Abstract
Tctex-1, a light-chain component of the cytoplasmic dynein motor complex, can function independently of dynein to regulate multiple steps in neuronal development. However, how dynein-associated and dynein-free pools of Tctex-1 are maintained in the cell is not known. Tctex-1 was recently identified as a Gβγ-binding protein and shown to be identical to the receptor-independent activator of G protein signaling AGS2. We propose a novel role for the interaction of Gβγ with Tctex-1 in neurite outgrowth. Ectopic expression of either Tctex-1 or Gβγ promotes neurite outgrowth whereas interfering with their function inhibits neuritogenesis. Using embryonic mouse brain extracts, we demonstrate an endogenous Gβγ–Tctex-1 complex and show that Gβγ co-segregates with dynein-free fractions of Tctex-1. Furthermore, Gβ competes with the dynein intermediate chain for binding to Tctex-1, regulating assembly of Tctex-1 into the dynein motor complex. We propose that Tctex-1 is a novel effector of Gβγ, and that Gβγ–Tctex-1 complex plays a key role in the dynein-independent function of Tctex-1 in regulating neurite outgrowth in primary hippocampal neurons, most likely by modulating actin and microtubule dynamics.
Keywords: AGS, dynein, heterotrimeric G protein, neurite outgrowth
Introduction
Signaling pathways that lead to neurite outgrowth and the establishment of neuronal polarity remain poorly understood. However, dynamic rearrangements of microtubules and actin filaments at the tips of growing axons are observed during neurite sprouting and elongation, suggesting that molecules that coordinate microtubule and actin microfilament dynamics play key roles in neurite extension and neuronal polarity determination (Fukata et al, 2002; Baas and Buster, 2004). Cytoplasmic dynein light-chain component, Tctex-1, was demonstrated recently to play key roles in initial neurite sprouting, axonal specification and elongation of hippocampal neurons in culture (Chuang et al, 2001, 2005). Cytoplasmic dynein is a microtubule-based, minus-end-directed motor complex involved in various cellular activities, including retrograde trafficking in neurons, Golgi maintenance, breakdown of the nuclear envelope and mitosis (Hirokawa, 1998; Sakato and King, 2004). Dynein comprises two ∼530 kDa heavy chains (DHCs) with ATPase and motor activities, two or three 74-kDa intermediate chains (DICs), and a group of accessory polypeptides including light intermediate chains and light chains (DLCs) (Vallee et al, 2004). DICs link accessory proteins, including the DLCs and the dynactin complex, to the DHC (Waterman-Storer et al, 1995; King, 2000). Three distinct DLC families have been identified: Tctex-1 (DYNTL1; Pfister et al, 2005), LC8 and LC7/Roadblock (Vallee et al, 2004).
Several lines of evidence suggest that Tctex-1 might function independently from dynein. First, there is strong biochemical evidence that a dynein-free pool of Tctex-1 exists independently of the dynein complex-associated Tctex-1 (Tai et al, 1998). Secondly, Tctex-1 is abundantly expressed in postmitotic, young neurons and was demonstrated recently to play a key role in neuritogenesis in hippocampal neurons in culture (Chuang et al, 2001, 2005). Cultured hippocampal neurons develop multiple abnormally long neurites when Tctex-1 is overexpressed and fail to develop neurites when Tctex-1 is suppressed (Chuang et al, 2005). The function of Tctex-1 in neuritogenesis was demonstrated to be dynein independent, since a mutant of Tctex-1 (Tctex-1 T94E), which failed to bind to DIC and therefore could not get incorporated into the dynein complex, induced a similar phenotype as the wild-type Tctex-1 protein (Chuang et al, 2005). Finally, Tctex-1 interacts with proteins besides DIC, including rhodopsin (Tai et al, 1999), parathyroid hormone receptor (PTHR) (Sugai et al, 2003), poliovirus receptor CD155 (Ohka et al, 2004), Herpes virus capsid protein VP26 (Douglas et al, 2004), bone morphogenetic receptor type II (BMPR-II) (Machado et al, 2003), the voltage-dependent anion channel (VDAC) (Schwarzer et al, 2002), Fyn kinase (Kai et al, 1997; Mou et al, 1998) and Trk neurotrophin receptor (Yano et al, 2001; Yano and Chao, 2004). How dynein-associated and dynein-free pools of Tctex-1 are maintained in the cell and how the assembly of Tctex-1 into the dynein complex is regulated is not known.
Tctex-1 was independently identified as an activator of G protein signaling 2 (AGS2), a receptor-independent activator of heterotrimeric guanine-nucleotide-binding regulatory proteins (G proteins), in a functional yeast screen in yeast (Takesono et al, 1999). AGS molecules can generally be divided into three subgroups: those that directly activate Gα, those that modulate Gα–Gβγ interaction by binding to Gα, and those that modulate Gα–Gβγ interaction by binding to Gβγ (Lanier, 2004). These AGS molecules have since demonstrated novel, non-canonical roles for G protein subunits in cell development and differentiation. For example, silencing of AGS3, a receptor-independent activator of Gβγ signaling, resulted in defects in mitotic spindle orientation and cleavage plane determination of neural progenitors in the developing neocortex demonstrating a novel role for G proteins in regulating neuronal cell fate (Sanada and Tsai, 2005).
AGS2/Tctex-1 was reported to bind Gβγ, but the molecular mechanism and functional consequences of the putative Tctex-1–Gβγ interaction have remained unknown (Takesono et al, 1999). Here, we studied the role of Gβγ in regulating the dynein-independent function of Tctex-1 in neuritogenesis. An endogenous Gβγ–Tctex-1 complex can be isolated from embryonic brain lysates and Gβ overlaps with the ‘dynein-free' Tctex-1 in cell fractionation experiments. Subcellular distribution of Gβγ and Tctex-1 overlap in the cell bodies as well as the growth cones of nascent axons in stage 3 primary cultured hippocampal neurons. Both Tctex-1 and Gβγ overexpression elicit similar phenotypes in primary hippocampal neurons. Interfering with Gβγ function inhibits neuritogenesis and diminishes the ability of Tctex-1 to induce neurite outgrowth. All known Gβ isoforms contain an identical consensus Tctex-1-binding motif first described in DIC (Mok et al, 2001). We show that full-length Gβ1, as well as a Gβ-peptide corresponding to the Tctex-1-binding region of Gβ1, compete with DIC for Tctex-1 binding. We propose that Gβγ binds to Tctex-1 to regulate the dynein-independent pool of Tctex-1 and its incorporation into the dynein motor complex. Finally, we demonstrate that Gβγ–Tctex-1 complex plays a key role in neuritogenesis in an established model of hippocampal neuron differentiation.
Results
Characterization of Gβγ–Tctex-1 interaction
Tctex-1 was shown to interact specifically with Gβγ and not with the Gα subunit of heterotrimeric G proteins (Takesono et al, 1999). We confirmed the interaction of Gβγ with Tctex-1 using purified components. We generated GST-tagged Tctex-1 and purified visual G protein transducin, Gtαβγ. GST–Tctex-1 was incubated with Gtαβγ and the bound Gtβγ subunit was detected using an anti-Gβ antibody. As shown in Figure 1A, GST–Tctex-1 but not GST alone, specifically bound Gtβγ. We also studied the Gβγ–Tctex-1 interaction in a mammalian cell expression system. We cotransfected human embryonic kidney (HEK) cells with Tctex-1 and Gβ1 expression plasmids. Confirming the in vitro data, we showed that Tctex-1 could robustly co-immunoprecipitate (co-IP) with Gβγ (Figure 1B). Tctex-1 did not co-IP with overexpressed Gtα in HEK cells (data not shown). The Gβ subunit consists of six different isoforms, including Gβ1 through 5 and a splice variant of Gβ5 called Gβ5L (long form). Gβ1–4 are about 80% identical to each other, whereas Gβ5 is only about 50% identical to the others. As shown in Figure 1C, all FLAG-tagged Gβ subunits (Gβ1, 2, 3, 5 and 5L) co-immunoprecipitated with Tctex-1 using an anti-Tctex-1 antibody. The expression of Gγ1 or Gγ2 together with Gβ1 did not affect the ability of Tctex-1 to associate with Gβ1. Gγ1 and Gγ2 expression was confirmed by stripping and reprobing the membranes with anti-Gγ1 and anti-Gγ2 antibodies, respectively (data not shown). Even though both Gβ and Tctex-1 are expressed in HEK 293 cells, we failed to co-IP a native endogenous Gβγ–Tctex-1 complex from these cells. Since Tctex-1 is abundantly expressed in postmitotic young neurons and several Gβγ combinations are found in brain, we employed embryonic mouse brain lysates in pull-down assays (Chuang et al, 2001). As shown in Figure 1C, we were able to pull down Gβ along with Tctex-1, demonstrating a native endogenous Gβγ–Tctex-1 complex in mouse embryonic brain lysate.
