Abstract
Members of the ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family of secreted proteins play important roles in animal development and pathogenesis. However, the lack of in vivo models has hampered elucidation of the mechanisms by which these enzymes are recruited to specific target tissues and the timing of their activation during development. Using transgenic worms and primary cell cultures, here we show that MIG-17, an ADAMTS family protein required for gonadal leader cell migration in Caenorhabditis elegans, is recruited to the gonadal basement membrane in a prodomain-dependent manner. The activation of MIG-17 to control leader cell migration requires prodomain removal, which is suggested to occur autocatalytically in vitro. Although the prodomains of ADAMTS proteases have been implicated in maintaining enzymatic latency, polypeptide folding and secretion, our findings demonstrate that the prodomain has an unexpected function in tissue-specific targeting of MIG-17; this prodomain targeting function may be shared by other ADAMTSs including those in vertebrates.
Keywords: ADAMTS protease, cell migration, glycosylation, MIG-17, prodomain
Introduction
ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family proteins are a group of zinc-dependent metalloproteases (MPs) that mainly degrade extracellular matrix (ECM) components such as proteoglycans and collagens (Colige et al, 1997; Tortorella et al, 1999, 2000; Kuno et al, 2000; Matthews et al, 2000; Fernandes et al, 2001; Sandy et al, 2001; Somerville et al, 2003; Wang et al, 2003). ADAMTSs have common structural features: a signal peptide, a prodomain, an MP domain, a disintegrin (DI) domain, a variable number of thrombospondin type I (TS) motifs and other ancillary domains near the C-terminus (Porter et al, 2005). The prodomain is required for maintaining enzymatic latency in ADAMTS-4 (Tortorella et al, 2005) and probably for polypeptide folding and secretion (Porter et al, 2005). The function of the DI domains is not known. Because ADAMTSs are secreted, they must be brought to their target sites of action during development.
In Caenorhabditis elegans, two ADAMTS family proteins, GON-1 and MIG-17, act in gonad development (Blelloch and Kimble, 1999; Nishiwaki et al, 2000). During larval development of C. elegans hermaphrodites, the anterior and posterior gonad arms elongate along the body wall and make two turns as they extend, finally forming the symmetrical U-shaped gonad arms. The migration of gonad arms is directed by two specialized gonadal leader cells, the distal tip cells (DTCs) (Kimble and White, 1981) (Figure 1A). GON-1 and MIG-17 are proposed to be required for proper remodeling of the gonadal basement membrane, which supports active morphogenesis of the gonad (Blelloch et al, 1999; Nishiwaki et al, 2000). GON-1 function is essential for the motility of DTCs and expansion of gonad arms, although the tissue distribution of GON-1 is not known (Blelloch and Kimble, 1999). Although lacking TS motifs, MIG-17 apparently belongs to the ADAMTS family based on significant homology between its MP, DI and PLAC (protease and lacunin) domains (of which the last was identified in this study) and those found in ADAMTS proteins (Nishiwaki et al, 2000). MIG-17 is secreted from body wall muscle cells and localizes to the gonadal basement membrane; it is required for directed DTC migration rather than motility per se (Nishiwaki et al, 2000).
Figure 1.
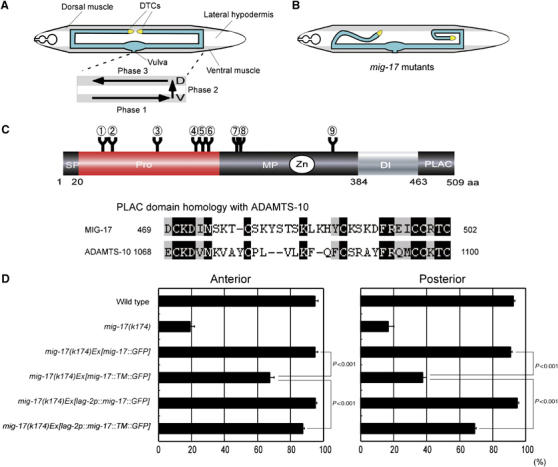
Tissue-specific expression of a membrane-anchored MIG-17. (A) The gonad morphology and the phases of DTC migration in wild-type hermaphrodites. (Upper) The gonad is shown in blue. Ventral and dorsal body wall muscles are shaded. Unshaded part between dorsal and ventral muscles corresponds to the hypodermis. (Lower) The phases of DTC migration are shown by arrows. (B) Abnormal gonad morphogenesis in mig-17 mutants. (C) (Upper) Domain structure and potential N-glycosylation sites of MIG-17. The circled numbers (1–9) indicate potential N-glycosylation sites (N-X-S/T). 1, N52; 2, N65; 3, N123; 4, N172; 5, N183; 6, N189; 7, N218; 8, N219; 9, N350. (Lower) Sequence alignment of the PLAC domains of MIG-17 and human ADAMTS-10. Identical and homologous amino acids are shown in black and gray, respectively. (D) A membrane-anchored MIG-17 can rescue mig-17 mutants when expressed in DTCs. The ratios of normal DTC migration are shown in bar graphs with the mean±s.e.m. For the animals carrying lag-2 promoter-driven transgenes, those with GFP expression in DTCs were scored. P-values from Fisher's exact test are shown for some combinations. n=120. DI, disintegrin domain; PLAC, protease and lacunin domain; MP, metalloprotease domain; Pro, prodomain; SP, signal peptide.
