurrent giant cell tumor of bone, given a variable length of follow-up.
Figure 3.
Treatment course and outcome at latest follow-up of patients with giant cell tumor of bone treated primarily with intralesional excisional curettage and adjuvant ethanol irrigation.
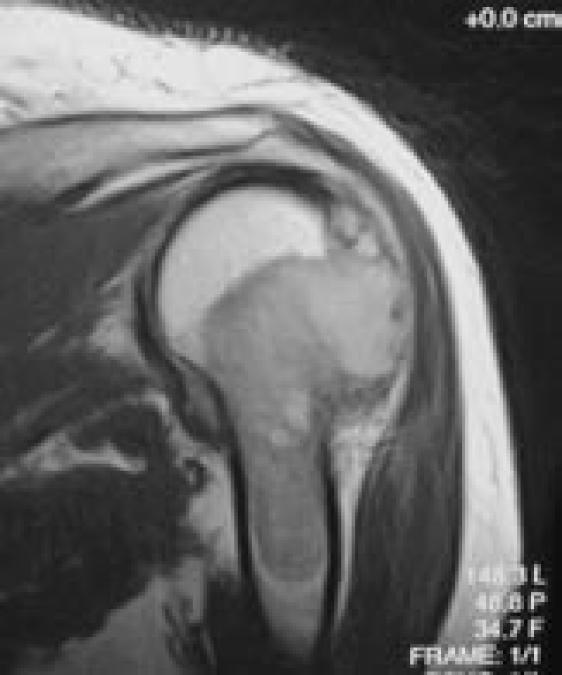
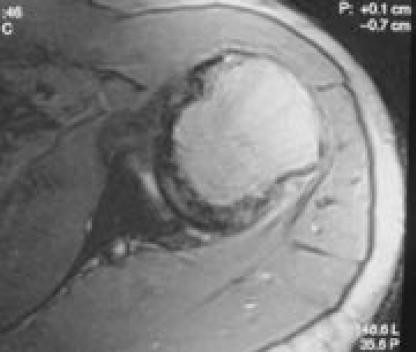
Soft-tissue involvement was noted on most of the 12 repeat excisional curettages for recurrence. A high-speed burr was used during nine of these repeat excisions, with the other three utilizing curettage alone prior to adjuvant ethanol. Defects were filled with allograft in two cases, calcium sulfate in two cases, a mix of allograft and calcium sulfate in four cases, and polymethylmethacrylate in four cases. One of the patients had moderate atypia noted histologically in his recurrent tumor. He was treated with adjuvant external beam irradiation after a brief delay for early graft incorporation (Figure 5).
Figure 5.
Magnetic resonance images (A and B) demonstrating the presentation of a Campanacci stage 3 giant cell tumor of the proximal humerus in a 32-year-old male. Four months after curettage, ethanol irrigation, and grafting, this lesion recurred. Histopathology from excisional curettage of the recurrence was atypical, prompting postoperative external-beam radiation therapy.
Figure 5A.
Figure 5B.
Three recurrences followed these 12 repeat excisional curettages (Figures 2, 3, and 4), two in a single patient (Figure 6). The other re-recurrence was also associated with benign pulmonary metastases. These metastases were wedge-resected and the recurrent bone lesion was widely resected prior to endoprosthetic reconstruction.
Figure 4.
Treatment course and outcome at latest follow-up of patients with recurrent giant cell tumor of bone treated with repeat intralesional excisional curettage and adjuvant ethanol irrigation.
Figure 6.
Images representing the clinical course of a 22-year-old male who presented with this Campanacci stage 3 giant cell tumor of bone (A and B). After curettage, ethanol irrigation, and grafting, it recurred (C). After repeat curettage, high-speed burring, ethanol irrigation, and grafting, it recurred two more times (D and E, respectively). Plain radiographs obtained 15 months after a fourth intralesional excisional curettage with high-speed burring, ethanol irrigation, and calcium sulfate grafting show no recurrence (F and G).