Figure 1.
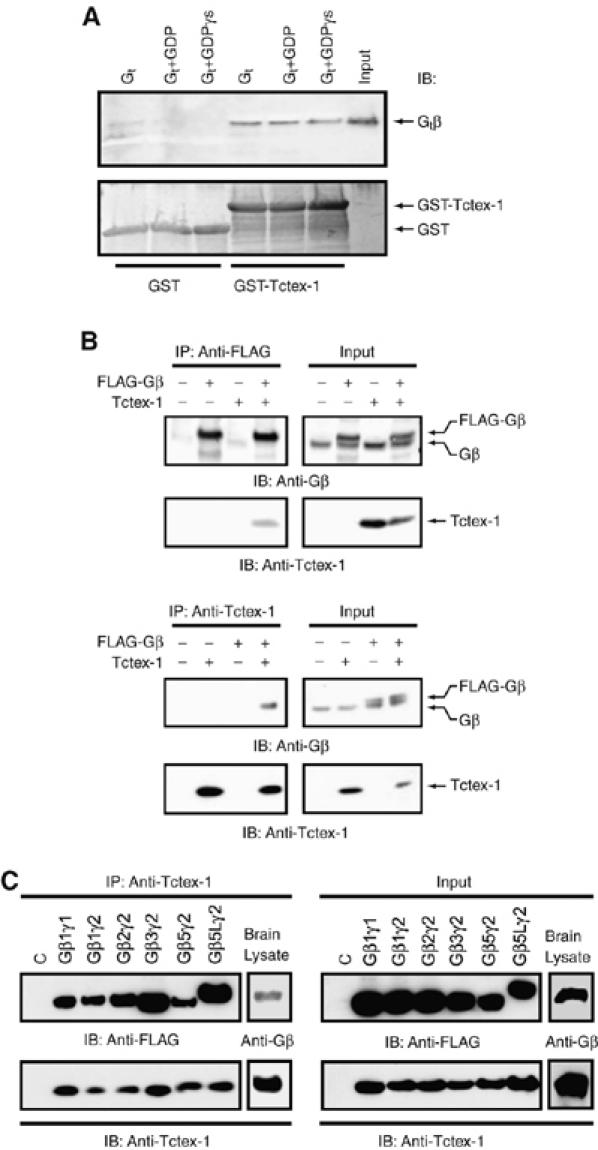
Gβγ interacts with Tctex-1. (A) Transducin Gαtβ1γ1 (Gt) interacts with GST-fused Tctex-1. GST alone (lanes 1–3) or GST-fused Tctex-1 (lanes 4–6) (300 nM) were incubated with Gt (40 nM) (lanes 1 and 4), plus 10 μM GDP (lanes 2 and 5) or plus 10 μM GTPγS and 5 mM MgCl2 (lanes 3 and 6). Purified Gt was loaded as a control in lane 7. The bound samples were analyzed by SDS–PAGE followed by immunoblotting (IB) for Gβ using anti-Gβ antibody (top panel). A Coomassie blue-stained gel in the bottom panel shows that equal amounts of the GST and GST–Tctex-1 were used. (B) Gβγ and Tctex-1 co-IP in HEK cells. HEK cells were transfected with FLAG-tagged Gβ1 and Tctex-1 cDNAs as indicated and cell lysates were immunoprecipitated (IP) with anti-Tctex-1 antibody (top panel) or with anti-FLAG antibody (bottom panel) and subjected to SDS–PAGE, followed by Western blot analysis (IB) using anti-Gβ and anti-Tctex-1 antibodies. A 20 μg weight of total cell lysate was analyzed as input (right half of each panel) to show expression levels of Gβ and Tctex-1. The doublet seen in the anti-Gβ blots correspond to the endogenous Gβ and the FLAG-tagged Gβ proteins. (C) Tctex-1 interacts with several overexpressed Gβ isoforms, and with endogenous Gβ in brain lysates. HEK293 cells were cotransfected with FLAG-tagged Gβ1, β2, β3, β5 or β5L, along with Gγ1 or Gγ2 and Tctex-1 expression vectors. Tctex-1 was IP with anti-Tctex-1 antibody and the immunocomplexes were analyzed by Western blotting using anti-Tctex-1 antibody to detect Tctex-1 and anti-FLAG antibody to detect the FLAG-tagged Gβ subunits that co-IP with Tctex-1. Endogenous Tctex-1 was immunoprecipitated from E15 embryonic mouse brain lysate using anti-Tctex-1 antibody and analyzed by Western blotting for Tctex-1 and the associated brain Gβ subunit using anti-Tctex-1 and anti-pan Gβ antibody, respectively. A 20 μg weight of total cell lysate was analyzed by as input to show protein expression levels of Tctex-1, FLAG-tagged Gβ and endogenous Gβ using anti-Tctex-1, anti-FLAG and anti-pan Gβ antibodies, respectively. All images are representative of three independent experiments.
To map the Gβγ-binding site on Tctex-1, we constructed N- and C-terminal truncation mutants of Tctex-1. FLAG-tagged full-length Tctex-1 and various truncation mutants of Tctex-1 were cotransfected with Gβ1 in HEK cells (Figure 2A). As shown in Figure 2B, full-length and the N-terminal truncated mutants of Tctex-1 were able to co-IP Gβγ, whereas the C-terminal truncation mutant of Tctex-1, 1–92, which is missing the last 21 amino acids of Tctex-1, failed to co-IP Gβγ (Figure 2B). Taken together, these results suggest that the C-terminal tail of Tctex-1 is required for the Gβγ–Tctex-1 interaction.
Figure 2.
Mapping of the Gβ-binding domain of Tctex-1. (A) Schematic representation of the N- and C-terminal truncation mutants of Tctex-1 used in the mapping of the Gβ-binding domain of Tctex-1. (B) C-terminal region of Tctex-1 is required for Gβ binding. HEK cells were cotransfected with the indicated truncation mutants of FLAG-tagged Tctex-1, along with Gβ cDNAs. Tctex-1 and its mutants were immunoprecipitated with anti-FLAG antibody and the immunocomplexes were analyzed by Western blotting with anti-Gβ antibody (top panels) and anti-FLAG antibody to detect FLAG-tagged Tctex-1 (bottom panels). The expression level of each protein was analyzed by direct Western using 20 μg of total cell lysate as input. This is a representative image of at least three independent experiments.
Consensus binding motif on Gβ is required for Tctex-1 binding
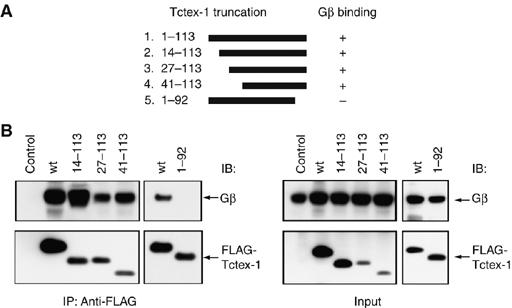
An 11-amino-acid peptide derived from DIC was used to identify residues on Tctex-1 that are involved in the DIC–Tctex-1 interaction (Mok et al, 2001). Comparison of this peptide sequence of DIC with sequences of other Tctex-1 target proteins helped to identify a motif of basic residues, R/K-R/K-X-X-R/K, as a consensus Tctex-1-binding motif (Mok et al, 2001). Additionally, a second Tctex-1-binding motif, V-S-K/H-T/S-X-V/T-T/S-N/Q-V, has also been identified in a subset of Tctex-1-interacting proteins (Sugai et al, 2003). We found a potential Tctex-1-binding motif in all six Gβ subunits, which maps to the outermost β-strand of the seventh blade of the Gβ propeller (Figure 3A and B). We generated a Gβ-peptide corresponding to amino acids 40–57 of Gβ1, which includes the basic Tctex-1-binding motif and surrounding residues either with no tag or with a thiol-specific, environmentally-sensitive, fluorescent compound, MIANS. We performed fluorescence anisotropy measurements to determine the binding affinity of the Gβ-peptide to Tctex-1. Keeping the MIANS-labeled Gβ-peptide constant and adding increasing concentrations of purified Tctex-1, we observed enhanced binding, which leveled off at high concentrations of Gβ-peptide (Figure 3C). The experimentally determined Kd for the Gβ-peptide–Tctex-1 interaction was 1.35 μM.