MIG-17 offers an excellent model to study the molecular behavior and function of ADAMTSs during organ morphogenesis. In the present study, we used MIG-17 transgenes having mutations in the domains and glycosylation sites to investigate the regions in MIG-17 that are responsible for its localization and function in vivo. Using embryonic cell cultures, we found that mutant MIG-17 proteins are secreted with kinetics similar to that of the wild-type protein. We showed that MIG-17 is secreted as a proform and that the glycosylated prodomain plays an important role in targeting MIG-17 to the gonad. MIG-17 appears to be converted from the proform to the mature form via intramolecular autocatalytic activity, a process that is essential for MIG-17 to control DTC migration. Our findings provide a model of the action of ADAMTS proteases during organ formation and shed new light on the function of prodomains in targeting ADAMTSs to specific tissues or cells.
Results
MIG-17 is primarily required on the surface of DTCs
The wild-type hermaphrodite gonad consists of two U-shaped arms formed by directed migration of DTCs. In mig-17 mutants, such U-shaped gonad morphogenesis is not achieved, and the gonad arms become deformed because of the aberrant migration of DTCs (Figure 1A and B). The domain structure of MIG-17 is shown in Figure 1C. We found that the C-terminal domain corresponds to the PLAC domain shared by several ADAMTSs. The PLAC domain of MIG-17 is most similar to that of human ADAMTS-10 (Somerville et al, 2004a) (Figure 1C).
MIG-17 is secreted from the body wall muscle cells and localizes to the gonadal basement membrane shortly after the first turn of the DTCs (Nishiwaki et al, 2000). As ectopic expression of MIG-17 by the lag-2 promoter, which drives expression in DTCs, can rescue the DTC migration defects in mig-17 mutants, it has been assumed that MIG-17 localization on the DTC surface is important for its function (Nishiwaki et al, 2000). However, because MIG-17 is a secreted protein, it is still possible that MIG-17 produced by DTCs diffuses to its natural target tissues where it controls DTC migration. To examine whether MIG-17 is actually required on the DTC surface, we constructed a membrane-bound MIG-17 using the transmembrane domain of integrin-α, INA-1 (Baum and Garriga, 1997). When we expressed this MIG-17 construct tagged with GFP (MIG-17-TM-GFP) in body wall muscles using the endogenous promoter, the DTC migration defects of mig-17(k174) null mutants were weakly rescued for anterior DTCs, whereas they were mostly unrescuable for posterior DTCs. On the other hand, the expression of the same construct using the lag-2 promoter efficiently rescued both the anterior and posterior DTC migration defects, although the rescue was weaker than that achieved using MIG-17-GFP without a transmembrane domain (Figure 1D). These results suggest that the DTC surface is the primary site of action for MIG-17. The weaker rescue by the transmembrane form compared with the secreted form may suggest that anchoring to the plasma membrane weakly impairs the activity or mode of localization of MIG-17 in the gonadal basement membrane.
N-glycosylation of the prodomain is essential for MIG-17 localization to the gonad
mig-23 encodes a Golgi nucleoside diphosphatase required for protein glycosylation. We previously showed that mutations in mig-23 prevent MIG-17 localization to the gonad because of its defective glycosylation (Nishiwaki et al, 2004). However, the function of each glycan modification in MIG-17 remains unknown. MIG-17 contains six potential N-glycosylation sites (N-X-S/T) in the prodomain, three in the MP domain, but none in the DI and PLAC domains (Figure 1C). To address the roles of N-glycosylation in MIG-17, we mutated the MIG-17 N-glycosylation sites. The asparagine residues in these sites were changed to glutamines in various combinations (Figure 2A), thereby prohibiting N-glycosylation, and these MIG-17 mutants were expressed as GFP fusion proteins.