Figure 6A.
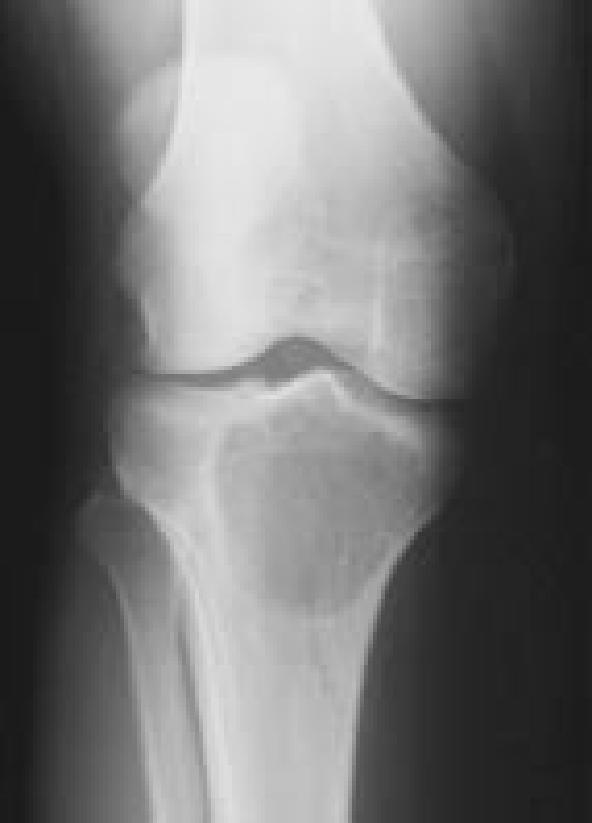
Figure 6B.
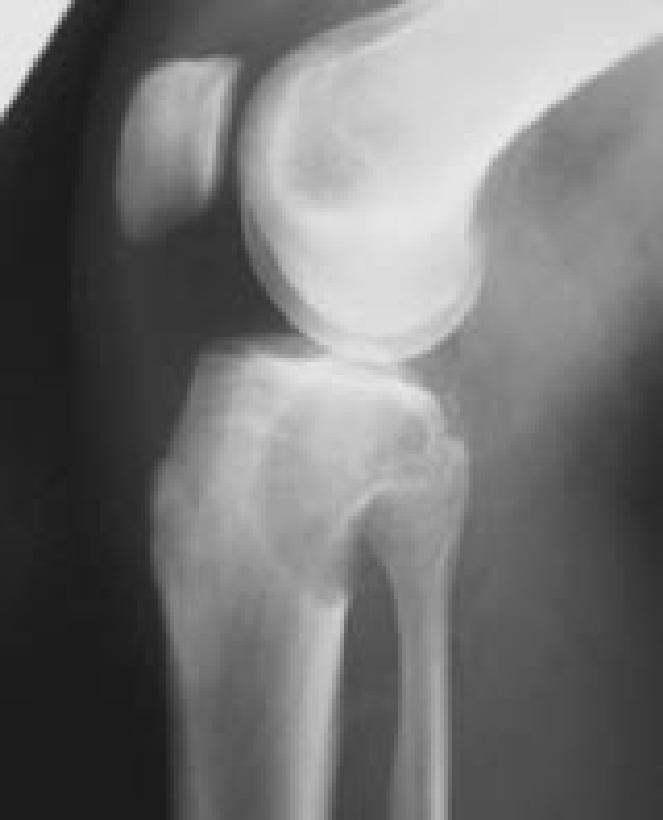
Figure 6C.