Figure 3.
Analysis of the Tctex-1-interacting motif on Gβ. (A) Structure of heterotrimer Gαβγ highlighting the Tctex-1-binding motif in Gβ. Gα and Gγ (gray) are shown as molecular surface representation, whereas Gβ (cyan) is shown as a secondary structure cartoon. Four β-strands that make up blade 7 of the Gβ propeller are highlighted (β-strands A–C in yellow and β-strand D in orange, left panel). Expanded region (right panel) shows the region of Gβ involved in the formation of blade 7. C-terminal residues 315–340 (yellow) form the A, B and C β-strands, whereas N-terminal residues 47–52 (orange) form the outermost D β-strand of this blade. Residues 47–52 comprise the consensus R/K-R/K-X-X-R/K Tctex-1-binding motif. Image created using VMD software (Humphrey et al, 1996). Crystal coordinates obtained from PDB file 1GOT (Lambright et al, 1996). (B) Consensus sequence R/K-R/K-X-X-R/K found in various Tctex-1-interacting proteins. (C) Fluorescence anisotropy of MIANS-labeled Gβ-peptide in the presence of Tctex-1. Tctex-1 was titrated to the MIANS Gβ-peptide, whose concentration was maintained at 0.7 μM. With increasing concentration of Tctex-1, there was an enhancement in the anisotropy value, indicative of specific binding of the peptide to the protein. In addition, the plot showed a sigmoidal curve, indicative of cooperative binding. Each data point is an average of five readings with standard error less than 5%. The Kd value for the Tctex-1-Gβ–peptide interaction from nonlinear regression analysis was calculated to be 1.35 μM. (D) Reduced interaction of Gβ AAA mutant with Tctex-1. HEK cells were cotransfected with Tctex-1 and the indicated FLAG-tagged Gβ mutants. The AAA Gβ1 mutant, wherein all three Arg residues (R48, R49 and R52) within the consensus Tctex-1-binding motif are mutated to alanine, showed significantly reduced ability to co-IP Tctex-1. The upper band observed in the top left panel of the anti-FLAG IP corresponds to the IgG heavy chain. This image is representative of three independent experiments.
We further dissected the binding motif to identify which particular residues within the binding motif are critical for the Gβ–Tctex-1 interaction. The cluster of basic residues within the Tctex-1-binding motif (R/K-R/K-X-X-R/K) prompted us to evaluate the role of charge-based regulation of the interaction. We generated site-directed mutants of the Tctex-1-binding motif in Gβ, where the Arg residues were replaced either individually or in combination, and tested their ability to interact with Tctex-1 in co-IP experiments. In accordance with our hypothesis, we found that mutation of all three basic residues (R48, R49 and R52) to alanines within the full-length Gβ protein (Gβ1 AAA) significantly decreased the ability of Gβγ to interact with Tctex-1 (Figure 3D).
Gβγ and Tctex-1 distribution overlaps in cultured hippocampal neurons
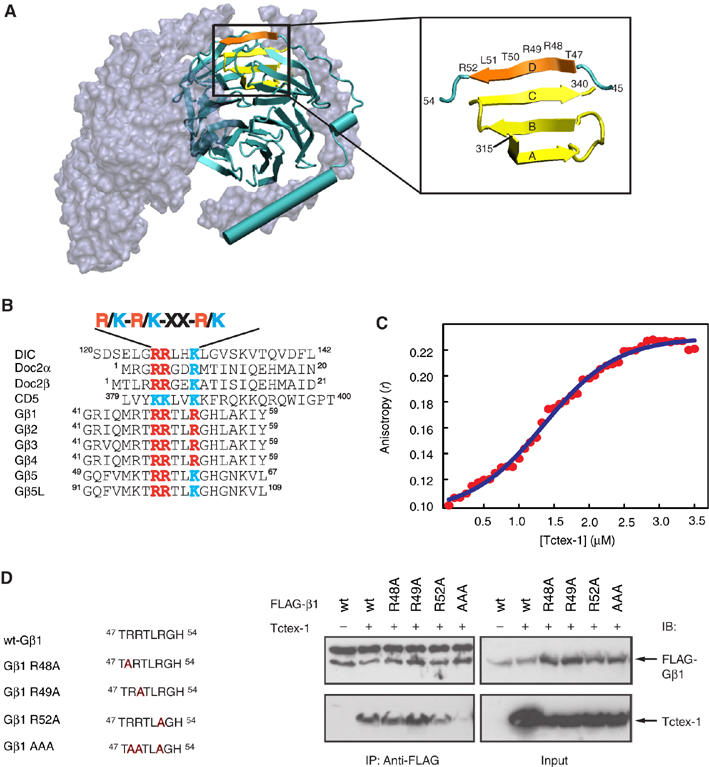
Tctex-1 was recently shown to regulate neurite outgrowth in primary hippocampal neurons (Chuang et al, 2005). We decided to investigate whether the endogenous Gβγ–Tctex-1 complex, isolated from mouse brain lysates, could play a role in Tctex-1-induced neuritogenesis. To this end, we first examined the expression pattern of Gβ in cultured primary hippocampal neurons. Primary hippocampal neurons adopt the characteristic polarized morphology in a well-defined sequence of developmental stages (Dotti et al, 1988; Fukata et al, 2002). Within 24 h of plating, hippocampal neurons send out several processes of relatively equal length (stage 2). Even though phenotypically the neurons still appear non-polarized, proteins known to be involved in cell polarization already start to display a polarized distribution pattern (Bradke and Dotti, 2000; Fukata et al, 2002; Banker, 2003; Schwamborn and Puschel, 2004). At this stage, Gβ displays diffuse labeling throughout the cell body and the minor processes (Figure 4A–C). As the neurons develop through stages 2–3 and reach stage 3, they adopt the final differentiated polarized neuronal phenotype with one long neurite (the nascent axon) and several minor processes. Approximately 40% of stage 3 neurons examined (n>50) displayed Gβ immunoreactivity in the central region of the axonal growth cones (Figure 4D–K), whereas the remaining neurons failed to show an enhanced labeling intensity of Gβ in the growth cones (Figure 4L–N).
Figure 4.
Expression pattern of Gβ in various stages of cultured primary hippocampal neuron differentiation. Gβ distributes diffusely throughout the cell body and the minor processes in stage 2 neurons (A–C). As the neurons develop through stages 2–3 and reach stage 3 and adopt the well-differentiated neuronal phenotype, Gβ shows two distinct labeling patterns. Approximately 40% of stage 3 neurons (n>50) showed Gβ labeling in the central region of the axonal growth cone (D–K). An example of a stage 3 neuron that failed to show any detectable enhancement of Gβ in the growth cones is represented (L–N). Neurons were colabeled for Gβ (green in A, D, H and L), Tyr-Tubulin (red in B, E, I and M) and actin (blue in G and K). Overlayed images are shown in C, F, J and N. Scale bars equal 10 μm in panels (A–C) and 20 μm in panels (D–N).
We then examined the expression patterns of Gβ and Tctex-1 in hippocampal neurons of various stages. Tctex-1 and Gβ are expressed essentially homogenously throughout the cell body and processes of stage 2 neurons (Figure 5Aa–c). As the neurons develop through stages 2–3, Gβ and Tctex-1 continue to overlap in the perinuclear Golgi region within the cell body. We also observed a strong colocalization of Tctex-1 and Gβ within the axonal growth cones of majority of stage 3 neurons (70%, n=100) (Figure 5Ad–i and 5B).
Figure 5.