Figure 2.
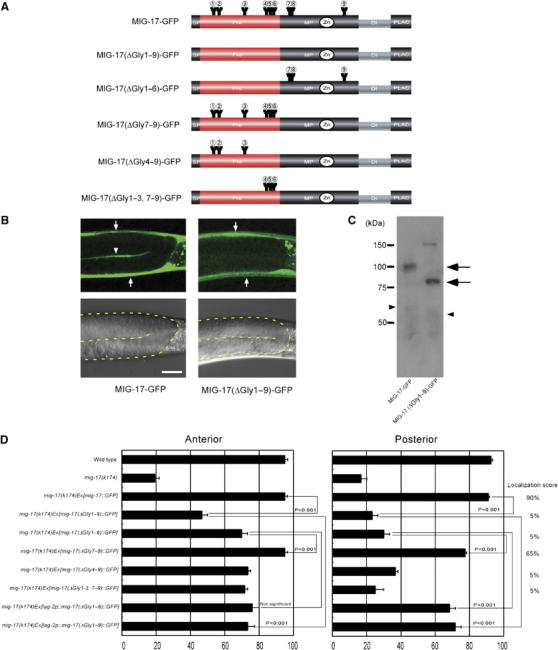
Requirement of N-glycosylation for MIG-17 localization and function. (A) Glycosylation mutant constructs. Only the intact potential glycosylation sites are indicated. All asparagines of potential N-glycosylation sites were changed to glutamines in MIG-17(ΔGly1–9)-GFP: N52Q (AAT to CAA), N65Q (AAC to CAA), N123Q (AAT to CAA), N172Q (AAT to CAA), N183Q (AAC to CAA), N189Q (AAT to CAA), N218Q (AAC to CAA), N219Q (AAT to CAA) and N350Q (AAT to CAA). (B) Confocal (upper) and Nomarski (lower) images of wild-type hermaphrodites expressing MIG-17-GFP (left) or MIG-17(ΔGly1–9)-GFP (right). The boundaries of the gonads are depicted by a dotted line in the Nomarski images. Lateral views of posterior gonads. Dorsal to the top, anterior to the left. The gonadal localization of MIG-17 can be detected by linear GFP fluorescence between the proximal and distal gonad arms in animals expressing MIG-17-GFP (arrowhead). The strong dorsal and ventral GFP fluorescence corresponds to expression of transgenes in the body wall muscles (arrows). Punctate fluorescence outside the gonads is gut autofluorescence. Scale bar, 20 μm. (C) Western blot analysis. The lysates from wild-type worms expressing MIG-17-GFP or MIG-17(ΔGly1–9)-GFP were immunoblotted with anti-GFP. Pro- and mature forms are shown by arrows and arrowheads, respectively. The band of about 150 kDa in the MIG-17(ΔGly1–9)-GFP lane is often detected even in non-transgenic worms and probably due to nonspecific binding of the secondary antibody. (D) Requirement for glycosylation of MIG-17 in MIG-17 localization and function. Data for DTC migration are shown as in Figure 1D (n=120). Percentage of posterior gonads localized with GFP is indicated as the localization score on the right (n=20).
We examined their localization to the gonad surface (gonadal basement membrane) in the wild-type background by confocal microscopy and their ability to rescue DTC migration defects in mig-17(k174) null mutants. Although the wild-type MIG-17-GFP efficiently localized to the gonad (90% of the gonads), the mutant protein that was disrupted at all nine potential glycosylation sites (MIG-17(ΔGly1–9)-GFP) failed to localize to the gonad (5% of the gonads) (Figure 2B and D). Because the lack of glycosylation could affect MIG-17-GFP secretion, we assessed whether MIG-17(ΔGly1–9)-GFP could be secreted from the muscle cells by examining endocytotic uptake of GFP fusion proteins by coelomocytes, which are scavenger cells in the body cavity (Fares and Greenwald, 2001a, 2001b). GFP fluorescence was observed in the coelomocytes in animals expressing MIG-17(ΔGly1–9)-GFP as well as those expressing MIG-17-GFP (data not shown). As discussed in a later section, the efficiency of secretion of the fusion protein was only slightly affected in MIG-17(ΔGly1–9)-GFP-expressing cells. Western blot analysis revealed that MIG-17(ΔGly1–9)-GFP migrates significantly faster than MIG-17-GFP, consistent with the idea that MIG-17 contains multiple N-glycosyl chains (Figure 2C). These results indicate that MIG-17 lacking N-glycosylation is secreted from the body wall muscle cells into the body cavity, and that N-glycosylation is essential for MIG-17 localization to the gonad.