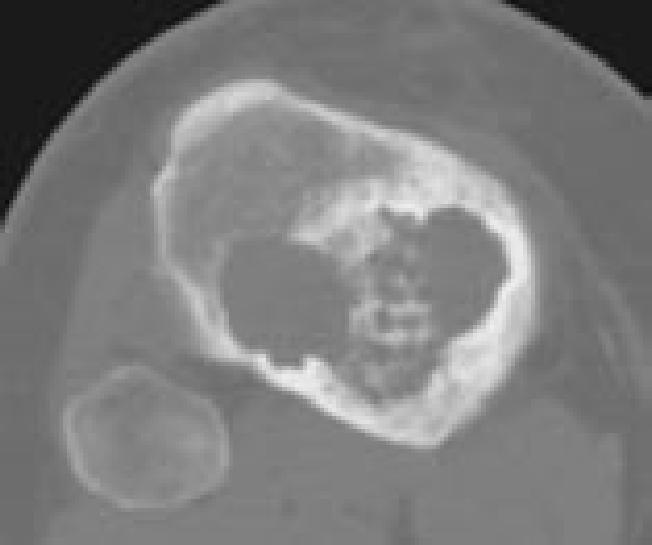
Figure 6D.
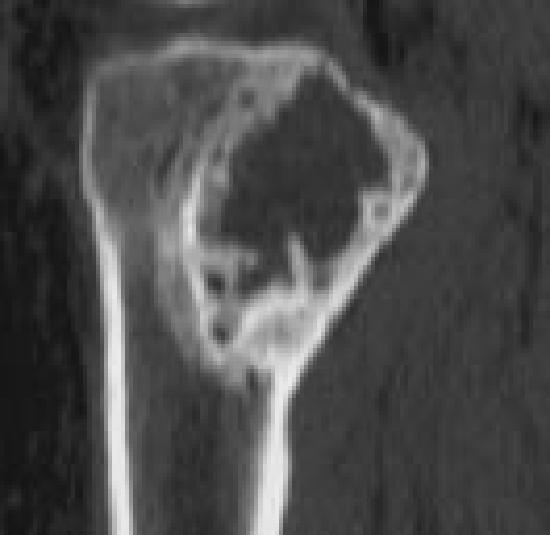
Figure 6E.
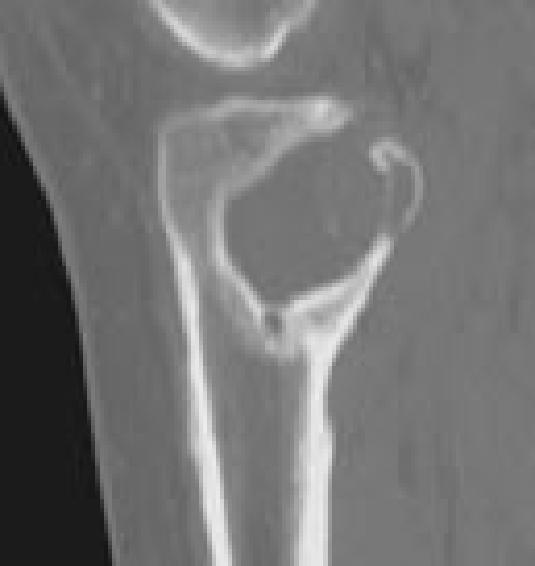
Figure 6F.