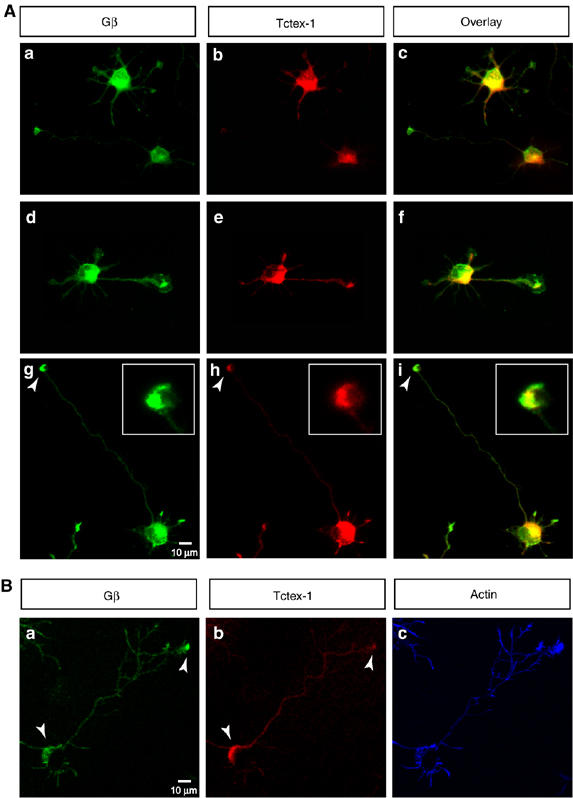
Overlapping distribution of Tctex-1 and Gβ in hippocampal neurons. (A) Cultured hippocampal neurons in various stages of differentiation were colabeled for Gβ (green in a, d and g) and Tctex-1 (red in b, e and h). The overlayed images are shown in c, f and i. Gβ and Tctex-1 show homogenous expression within the cell body and all the neurites in typical stage 2 cells (a–c). As the neurons progress through stage 3, Gβ and Tctex-1 continue to overlap within the cell body, but in addition, show strong co-distribution at the growth cones of the future axon (a–i). Majority of the stage 3 neurons examined (70%, n=100) (a–f) show enhanced colabeling of Gβ and Tctex-1 in axonal growth cones as compared with the minor neurites that do not show an enrichment of Gβ or Tctex-1 at the tips. A small subset of the stage 3 neurons examined (30%, n=100) continued to show colabeling of Gβ and Tctex-1 in the cell body and at the axonal growth cones, and also showed some colabeling at the tips of the minor neurites (g–i). A magnified view of the growth cone of a stage 3 neuron is shown in the inset (g–i) Greater than 100 individual neurons were examined. Scale bar equals 10 μm (a–i) and 3 μm in the magnified insets (g–i). (B) Confocal image of a representative stage 3 neuron shows perinuclear, cytoplasmic staining for Gβ and Tctex-1 within the cell body and an enrichment at the tips of some of the axonal growth cones (panels A–C). Scale bar equals 10 μm.
Role of Gβγ and Tctex-1 in neuronal differentiation
To study the role of Gβγ in hippocampal neuronal differentiation, we used a Gβγ-specific antagonist, βARKct, to perform loss-of-function experiments. βARKct contains the Gβγ-binding domain of β-adrenergic receptor kinase 1 and when expressed in intact cells, it inhibits Gβγ-dependent signaling by binding to and sequestering Gβγ (Koch et al, 1994). Hippocampal neurons transfected with EGFP expression vector alone were indistinguishable from non-transfected cells and developed normally through stage 2 and stage 3 (Figure 6A). Quantification of the different stages showed that a significant fraction of neurons transfected with EGFP alone were found in stage 2 (52%) and stage 3 (40%) (Table I). To study the effect of βARKct on neurite outgrowth, hippocampal neurons were transfected either 2 h after plating or 12 h after plating with expression vectors for βARKct together with EGFP (Figure 6B and Supplementary Figure 1). As seen in Figure 6B, neurons that were transfected with the EGFP plus βARKct 2 h after plating failed to develop neurites and seemed to be arrested in stage 1 (78%) (Figure 6B and Supplementary Figure 1). The neighboring, untransfected cells (observed within the same field) appear healthy and proceed normally through development reaching stage 3 (Figure 6B). As compared with EGFP alone or un-transfected cells, a significantly smaller fraction of βARKct-transfected cells reached stage 2 (17%) or stage 3 (4%) (Table I). Neurons transfected 12 h after plating seemed to be arrested in stage 2 (Figure 6B). This result is very similar to what was seen with loss of Tctex-1 function wherein most Tctex-1-suppressed neurons had segmented lamellipodia but neither typical neurites nor growth cones (Chuang et al, 2005).
Figure 6.
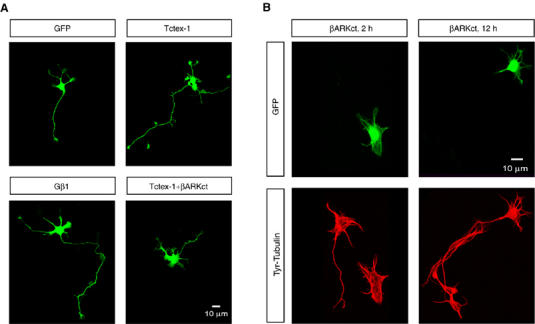
Role of Gβγ and Tctex-1 in neuronal differentiation. (A) Ectopic expression of Gβ1 and Tctex-1 induces multiple, long neurites in hippocampal neurons. Hippocampal neurons were transfected as indicated, with expression vectors for GFP, GFP+FLAG-Gβ1, GFP+FLAG-Tctex-1 and GFP+Tctex-1+βARKct 2 h after plating. The transfected neurons were fixed and processed for GFP fluorescence 24 h after transfection. (B) Expression of Gβγ-sequestering reagent, βARKct inhibits neurite outgrowth. Hippocampal neurons were transfected either 2 h or 12 h after plating, with expression vectors for GFP and βARKct and analyzed 24 h after transfection for GFP expression (green) and Tyr-Tubulin labeling (red). βARKct expression results in arresting the cells in stage 1 when transfected 2 h after plating and in stage 2 when transfected 12 h after plating. Note that, under both conditions, the untransfected cells within the same field appear healthy and have reached stage 3. The images shown here are representative of three independent transfections. Quantification of the images from these transfections is shown in Table I.
Table 1.
Quantitative analyses of morphological changes of transfected neurons
| Overexpressed protein | % cells (stage 1) | % cells (stage 2) | % cells with a single neurite (stage 3) | % cells with multiple neurites >70–80 μm |
|---|---|---|---|---|
| GFP alone | 8±4 | 52±8 | 40±6 | 0.6±01 |
| βARKct | 78±6* | 17±3* | 4±2* | ND |
| FLAG-Gβ1 | 2±1 | 36±9* | 42±14 | 22±6* |
| FLAG-Tctex1 | 2±1 | 24±8* | 28±6 | 38±8* |
| FLAG-Tctex1+βARKct | 10±4 | 38±4* | 40±12 | 12±6* |
| Cells were transfected at 2 h after plating and fixed 24 h later. Each transfection received 1 μg of GFP expressing vector for visualizing the transfected cells. For all other constructs, 2 μg of plasmid were typically used. The total amount of DNA added was kept constant by adding appropriate amount of control vector. A neurite longer than 70–80 μm was considered to be an axon in these analyses. Each value represents the mean±s.e.m. of at least 50–75 cells for each experimental condition. Asterisk represents value significantly different from that of the GFP-transfected group (P<0.01). | ||||
| ND, not detected. |
We then performed gain-of-function studies to examine the phenotype of neurons overexpressing Gβ1. Primary hippocampal neurons were transfected 2 h after plating with FLAG-Gβ1 and EGFP expression vectors. In contrast to cells expressing EGFP alone, a significantly greater number of FLAG-Gβ1-transfected neurons developed multiple long neurites (Figure 6A and Table I). Similar to the case of Gβ1 overexpression and as demonstrated previously (Chuang et al, 2005), Tctex-1 overexpression also elicited the same phenotype of multiple long neurites (Figure 6A and Table I), demonstrating that ectopic overexpression of either Gβ1 or Tctex-1 promotes neurite outgrowth. Coexpression of Tctex-1 along with the Gβγ-sequestering reagent βARKct seems to partially revert the phenotype induced by βARKct by 24 h after transfection (Figure 6A, Table I and Supplementary Figure 1).