In contrast to the mostly normal DTC migration in wild-type worms, about 80% of the anterior or posterior gonads exhibited abnormal migration in mig-17(k174) mutants (Figure 2D). When MIG-17-GFP was expressed in the mig-17(k174) mutant, the migration defects were mostly rescued in both anterior and posterior gonads. When MIG-17(ΔGly1–9)-GFP was expressed in mig-17 mutants, although the migration defects of anterior DTCs were weakly rescued, the defects of the posterior DTCs were not (Figure 2D). Therefore, posterior DTC migration is strongly dependent on N-glycosylation of MIG-17.
To determine which N-glycosylation site(s) (i.e., in the prodomain or MP domain) is important for gonadal localization of MIG-17, we constructed MIG-17(ΔGly1–6)-GFP, which lacks glycosylation in the prodomain, and MIG-17(ΔGly7–9)-GFP, which lacks glycosylation in the MP domain (Figure 2A). Although MIG-17(ΔGly7–9)-GFP localized to the wild-type gonad, MIG-17(ΔGly1–6)-GFP failed to do so (Figure 2D and Supplementary Figure S1). The mutant proteins having only either the first or last three glycosylation sites of the prodomain also failed to localize (Figure 2D). When these mutant constructs were expressed in mig-17 mutant animals, only MIG-17(ΔGly7–9)-GFP rescued the DTC migration defect (Figure 2D). These mutant proteins migrated faster than wild-type MIG-17 in Western blots (Supplementary Figure S2A). Examination of individual N-glycosylation sites revealed that the first, second, fourth and sixth sites in the prodomain are especially important for MIG-17 function as well as for localization (Supplementary Figure S3, Western blot in Supplementary Figure S2B). These results indicate that N-glycosylation of the prodomain is crucial for gonadal localization of MIG-17, whereas glycosylation of the MP domain has a minor role in this process.
Prodomain glycosylation is required for MIG-17 localization but not for control of DTC migration
We have shown that N-glycosylation of the prodomain is essential for MIG-17 localization and function. It is not clear, however, whether N-glycosylation is actually required for MIG-17 activity to control DTC migration, because the failure to localize appropriately precludes MIG-17 function on the gonad surface. Therefore, we expressed MIG-17(ΔGly1–6)-GFP or MIG-17(ΔGly1–9)-GFP in DTCs using the lag-2 promoter. Surprisingly, we found that both of these mutant proteins significantly rescued mig-17 mutants (Figure 2D). These results indicate that prodomain glycosylation is essential to recruit MIG-17 to the gonad surface but that it is dispensable for DTC migration after MIG-17 is localized to the gonad. Non-N-glycosylated MIG-17 probably has normal enzymatic activity against the physiological substrate required for DTC migration. A serine protease, matriptase, is also reported to retain normal substrate specificity after removal of N-linked polysaccharides (Ihara et al, 2004).
Functions of MIG-17 domains
Using transgenes mutated for each domain of MIG-17, we previously reported that the prodomain, DI domain and enzymatic activity are involved in MIG-17 localization to the gonad and that all domains are required for MIG-17's ability to control DTC migration (Nishiwaki et al, 2000). These analyses were qualitative, however, and thus we quantitatively re-evaluated the same constructs and several additional constructs (Figure 3A). The PLAC domain was completely dispensable for localization (Figure 3B). Although deletion of the DI domain (MIG-17(ΔDI)-GFP) or loss of proteinase activity (MIG-17(E303Q)-GFP) substantially weakened the GFP signal on the gonad surface, the signal was still detected in about half of the gonads examined. Deletion of the prodomain (MIG-17(ΔPro)-GFP) completely abolished localization. We generated MIG-17 mutant constructs D79N and G292E corresponding to the mig-17 alleles k135 and k176, which encode missense mutations within the pro- and MP domains, respectively, and have strong DTC migration defects similar to mig-17(k174) (Figure 3A). MIG-17(D79N)-GFP failed to localize, whereas MIG-17(G292E)-GFP localized normally (Figure 3B). Although these results raised the possibility that GFP fused with the prodomain alone (MIG-17(Pro1–209)-GFP) could localize to the gonad, this was not the case. The GFP fluorescence in coelomocytes was observed at similar levels in all transgenic animals for mutant constructs as well as in those for the wild-type construct (data not shown). We observed that secretion efficiency was only slightly affected in MIG-17(ΔPro)-GFP-expressing cells, as discussed later. These results indicate that the prodomain is very important for MIG-17 localization to the gonad, but it is not sufficient. When these constructs were introduced into mig-17 mutants, they all exhibited very little rescue activity—especially for posterior DTC migration abnormalities (Figure 3B).