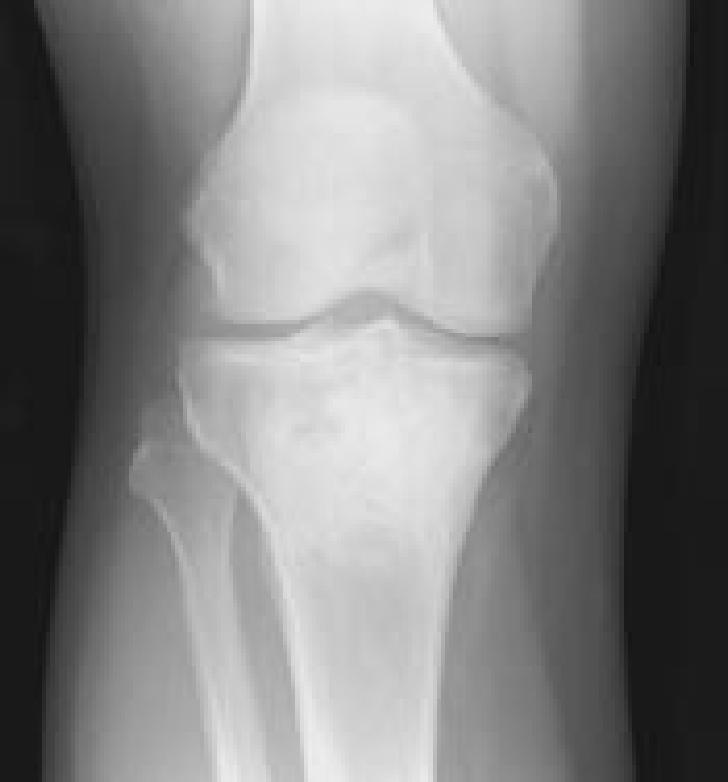
Figure 6G.
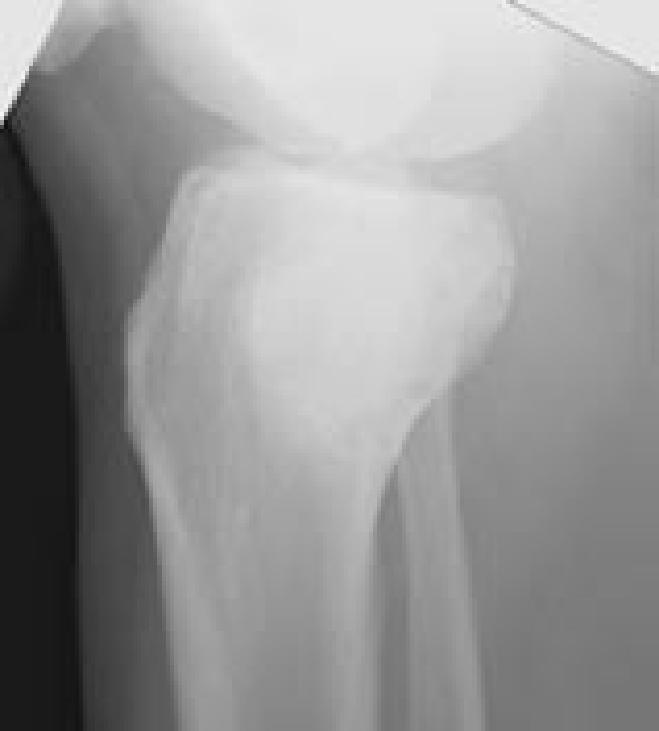
Two patients without recurrences had noteworthy complications after repeat excisional curettage. One patient sustained an intra-articular fracture around the cemented defect, which was treated conservatively, but led to significant osteoarthritis 12 years later. Another patient had persistent wound drainage, which was treated with graft removal, antibiotics, and delayed re-grafting. Whether this represented a low-grade infection or the wound drainage occasionally associated with calcium sulfate filling of non-contained bone defects9 was never concluded, but all cultures were negative.
Fisher's exact test noted statistically insignificant trends toward use of a high-speed burr associating with lower recurrence rate (p = 0.16), and higher Campanacci stage associating with a higher recurrence rate (p = 0.32) for primary excisions. Defect filling material did not appreciably correlate with recurrence rate.
DISCUSSION
Early reports of intralesional curettage for GCTs of bone noted recurrence rates ranging greater than 50 percent.4–7 As recurrence can make joint-preserving strategies much more difficult, such frequent recurrence is to be avoided if possible.