Gβγ overlaps with the ‘dynein-free' pool of Tctex-1 and regulates assembly of Tctex-1 into the dynein motor complex
Previous studies demonstrated that Tctex-1-mediated neurite outgrowth proceeds via a dynein-independent mechanism (Chuang et al, 2005). However, how dynein-bound and dynein-free pools of Tctex-1 are regulated in neurons remains unclear. We are interested in testing whether the Gβ–Tctex-1 complex may modulate the partition of Tctex-1 into dynein-associated and dynein-free pools. Toward this end, we first examined whether Gβγ overlaps with the dynein-associated or dynein-independent pool of Tctex-1. Using velocity density gradient sedimentation of lysates generated from embryonic E15 mouse brains, we demonstrated that, as shown previously, in addition to co-sedimenting with the DIC-containing, 19–20S fractions, Tctex-1 can also be found in the dynein-free, lighter-density fractions (Figure 7A). Interestingly, we show that the majority of Gβ-containing fractions overlap with the lighter, non-DIC containing fraction of Tctex-1. Very little, if any, Gβ can be found in the DIC-containing, higher-density fractions (Figure 7A).
Figure 7.
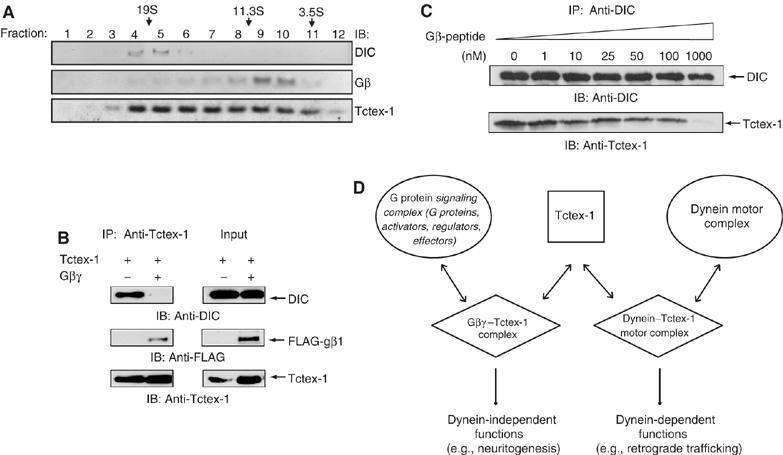
Gβ overlaps with dynein-free pool of Tctex-1 and competes with DIC for Tctex-1 binding. (A) Gβ co-sediments with the dynein-free lighter fractions of Tctex-1. Homogenates from E15 mouse brain were sedimented in a 5–20% linear sucrose gradient. Each fraction was analyzed by SDS–PAGE and immunoblotted with the indicated antibodies. (B) Coexpression of Gβγ along with Tctex-1 resulted in decreased amounts of DIC associated with Tctex-1. HEK cells were transfected with either Tctex-1 alone (lane 1) or cotransfected with Tctex-1 along with FLAG-Gβ1 (lane 2). Tctex-1 was immunoprecipitated with anti-Tctex-1 antibody and the immunocomplexes were analyzed with anti-DIC antibody (top), anti-FLAG antibody (middle) and anti-Tctex-1 antibodies (bottom). Total cell lysate (TCL) was subjected to direct Western analysis to show expression levels of DIC, FL-Gβ1 and Tctex-1 (right panel). (C) Competition of dynein–Tctex-1 interaction by Gβ-peptide. DIC was immunoprecipitated (IP) from lysates generated from HEK cells transfected with Tctex-1 cDNA. The DIC IP was titrated with increasing concentration of unlabeled Gβ-peptide (0–1000 nM) as indicated. The amino-acid sequence of the Gβ-peptide is provided in Materials and methods. As the concentration of the peptide increased, a decreasing amount of Tctex-1 was detected in the immune complex, as measured using an anti-Tctex-1 antibody. The filter was probed with anti-DIC antibody to demonstrate that equivalent amounts of DIC were immunoprecipitated. (D) Model of Gβγ-dependent regulation of dynein-free function of Tctex-1 in inducing neurite outgrowth. Tctex-1 binding to DIC is required for incorporation of Tctex-1 in the dynein motor complex. Tctex-1 has been shown to exist free of dynein in cells. The dynein motor function is dispensable for the ability of Tctex-1 to induce neuritogenesis in primary hippocampal neurons. In embryonic mouse brain lysates, Gβγ overlaps with the dynein-free fractions of Tctex-1. We propose that G protein βγ subunit, which contains the DIC-like Tctex-1-binding motif and competes with DIC for Tctex-1 binding, serves to regulate the dynein-free pool of Tctex-1. The Gβγ–Tctex-1 complex exists free of the dynein–Tctex-1 complex and regulates the dynein-independent function of Tctex-1.
Tctex-1 binding to DIC is required for its incorporation into the dynein motor complex. We observed that all known Gβ isoforms contain a DIC-like, consensus basic Tctex-1-binding motif (R/K-R/K-X-X-R/K) (Figure 3B) and (Mok et al, 2001). Since Gβ contains the same Tctex-1-binding motif as DIC, we investigated whether Tctex-1 can simultaneously bind to Gβ and DIC. We investigated the effect of overexpression of full-length FLAG-tagged Gβ1 on the ability of Tctex-1 to interact with DIC. Figure 7B shows that Gβ1 overexpression results in dramatically reduced Tctex-1 binding to endogenous DIC in HEK 293 cells. We further assessed the DIC–Tctex-1 interaction in the presence of a Gβ-peptide corresponding to amino acids 40–57 of Gβ1, which includes the basic Tctex-1-binding motif and surrounding residues. As shown in Figure 7C, the presence of increasing concentration of the Gβ-peptide leads to decreased amounts of Tctex-1 that can be co-immunoprecipitated with DIC in an anti-DIC pull down.
Discussion
The recent discoveries of novel and non-canonical modes of G protein signaling and regulation have expanded the classical repertoire of receptor-dependent G protein activation (Cismowski and Lanier, 2005; McCudden et al, 2005). AGS proteins are a functionally defined group of proteins that activate G protein signaling in the absence of a classical G protein-coupled receptor (GPCR). It has been proposed that AGS proteins play important roles in the generation or positioning of signaling complexes and may serve as alternative binding partners for G protein subunits. Notably, receptor-independent activation of G proteins has been implicated in proper positioning of the mitotic spindle and asymmetric cell division (Willard et al, 2004). Silencing of AGS3, a receptor-independent activator of Gβγ signaling, resulted in defects in mitotic spindle orientation and cleavage plane determination of neural progenitors in the developing neocortex affecting neuronal cell fate (Sanada and Tsai, 2005). AGS2 was identified as a Gβγ-binding protein and was shown to be identical to cytoplasmic dynein light-chain Tctex-1 (Takesono et al, 1999). Interestingly, Tctex-1 has been shown to have both dynein-dependent and dynein-independent functions, and there is biochemical evidence for a dynein-free pool of Tctex-1 (Tai et al, 1998; Chuang et al, 2005). It is completely unclear how the dynein-associated and dynein-free pools of Tctex-1 are maintained in the cell.
In this study, we have investigated the functional role of the AGS2/Tctex-1–Gβγ complex. We demonstrate that both Gβγ and Tctex-1 distribute in primary hippocampal neurons similarly within the cell body and in the growth cone of the future axon of differentiated neurons. As with Tctex-1, ectopic expression of Gβγ induces multiple neurites, whereas interfering with Gβγ signaling, using a Gβ-sequestering reagent βARKct, inhibits neurite outgrowth. We demonstrate that all known isoforms of Gβ contain a DIC-like Tctex-1-binding motif and ectopic expression of Gβ1 interferes with the Tctex-1–DIC interaction. Moreover a Gβ-peptide, spanning the Tctex-1-binding motif, competes with DIC for Tctex-1 binding, suggesting that Gβγ–Tctex-1 interaction may modulate the dynein–Tctex-1 motor complex formation. Since the neuritogenic effects of Tctex-1 are dynein motor activity independent, Gβγ binding to Tctex-1 provides a mechanistic model for recruiting and maintaining a dynein-free pool of Tctex-1 and the role of this complex in Tctex-1-mediated neurite outgrowth.