Figure 3.
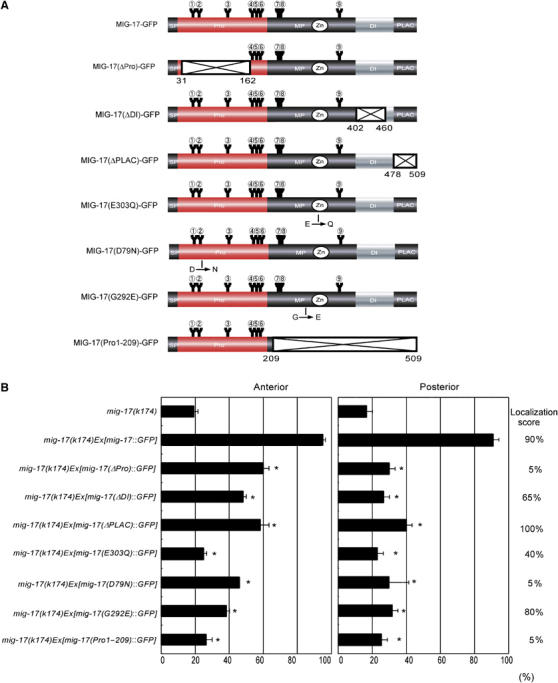
Requirement of domains for localization and function of MIG-17. (A) MIG-17 mutant constructs. Deleted regions are shown as crosshatched boxes. The amino-acid positions of the N and C termini of the deleted regions are indicated. Positions for amino-acid changes for MIG-17(E303Q)-GFP, MIG-17(D79N) and MIG-17(G292E) are shown. (B) mig-17 rescue experiments. Data for DTC migration are shown as in Figure 1D (n=120). Asterisks indicate P<0.001 in Fisher's exact test against the score of mig-17(k174)Ex[mig-17∷GFP]. Localization scores are shown on the right (n=20).
Prodomain processing requires the autocatalytic activity of MIG-17
Most of the ADAMs and ADAMTSs reportedly are cleaved between the prodomain and MP domain as they progress through the secretory pathway. Prodomain processing can be recapitulated in vitro with cell lysates in some cases (Schlomann et al, 2002). Therefore, we examined prodomain processing of various MIG-17-GFP constructs using worm lysates.
Western blotting of extracts of wild-type animals expressing MIG-17-GFP showed mostly the proform with only a small amount of the mature form (Figure 2C). However, when the extracts were incubated at room temperature, the amount of the mature form gradually increased (Figure 4A, left panel). In contrast, the protease-deficient form, MIG-17(E303Q)-GFP, existed only as a proform and was never processed to maturity during incubation, suggesting that autocatalytic activity is necessary for conversion to the mature form and that endogenous wild-type MIG-17 cannot process the mutant MIG-17(E303Q)-GFP (Figure 4A, right panel). Therefore, the prodomain of MIG-17 is probably removed via an intramolecular autocatalytic activity, a conclusion that is supported by the fact that the serine and cysteine protease (but not MP) inhibitors aprotinin and leupeptin failed to inhibit the reaction (data not shown).
Figure 4.