A number of different techniques (Table 1) and chemical agents have been used as adjuvants to intralesional curettage of benign aggressive bone tumors such as GCT. These have included the use of a high speed burr, painting or irrigating with phenol,3,6,10–16 cryotherapy with liquid nitrogen,17–19 irrigation with hydrogen peroxide,20 irrigation with aqueous zinc chloride,21 thermal cautery with a carbon dioxide laser,22 defect filling with poly-methylmethacrylate (for its heating properties),3,11,12,23–26 and the use of defect-filling agents that elute methotrexate27 or adriamycin.28
TABLE 1. Recurrence rates reported after curettage of giant cell tumors of bone.
| Author | Year | Tumor Characteristics | Adjuvant Treatments | Number of Patients | Recurrence Rate |
|---|---|---|---|---|---|
| Capanna et al.3 | 1990 | none | 280 | 45% | |
| Shih et al.33 | 1998 | none* | 22 | 0% | |
| Richardson et al.32 | 1998 | none | 16 | 0% | |
| Blackley et al.29 | 1999 | none | 59 | 12% | |
| Durr et al.10 | 1999 | none | 7 | 43% | |
| Saglik et al.41 | 1999 | none | 21 | 33% | |
| Trieb et al.16 | 2001 | none | 14 | 21% | |
| Turcotte et al.40 | 2002 | none | ~50 | 17% | |
| Khan et al.30 | 2004 | distal radius only | none | 23 | 17% |
| Prosser et al.31 | 2005 | stage 1 &2 | none | 61 | 7% |
| stage 3 | none | 52 | 29% | ||
| recurrent | none | 29 | 34% | ||
| Capanna et al.3 | 1990 | PMMA | 187 | 19% | |
| O'Donnell et al.12 | 1994 | PMMA | 49 | 24% | |
| Bini et al.23 | 1995 | PMMA | 38 | 8% | |
| Saglik et al.41 | 1999 | PMMA | 6 | 0% | |
| Wada et al.42 | 2002 | PMMA | 15 | 7% | |
| Turcotte et al.40 | 2002 | PMMA | 62 | 19% | |
| McDonald et al.5 | 1986 | phenol | 80 | 34% | |
| Capanna et al.3 | 1990 | phenol | 147 | 19% | |
| Durr et al.10 | 1999 | phenol | 11 | 9% | |
| Trieb et al.16 | 2001 | phenol | 12 | 25% | |
| Turcotte et al.40 | 2002 | phenol | 37 | 19% | |
| Su et al.15 | 2004 | phenol | 56 | 18% | |
| Capanna et al.3 | 1990 | cryotherapy | 20 | 19% | |
| Sheth et al.19 | 1995 | distal radius only | cryotherapy | 12 | 25% |
| Malawer et al.17 | 1999 | primary | cryotherapy | 86 | 2.3% |
| recurrent | cryotherapy | 16 | 37.5% | ||
| Turcotte et al.40 | 2002 | cryotherapy | 10 | 0% | |
| Zhen et al.21 | 2004 | Zinc Chloride | 92 | 13% | |
| Capanna et al.3 | 1990 | phenol+PMMA | 33 | 3% | |
| Ghert et al.43 | 2002 | phenol+PMMA | 47 | 13% | |
| Lackman et al.11 | 2005 | stage 2 & 3 only | phenol+PMMA | 63 | 6% |
| O'Donnell et al.12 | 1994 | phenol+PMMA | 11 | 27% | |
| Saiz et al. 14 | 2004 | phenol+PMMA | 40 | 13% | |
| Ward and Li20 | 2002 | H2O2+phenol+ electrocautery+ PMMA (in half) |
24 | 8% |
PMMA = polymethylmethacrylate cement.
"none" may include the use of a high speed burr, which some authors consider an adjuvant.
Most surgeons agree that aggressive curettage through a sufficiently wide cortical window for visibility is of paramount importance. Typically, a high-speed burr is used to extend the intralesional margins after removal of the gross tumor. Some authors argue that these more aggressive excision techniques are sufficient to achieve an acceptably low frequency of recurrence, ranging from 0 to 19 percent.29–33 These authors argue that benefits attributed to chemical adjuvants may stem from their association with more recent curettage and burr techniques.