Dual role of Tctex-1
Tctex-1 has been identified as a light-chain subunit of the cytoplasmic dynein motor complex (Vallee et al, 2004), where it acts as a cargo adaptor for the dynein motor (Tai et al, 1999). However, Tctex-1 seems to have two functional roles—one as a dynein motor component and the second as a dynein-independent regulator of cell fate. A dynein-free pool of Tctex-1 has been confirmed biochemically (Tai et al, 1998; Li et al, 2004). Dynein motor activity is dispensable for Tctex-1-mediated modulation of cortical microfilament dynamics and Rac1 activity in embryonic hippocampal neurons (Chuang et al, 2005). Gβγ overlaps with dynein-free, less-dense fractions of Tctex-1 in embryonic brain lysates, suggestive of a dynein-independent role for the Gβγ–Tctex-1 complex.
We have identified a common Tctex-1-binding motif, which was initially defined by comparing the minimum Tctex-1-interacting sequence of DIC with other known Tctex-1-interacting proteins (Mok et al, 2001), in all known isoforms of Gβ. The sequence alignment demonstrates that all known Gβ subunits contain a DIC-like Tctex-1-binding motif. This region of Gβ maps to the outermost β-strand of the seventh blade of the Gβ propeller and has been previously shown to contain residues important for interaction with other known Gβγ effectors, including PLCβ2 and RACK1 (Panchenko et al, 1998; Buck and Iyengar, 2001; Chen et al, 2005). A second Tctex1-binding motif has also been identified, although it was often found that gaps of varying lengths had to be inserted and parts of the sequence were missing (Sugai et al, 2003). Overall, Tctex-1-interacting proteins fall into following three broad subsets: proteins that contain only the core basic R/K-R/K-X-X-R/K consensus motif; proteins that contain only the second Tctex-1-binding motif and proteins that contain both binding motifs. Gβ belongs to the first subset. We show both full-length Gβ and a Gβ-peptide, corresponding to amino acids derived from Gβ1 spanning the putative Tctex-1-binding motif, compete with DIC for Tctex-1 binding.
According to a model proposed by Wu et al (2005), DIC and cargo proteins would bind to opposite ends of the Tctex-1 dimer. However, Tctex-1-interacting proteins that contain only the core, DIC-like Tctex-1-binding motif, present a paradox, since these proteins must interact with Tctex-1 in the same region as DIC (Wu et al, 2005). It is therefore unclear whether proteins containing only the same core binding motif can be bound to the dynein–Tctex-1 complex and serve as cargo for transport by the dynein motor complex. Our data demonstrate that Gβγ competes with DIC for Tctex-1 binding. We propose that the Gβγ–Tctex-1 complex cannot itself be incorporated in the dynein motor complex. However, Gβγ regulates incorporation of Tctex-1 into the dynein motor complex and thereby regulates dynamics of the dynein-free pool of Tctex-1. Structural studies of the Tctex-1–Gβγ co-complex are underway to help clarify whether Gβ and DIC can simultaneously bind Tctex-1.
Role of Gβγ and Tctex-1 in regulating neuronal differentiation
Several lines of evidence suggest a role for the Gβγ–Tctex-1 interaction in neuronal differentiation. Tctex-1 was recently shown to play an important role in multiple steps of neuronal development, including neurite sprouting and extension, axonal polarity and dendritic arbor elaboration (Chuang et al, 2005). Moreover, Tctex-1 is highly abundant in fetal brains and in postmitotic young neurons in adult brain (Chuang et al, 2001). We report that Gβγ and Tctex-1 share overlapping expression patterns in primary hippocampal neurons. Both Tctex-1 and Gβγ localize in the growth cone of the axons in stage 3 neurons. Disruption of Tctex-1 expression and interfering with Gβγ function results in inhibition of neurite outgrowth, whereas ectopic expression of Gβγ and Tctex-1 results in multiple axon-like long neurites. Moreover, interfering with Gβγ function partially reduced the ability of Tctex-1 to induce its neuritogenic effects, suggesting that the Gβγ–Tctex-1 complex is required for full activity of Tctex-1. But how does Gβγ regulate Tctex-1-induced neuronal effects? We show that Gβγ competes with DIC for Tctex-1 binding and thus regulates the incorporation of Tctex-1 into the dynein motor complex. Since motor activity is dispensable for Tctex-1-mediated neurite outgrowth and it is the dynein-free pool of Tctex-1 that promotes its neuritogenic effects, according to our model (see Figure 7D), Gβγ binding to Tctex-1 prevents its incorporation into the dynein complex and thereby promotes its dynein-independent activity.
Ectopic expression of Tctex-1 mostly reverts the dramatic inhibition of neurite outgrowth observed in the presence of the Gβγ-sequestering reagent, βARKct. Since both Gβγ and Tctex-1 interact with several proteins and regulate various signaling pathways, it is probable that the Gβγ–Tctex-1 complex could be one of several signaling complexes leading to regulation of neurite induction and neuronal differentiation. For example, Tctex-1 stimulation of actin dynamics in neurons seems to be Rac dependent (Chuang et al, 2005). Tctex-1 may directly interact with a Rac-GEF to modulate Rac activity. Rho family GTPases are key regulators of actin dynamics that lead to actin-based assemblies associated with the structure and motility of cells. Rho GTPases have emerged as common regulators of actin dynamics that drive growth cone motility. The activity of Rho GTPases is regulated by a variety of proteins that either promote GTP uptake (GEFs) or stimulate hydrolysis (GAPs). Sequential activation of Rap1B and Cdc42 was shown to be essential for axonal specification and the establishment of neuronal polarity in hippocampal neurons (Schwamborn et al, 2004, 2006). Gβγ drives the membrane recruitment and activation of PAK-associated PIXα, an exchange factor for Cdc42 (Li et al, 2003). This Gβγ-PAK1/PIXα/Cdc42 complex and phosphatidylinositol 3-kinase (PI3K) product PI(3,4,5)P3 (PIP3) were demonstrated to be essential for actin polymerization at the leading edge and persistent directional migration of myeloid cells (Li et al, 2003). Gβγ subunit might serve to link extracellular cues to localized regulation of actin and microtubule dynamics. Rho GTPases and their modulators provide a direct link between cell surface receptors, including growth factor receptors and GPCRs, and cytoskeletal rearrangements, leading to cell motility and cell polarity.
Tctex-1, but not the other dynein subunits, is highly abundant in the two germinal zones of adult brain, namely dentate gyrus and subventricular zone (SVZ) (Dedesma et al, 2006). In the developing neocortex, neural stem cells at the ventricular zone (VZ) either symmetrically divide into two identical daughter cells (e.g., progenitors or postmitotic neurons), or asymmetrically divide into one progenitor and one postmitotic neuron. Sanada and Tsai (2005) showed that perturbation of Gβγ signaling resulted in significantly decreased frequency of asymmetric cell division causing an overproduction of postmitotic neurons at the expense of mitogenic neural stem cells (Sanada and Tsai, 2005). Disruption of AGS3, which binds to Gαi-GDP, preventing the reassociation of Gβγ with Gα to reform the heterotrimer, leads to a phenotype resembling perturbation of Gβγ signaling (Sanada and Tsai, 2005). In the cortical neural progenitor cells, interfering with Gβγ function resulted in defects associated with mitotic spindle orientation and cleavage plane determination. But exactly how Gβγ regulates mitotic spindle alignment and cleavage orientation and thereby asymmetric cell division of neural stem cells is still unclear. Taken together, it can be concluded that G proteins and molecules that regulate G protein signaling are important players in neuronal development. We show specifically that Gβγ and Tctex-1, which individually are important in various pathways involving neuronal development, act in concert to regulate neuritogenesis.