Of chemical techniques, adjuvant phenolization and cryotherapy have surfaced as the most popular. Phenol, which has been shown to be cytotoxic to GCT cells in vitro,34 has been associated with favorable results ranging from six to 18 percent recurrence rates in recent series.10,11,14,15 While some data exist to confirm low systemic toxicity from the use of phenol as a local adjuvant,35 it is a caustic substance and must be handled carefully with respect to the patient's adjacent tissues and operating suite personnel. Cryotherapy with liquid nitrogen also results in reportedly low recurrence rates, but has associated risks of fracture and skin necrosis.17,18
We are unaware of any previous reports of the use of ethanol irrigation as an adjuvant to intralesional curettage for GCT of bone. High concentration ethanol is readily available in most surgical suites and relatively safe to use. The cytotoxicity from ethanol does not likely extend deeply into surrounding bone, but its adverse effects on adjacent tissues are also minimal.
Overall, the recurrence rate after the use of adjuvant ethanol is not widely different from the use of other adjuvants for GCT of bone. This series does reiterate the argument for the use of a high-speed burr, regardless of the chemical adjuvant selected. While numbers were too small to reach statistical significance, of the 12 primary intralesional curettages that utilized a high-speed burr and adjuvant ethanol, only one led to lesion recurrence. Only one of the 12 patients was followed for less than two years.
A number of factors must be considered in comparing different series of GCT patients for rates of recurrence. While histologic grading (other than malignancy) is not predictive of recurrence in GCT of bone,36 Campanacci staging is considered to be important, as stage 3 lesions, or those that have breached the cortex and involved the adjacent soft tissues, have a higher recurrence rate in series that distinguish them from lower stage lesions.31 Unfortunately, not all series distinguish them. Many others have skewed numbers due to the institutional practice of treating most stage 3 GCTs with wide resection rather than intralesional curettage. Our series had more recurrences after stage 3 primary lesions (three of 10) than after stage 2 lesions (two of 11), but the difference did not reach statistical significance.
Others have noted preoperative fractures as a major risk for recurrence.37 Three patients in the study group had preoperative fractures with significant displacement but none had a recurrence of their tumor.
Location can also play a role in prognosis, with the distal radius being a location notorious for more rampant soft tissue involvement and frequent recurrence.12,19,30,38,39 Only one of the four distal radius GCTs in this series recurred. However, notably, it was the only recurrence after use of a high-speed burr and adjuvant ethanol.
Treatment of recurrent lesions with repeat intralesional curettage is debated by some practitioners who believe that GCT recurrence merits wide excision. Rates of re-recurrence after repeat intralesional curettage range between 30 and 40 percent among the varied techniques reported.5,17,19,31,40 The three re-recurrences of 12 repeat intralesional curettages represent a respectable local control rate with the use of ethanol as an adjuvant. The contribution of the use of acrylic cement as the filling material more frequently in these repeat surgeries is difficult to isolate given the small numbers.
In conclusion, we feel that high concentration ethanol is an effective and safe adjuvant for the treatment of GCT when used in conjunction with aggressive curettage including high-speed burring. Whether any chemical adjuvant is necessary after performance of an appropriately aggressive curettage can probably only be answered definitively with a prospective, randomized comparison including many centers. Until such evidence becomes available, we feel that the use of ethanol is a safe compromise between higher-toxicity adjuvants and no adjuvant at all.