Materials and methods
Antibodies, chemical reagents and plasmid constructs
DIC mAb (clone 74.1) was obtained from Chemicon (Temecula, CA), anti-FLAG pAb and tyrosinated-tubulin (Tyr-Tubulin) (clone TUB1A2) mAb from Sigma-Aldrich (St Louis, MO), anti-pan Gβ antibody, (cat. no. sc-378), which recognizes all Gβ isoforms and anti-Gγ1 and anti-Gγ2, from Santa Cruz Biotechnology (Santa Cruz, CA), horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit IgG from Jackson Immunoresearch Laboratories (Bar Harbor, ME) and Alexa 488-conjugated goat anti-rabbit and Alexa 594-conjugated goat anti-mouse secondary antibodies from Invitrogen (Carlsbad, CA). Isopropyl-1-thio-β-D-galactopyranoside (IPTG) was obtained from US Biological (Swampscott, MA). Affinity-purified anti-Tctex-1 pAb was described in Tai et al (1998), and additionally, an anti-Tctex-1 mAb antibody was also generated (see Supplementary Figure 2, and J-Z Chuang and C-H Sung, unpublished data). Untagged Tctex-1 cloned into pET-3a was a gift from Dr Zhang (Mok et al, 2001). Expression vectors encoding EGFP, pEGFP-C1 (Clontech Laboratories Inc., Mountain View, CA), untagged and FLAG-tagged Tctex-1 (Chuang et al, 2001), untagged and FLAG-tagged Gβ1 and FLAG-tagged Gβ2, Gβ3, Gβ5 and Gβ5L (UMR cDNA Resource Center, www.cdna.org), and control vector pcDNA3.1 (+) (Invitrogen, Carlsbad, CA) were used.
Peptide synthesis and purification
Unlabeled Gβ-peptide, 40-VGRIQMRTRRTLRGHLAK-57 and labeled Gβ-peptide, C(MIANS)-40VGRIQMRTRRTLRGH-LAK-57 [MIANS=2-(4′-maleimidylanilino)napthalene-6-sulfonic acid] (corresponding to the amino-acid sequence of Gβ1) were obtained from the Proteomics Resource Center of Rockefeller University. Peptides were synthesized using F-moc amino acids and had amidated C-terminus. Molecular mass was confirmed by MALDI mass spectrometry, purified to greater than 95% purity by reverse-phase high-performance liquid chromatography (RP-HPLC) and was highly soluble in water.
Protein expression and purification
Tctex-1 was expressed and purified both as a GST-tagged protein as well as an untagged protein as described in Mok et al (2001). Briefly, a three-step FPLC-based column chromatography procedure was used. pET-3a vector encoding mouse Tctex-1 cDNA transformed into Escherichia coli BL21(DE3) host cells and Tctex-1 expression was induced by the addition of 400 μM IPTG. The cells were harvested and lysed 3 h after induction and the cleared lysate was loaded onto a HiPrep 16/10 DEAE Sepharose (GE Healthcare) column and the protein was eluted with a linear NaCl gradient of 0–0.3 M. Fractions containing Tctex-1 were pooled, concentrated and loaded onto a sizing column (HiLoad 16/60 Superdex 75 gel filtration column) and excess salt was removed by desalting chromatography (Hi Prep 26/10 desalting column). Fractions from the desalting column were loaded on to a MonoQ HR 5/5 and eluted with a gradient of 0–0.35 M NaCl. The purified Tctex-1 was dialyzed and snap frozen in working aliquots and stored at −80°C. Transducin (Gtαβγ) was purified from frozen bovine retinas (Min et al, 1993; Marin et al, 2000). The purified proteins were snap frozen in working aliquots and stored at −80°C.
Fluorescence anisotropy
Interaction between MIANS-labeled Gβ-peptide and Tctex-1 was monitored by fluorescence anisotropy. Anisotropy values were measured as described previously (Krishna et al, 2002). Briefly, the peptide was excited at 325 nm and the fluorescence anisotropy was measured in the absence or presence of Tctex-1 at 440 nm. Ludox was used as a standard in the anisotropy experiments (with an anisotropy value of ∼0.97).
Cell culture and transfection
HEK 293 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% (v/v) fetal calf serum. All cell cultures were maintained in 5% CO2 at 37°C. For immunoprecipitation assays, HEK293 cells were plated in 10-cm plates and transfected with 3.5 μg DNA/plate using Lipofectamine Plus (Invitrogen). Cell extracts were generated 48 h after transfection.
Immunoprecipitation and peptide competition assay
Cells were lysed in lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, EDTA, 2 mM EDTA, 1.0% (v/v) Triton X-100) plus protease inhibitor mixture (1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin and 0.7 μg/ml pepstatin). Cell lysates were cleared by centrifugation and the supernatant fractions were then incubated with antibody and Protein A–agarose beads (Repligen, Cambridge, MA) for 2 h at 4°C. For Gβ-peptide competition assay, cell lysates were incubated with either anti-DIC antibody alone or in the presence of increasing concentration of unlabeled Gβ-peptide (1, 10, 25, 50, 100 or 1000 nM). The immunoprecipitates were washed three times with cold lysis buffer. The proteins were released from beads by boiling in SDS sample buffer and separated by SDS–PAGE. Proteins were transferred to nitrocellulose and detected using anti-Gβ (1:1000), anti-Tctex-1 (1:2000), anti-FLAG (1:5000) or anti-DIC (1:1000) antibodies.
Culture and immunofluorescence analyses of hippocampal neurons
Cultures of dissociated embryonic hippocampal neurons were prepared as described previously (Goslin and Banker, 1991; Chuang et al, 2005). Briefly, embryonic E18 rat brains were dissected, the isolated hippocampi were incubated in 1 × HBSS (Invitrogen) containing 0.25% trypsin for 15 min at 37°C and dissociated by pipetting in plating medium (minimum essential medium (MEM) supplemented with 10% horse serum, glucose and 100 U/ml penicillin/streptomycin; Invitrogen). Freshly trypsin-dissociated neurons were plated onto glass coverslips coated with poly-L-lysine (Sigma P2636) and cultured at 37°C and 5% CO2. For endogenous expression analysis, neurons were allowed to attach to the substrate followed by incubation in maintenance medium (MEM supplemented with ovalbumin, B27 and N2.1 supplement, pyruvate, and 100 U/ml penicillin/streptomycin; Invitrogen). Neurons were fixed at 1–3 days in vitro (d.i.v.) with 4% paraformaldehyde/sucrose solution in phosphate-buffered saline (PBS) for 15 min at room temperature (RT) and processed for immunohistochemistry. For transfection studies, neurons were transfected either at 2 h after plating or 12 h after plating using Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol, with minor adjustments. Total DNA amount for each transfection was kept constant using the empty backbone control vector, pcDNA3.1(+). Neurons were incubated with the DNA:lipid mixture for 2 h at 37°C and the medium was then removed and replaced with maintenance medium. Neurons were fixed at the indicated times and processed for immunohistochemistry following published protocols (Chuang et al, 2005). Primary antibody was followed by goat anti-rabbit or anti-mouse secondary antibodies coupled to Alexa 488 or Alexa 594 (Invitrogen). The coverslips were mounted in Vectashield mounting medium (Vector Labs Inc., Burlingame, CA) with DAPI. All immunostained cells were analyzed either using a Zeiss confocal microscope or a Zeiss Axiovert 200 M equipped with an Axiocam HR camera. At least three independent experiments were conducted for each manipulation and 50–100 cells on 15–40 coverslips were examined in each experiment. Different stages of neuritic development were classified based on established guidelines (Dotti et al, 1988; Chuang et al, 2005). Quantification of labeling intensities and morphometric analyses were carried out using Metamorph software (Universal Imaging Co., Downingtown, PA) as described (Chuang et al, 2005) and the images were processed using Adobe Photoshop.
Velocity density gradient sedimentation
Embryonic E15 mouse brains were harvested and rinsed with ice-cold PBS twice. Cells were broken using the Dounce homogenizer (Kimble Kontes, Vineland, NJ) with pestle A (10 times) followed by pestle B (20 times) in 1 ml PEM buffer (80 mM PIPES, pH 6.8, 1 mM EGTA, 1 mM MgSO4, 0.5 mM DTT and protease inhibitors) on ice. Homogenates were cleared by centrifugation at 2000 r.p.m. for 5 min to remove nuclei followed by centrifugation at 100 000 g at 4°C for 1 h. The high-speed supernatant fraction was fractionated in a 5–20% linear sucrose gradient in 11 ml of Tris–KCl buffer (20 mM Tris, pH 7.5, 50 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 0.5 mM DTT and protease inhibitors) using a SW41 Ti rotor (Beckman) at 32 000 r.p.m. in 4°C for 16 h. The S-values were determined by measuring the reflective index of each fraction.
Supplementary Material
Supplementary Figure Legends
Supplementary Figure 1
Supplementary Figure 2
Acknowledgments
PS was supported earlier by the NIH Training Grant EY07138 and is currently a fellow of the Murray Foundation. TPS was formerly an Ellison Medical Foundation senior scholar. This work was supported in part by NIH EY11307 to C-HS and the Alene Reuss Memorial Trust and the Howard Hughes Medical Institute.
References
- Baas PW, Buster DW (2004) Slow axonal transport and the genesis of neuronal morphology. J Neurobiol 58: 3–17 [DOI] [PubMed] [Google Scholar]
- Banker G (2003) Pars, PI 3-kinase, and the establishment of neuronal polarity. Cell 112: 4–5 [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG (2000) Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol 10: 574–581 [DOI] [PubMed] [Google Scholar]
- Buck E, Iyengar R (2001) Modular design of Gbeta as the basis for reversible specificity in effector stimulation. J Biol Chem 276: 36014–36019 [DOI] [PubMed] [Google Scholar]
- Chen S, Lin F, Hamm HE (2005) RACK1 binds to a signal transfer region of Gbeta gamma and inhibits PCLbeta 2 activation. J Biol Chem 280: 33445–33452 [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Milner TA, Sung CH (2001) Subunit heterogeneity of cytoplasmic dynein: differential expression of 14 kDa dynein light chains in rat hippocampus. J Neurosci 21: 5501–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH (2005) The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell 9: 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski MJ, Lanier SM (2005) Activation of heterotrimeric G-proteins independent of a G-protein coupled receptor and the implications for signal processing. Rev Physiol Biochem Pharmacol 155: 57–80 [DOI] [PubMed] [Google Scholar]
- Dedesma C, Chuang JZ, Alfinito PD, Sung CH (2006) Dynein light chain Tctex-1 identifies neural progenitors in adult brain. J Comp Neurol 496: 773–786 [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas MW, Diefenbach RJ, Homa FL, Miranda-Saksena M, Rixon FJ, Vittone V, Byth K, Cunningham AL (2004) Herpes simplex virus type 1 capsid protein VP26 interacts with dynein light chains RP3 and Tctex1 and plays a role in retrograde cellular transport. J Biol Chem 279: 28522–28530 [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kimura T, Kaibuchi K (2002) Axon specification in hippocampal neurons. Neurosci Res 43: 305–315 [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G (1991) Rat Hippocampal Neurons in Low-Density Culture. The MIT Press: Cambridge, MA [Google Scholar]
- Hirokawa N (1998) Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279: 519–526 [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14: 33–38, 27–38 [DOI] [PubMed] [Google Scholar]
- Kai N, Mishina M, Yagi T (1997) Molecular cloning of Fyn-associated molecules in the mouse central nervous system. J Neurosci Res 48: 407–424 [PubMed] [Google Scholar]
- King SM (2000) The dynein microtubule motor. Biochim Biophys Acta 1496: 60–75 [DOI] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ (1994) Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci USA 91: 12706–12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna AG, Menon ST, Terry TJ, Sakmar TP (2002) Evidence that helix 8 of rhodopsin acts as a membrane-dependent conformational switch. Biochemistry 41: 8298–8309 [DOI] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB (1996) The 2.0 Å crystal structure of a heterotrimeric G protein. Nature 379: 311–319 [DOI] [PubMed] [Google Scholar]
- Lanier SM (2004) AGS proteins, GPR motifs and the signals processed by heterotrimeric G proteins. Biol Cell 96: 369–372 [DOI] [PubMed] [Google Scholar]
- Li MG, Serr M, Newman EA, Hays TS (2004) The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol Biol Cell 15: 3005–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D (2003) Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114: 215–227 [DOI] [PubMed] [Google Scholar]
- Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC (2003) Functional interaction between BMPR-II and Tctex-1, a light chain of dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet 12: 3277–3286 [DOI] [PubMed] [Google Scholar]
- Marin EP, Krishna AG, Zvyaga TA, Isele J, Siebert F, Sakmar TP (2000) The amino terminus of the fourth cytoplasmic loop of rhodopsin modulates rhodopsin–transducin interaction. J Biol Chem 275: 1930–1936 [DOI] [PubMed] [Google Scholar]
- McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS (2005) G-protein signaling: back to the future. Cell Mol Life Sci 62: 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KC, Zvyaga TA, Cypess AM, Sakmar TP (1993) Characterization of mutant rhodopsins responsible for autosomal dominant retinitis pigmentosa. Mutations on the cytoplasmic surface affect transducin activation. J Biol Chem 268: 9400–9404 [PubMed] [Google Scholar]
- Mok YK, Lo KW, Zhang M (2001) Structure of Tctex-1 and its interaction with cytoplasmic dynein intermediate chain. J Biol Chem 276: 14067–14074 [DOI] [PubMed] [Google Scholar]
- Mou T, Kraas JR, Fung ET, Swope SL (1998) Identification of a dynein molecular motor component in Torpedo electroplax; binding and phosphorylation of Tctex-1 by Fyn. FEBS Lett 435: 275–281 [DOI] [PubMed] [Google Scholar]
- Ohka S, Matsuda N, Tohyama K, Oda T, Morikawa M, Kuge S, Nomoto A (2004) Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J Virol 78: 7186–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko MP, Saxena K, Li Y, Charnecki S, Sternweis PM, Smith TF, Gilman AG, Kozasa T, Neer EJ (1998) Sites important for PLCbeta2 activation by the G protein betagamma subunit map to the sides of the beta propeller structure. J Biol Chem 273: 28298–28304 [DOI] [PubMed] [Google Scholar]
- Pfister KK, Fisher EMC, Gibbons IR, Hays TS, Holzbaur ELF, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, King SM, Vallee RB (2005) Cytoplasmic dynein nomenclature. J Cell Biol 171: 411–413. 10.1083/jcb.200508078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato M, King SM (2004) Design and regulation of the AAA+ microtubule motor dynein. J Struct Biol 146: 58–71 [DOI] [PubMed] [Google Scholar]
- Sanada K, Tsai LH (2005) G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122: 119–131 [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Fiore R, Bagnard D, Kappler J, Kaltschmidt C, Puschel AW (2004) Semaphorin 3A stimulates neurite extension and regulates gene expression in PC12 cells. J Biol Chem 279: 30923–30926 [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Li Y, Puschel AW (2006) GTPases and the control of neuronal polarity. Methods Enzymol 406: 715–727 [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Puschel AW (2004) The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 7: 923–929 [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Barnikol-Watanabe S, Thinnes FP, Hilschmann N (2002) Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int J Biochem Cell Biol 34: 1059–1070 [DOI] [PubMed] [Google Scholar]
- Sugai M, Saito M, Sukegawa I, Katsushima Y, Kinouchi Y, Nakahata N, Shimosegawa T, Yanagisawa T, Sukegawa J (2003) PTH/PTH-related protein receptor interacts directly with Tctex-1 through its COOH terminus. Biochem Biophys Res Commun 311: 24–31 [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH (1999) Rhodopsin's carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell 97: 877–887 [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Sung CH (1998) Localization of Tctex-1, a cytoplasmic dynein light chain, to the Golgi apparatus and evidence for dynein complex heterogeneity. J Biol Chem 273: 19639–19649 [DOI] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S III, Duzic E, Lanier SM (1999) Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem 274: 33202–33205 [DOI] [PubMed] [Google Scholar]
- Vallee RB, Williams JC, Varma D, Barnhart LE (2004) Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol 58: 189–200 [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL (1995) The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci USA 92: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Kimple RJ, Siderovski DP (2004) RETURN OF THE GDI: the GoLoco motif in cell division. Annu Rev Biochem 73: 925–951 [DOI] [PubMed] [Google Scholar]
- Wu H, Maciejewski MW, Takebe S, King SM (2005) Solution structure of the tctex1 dimer reveals a mechanism for Dynein–cargo interactions. Structure (Camb) 13: 213–223 [DOI] [PubMed] [Google Scholar]
- Yano H, Chao MV (2004) Mechanisms of neurotrophin receptor vesicular transport. J Neurobiol 58: 244–257 [DOI] [PubMed] [Google Scholar]
- Yano H, Lee FS, Kong H, Chuang J, Arevalo J, Perez P, Sung C, Chao MV (2001) Association of Trk neurotrophin receptors with components of the cytoplasmic dynein motor. J Neurosci 21: RC125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure Legends
Supplementary Figure 1
Supplementary Figure 2