References
- 1.Jaffe HL, Lichtenstein L, Portis RB. Giant cell tumor of bone: its pathologic appearance, grading, supposed variants, and treatment. Arch Pathol. 1940;30:993–1031. [Google Scholar]
- 2.Dahlin DC. Caldwell Lecture. Giant cell tumor of bone: highlights of 407 cases. Am J Roentgenol. 1985;144(5):955–960. doi: 10.2214/ajr.144.5.955. [DOI] [PubMed] [Google Scholar]
- 3.Capanna R, Fabbri N, Bettelli G. Curettage of giant cell tumor of bone. The effect of surgical technique and adjuvants on local recurrence rate. Chir Organi Mov. 1990;75(1 Suppl):206. [PubMed] [Google Scholar]
- 4.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52(4):619–664. [PubMed] [Google Scholar]
- 5.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235–242. [PubMed] [Google Scholar]
- 6.Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long-term follow-up. Jpn J Clin Oncol. 1998;28(5):323–328. doi: 10.1093/jjco/28.5.323. [DOI] [PubMed] [Google Scholar]
- 7.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64(5):755–761. [PubMed] [Google Scholar]
- 8.Campanacci M, Capanna R, Fabbri N, Bettelli G. Curettage of giant cell tumor of bone. Reconstruction with subchondral grafts and cement. Chir Organi Mov. 1990;75(1 Suppl):212–213. [PubMed] [Google Scholar]
- 9.Lee GH, Khoury JG, Bell JE, Buckwalter JA. Adverse reactions to OsteoSet bone graft substitute, the incidence in a consecutive series. Iowa Orthop J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Durr HR, Maier M, Jansson V, Baur A, Refior HJ. Phenol as an adjuvant for local control in the treatment of giant cell tumour of the bone. Eur J Surg Oncol. 1999;25(6):610–618. doi: 10.1053/ejso.1999.0716. [DOI] [PubMed] [Google Scholar]
- 11.Lackman RD, Hosalkar HS, Ogilvie CM, Torbert JT, Fox EJ. Intralesional curettage for grades II and III giant cell tumors of bone. Clin Orthop Relat Res. 2005;438:123–127. doi: 10.1097/01.blo.0000180051.27961.c3. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76(12):1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Picci P, Manfrini M, Zucchi V, Gherlinzoni F, Rock M, Bertoni F, Neff JR. Giant-cell tumor of bone in skeletally immature patients. J Bone Joint Surg Am. 1983;65(4):486–490. [PubMed] [Google Scholar]
- 14.Saiz P, Virkus W, Piasecki P, Templeton A, Shott S, Gitelis S. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop Relat Res. 2004. pp. 221–226. [DOI] [PubMed]
- 15.Su YP, Chen WM, Chen TH. Giant-cell tumors of bone: an analysis of 87 cases. Int Orthop. 2004;28(4):239–243. doi: 10.1007/s00264-004-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trieb K, Bitzan P, Lang S, Dominkus M, Kotz R. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur J Surg Oncol. 2001;27(2):200–202. doi: 10.1053/ejso.2000.1086. [DOI] [PubMed] [Google Scholar]
- 17.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin Orthop Relat Res. 1999. pp. 176–188. [DOI] [PubMed]
- 18.Marcove RC, Weis LD, Vaghaiwalla MR, Pearson R, Huvos AG. Cryosurgery in the treatment of giant cell tumors of bone. A report of 52 consecutive cases. Cancer. 1978;41(3):957–969. doi: 10.1002/1097-0142(197803)41:3<957::aid-cncr2820410325>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Sheth DS, Healey JH, Sobel M, Lane JM, Marcove RC. Giant cell tumor of the distal radius. J Hand Surg. (Am) 1995;20(3):432–440. doi: 10.1016/S0363-5023(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 20.Ward WG, Sr, Li G., III Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002. pp. 259–270. [DOI] [PubMed]
- 21.Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br. 2004;86(2):212–216. doi: 10.1302/0301-620x.86b2.14362. [DOI] [PubMed] [Google Scholar]
- 22.Kenan S, Kirby EJ, Buchalter J, Lewis MM. The potential role of the laser in marginal sterilization of giant cell tumor following curettage. Bull Hosp Jt Dis Orthop Inst. 1988;48(1):93–101. [PubMed] [Google Scholar]
- 23.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop Relat Res. 1995. pp. 245–250. [PubMed]
- 24.Liu HS, Wang JW. Treatment of giant cell tumor of bone: a comparison of local curettage and wide resection. Changgeng Yi Xue Za Zhi. 1998;21(1):37–43. [PubMed] [Google Scholar]
- 25.Persson BM, Ekelund L, Lovdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand. 1984;55(2):209–214. doi: 10.3109/17453678408992339. [DOI] [PubMed] [Google Scholar]
- 26.Rock M. Curettage of giant cell tumor of bone. Factors influencing local recurrences and metastasis. Chir Organi Mov. 1990;75(1 Suppl):204–205. [PubMed] [Google Scholar]
- 27.Kirchen ME, Menendez LR, Lee JH, Marshall GJ. Methotrexate eluted from bone cement: effect on giant cell tumor of bone in vitro. Clin Orthop Relat Res. 1996. pp. 294–303. [PubMed]
- 28.Zhang Y, Hou C, Chen A. [A preliminary clinical observation of giant cell tumor of bone treated by adriamycin-loaded chitosan drug delivery system]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1998;12(5):280–282. [PubMed] [Google Scholar]
- 29.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81(6):811–820. doi: 10.2106/00004623-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Khan MT, Gray JM, Carter SR, Grimer RJ, Tillman RM. Management of the giant-cell tumours of the distal radius. Ann R Coll Surg Engl. 2004;86(1):18–24. doi: 10.1308/003588404772614632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005. pp. 211–218. [DOI] [PubMed]
- 32.Richardson MJ, Dickinson IC. Giant cell tumor of bone. Bull Hosp Jt Dis. 1998;57(1):6–10. [PubMed] [Google Scholar]
- 33.Shih HN, Hsu RW, Sim FH. Excision curettage and allografting of giant cell tumor. World J Surg. 1998;22(5):432–437. doi: 10.1007/s002689900411. [DOI] [PubMed] [Google Scholar]
- 34.Quint U, Vanhofer U, Harstrick A, Muller RT. Cytotoxicity of phenol to musculoskeletal tumours. J Bone Joint Surg Br. 1996;78(6):984–985. doi: 10.1302/0301-620x78b6.1281. [DOI] [PubMed] [Google Scholar]
- 35.Quint U, Muller RT, Muller G. Characteristics of phenol. Instillation in intralesional tumor excision of chondroblastoma, osteoclastoma and enchondroma. Arch Orthop Trauma Surg. 1998;117(1-2):43–46. doi: 10.1007/s004020050188. [DOI] [PubMed] [Google Scholar]
- 36.Sanerkin NG. Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer. 1980;46(7):1641–1649. doi: 10.1002/1097-0142(19801001)46:7<1641::aid-cncr2820460725>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Dreinhofer KE, Rydholm A, Bauer HC, Kreicbergs A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. J Bone Joint Surg Br. 1995;77(2):189–193. [PubMed] [Google Scholar]
- 38.Cheng CY, Shih HN, Hsu KY, Hsu RW. Treatment of giant cell tumor of the distal radius. Clin Orthop Relat Res. 2001. pp. 221–228. [DOI] [PubMed]
- 39.Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg. (Am) 2004;29(2):188–193. doi: 10.1016/j.jhsa.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002. pp. 248–258. [DOI] [PubMed]
- 41.Saglik Y, Yildiz Y, Karakas A, Ogut H, Erekul S. Giant cell tumor of bone. Bull Hosp Jt Dis. 1999;58(2):98–104. [PubMed] [Google Scholar]
- 42.Wada T, Kaya M, Nagoya S, Kawaguchi S, Isu K, Yamashita T, Yamawaki S, Ishii S. Complications associated with bone cementing for the treatment of giant cell tumors of bone. J Orthop Sci. 2002;7(2):194–198. doi: 10.1007/s007760200033. [DOI] [PubMed] [Google Scholar]
- 43.Ghert MA, Rizzo M, Harrelson JM, Scully SP. Giant-cell tumor of the appendicular skeleton. Clin Orthop Relat Res. 2002. pp. 201–210. [DOI] [PubMed]