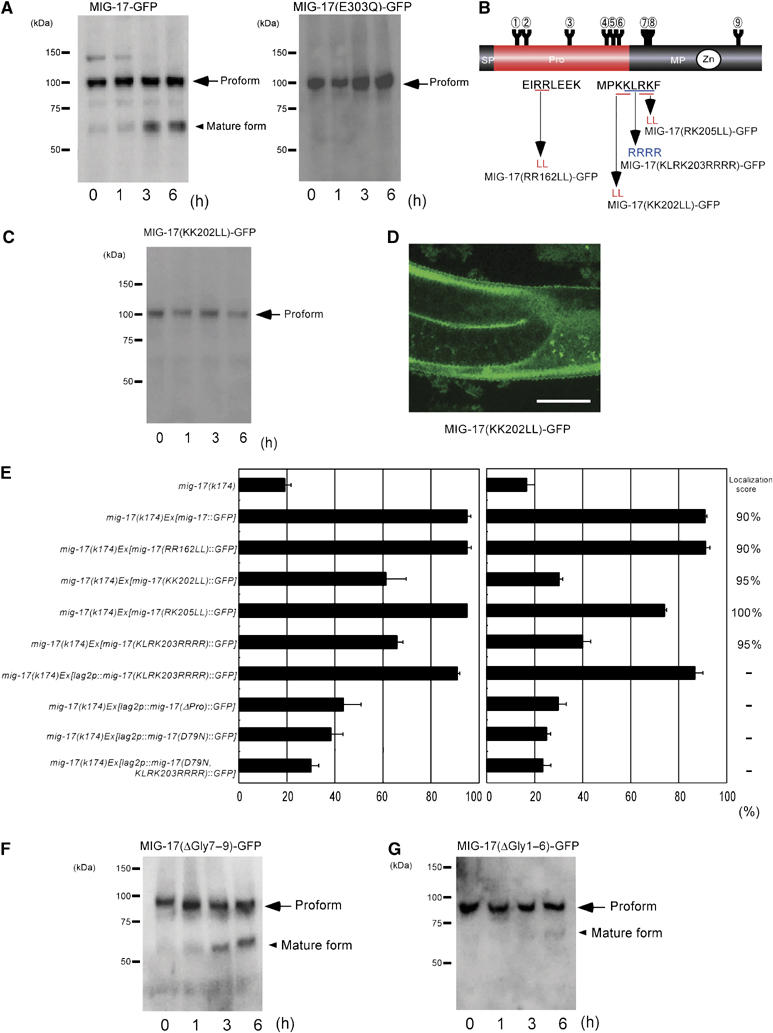
Prodomain processing is essential for MIG-17 function in cell migration. (A) Processing of MIG-17 in vitro. The lysates from wild-type worms expressing MIG-17-GFP or MIG-17(E303Q)-GFP were incubated at room temperature for the indicated periods and immunoblotted with anti-GFP. (B) Mutant constructs of potential processing sites. (C) The extracts from wild-type animals expressing MIG-17(KK202LL)-GFP were analyzed as in (A). (D) Gonadal localization of MIG-17(KK202LL)-GFP. Scale bar, 20 μm. (E) mig-17 rescue experiments using the constructs in (B). Data for DTC migration are shown as in Figure 1D (n=120). The localization scores are shown on the right (n=20). (F, G) Processing of glycosylation mutants MIG-17(ΔGly7–9)-GFP and MIG-17(ΔGly1–6)-GFP in vitro. Experiments were preformed as in (A). The mature form of MIG-17(ΔGly1–6)-GFP can be detected after a 6-h incubation.
Prodomain processing is essential for MIG-17 activity in controlling DTC migration
Although the actual processing site of MIG-17 is not known, prodomain processing of ADAMTS proteases often occurs in the region of consecutive basic amino acids (Cal et al, 2001; Longpre and Leduc, 2004; Somerville et al, 2004b). To determine the processing site, we individually altered three sets of two consecutive basic residues, Arg162Arg163, Lys202Lys203 and Arg205Lys206, to leucines (Figure 4B). We introduced these mutant constructs into wild-type worms. Although the proteins carrying substitutions at Arg162Arg163 (MIG-17(RR162LL)-GFP) and Arg205Lys206(MIG-17(RK205LL)-GFP) were processed (data not shown), the protein carrying substitutions at Lys202Lys203 (MIG-17(KK202LL)-GFP) was not processed (Figure 4C). Therefore, it is likely that the processing occurs at or near Lys202Lys203. These three constructs localized to the gonad surface (Figure 4D and E) and, when expressed in mig-17 mutants, MIG-17(RR162LL)-GFP and MIG-17(RK205LL)-GFP rescued the DTC migration defects, whereas the rescue was severely impaired in mutants expressing MIG-17(KK202LL)-GFP (Figure 4E). These results indicate that MIG-17 prodomain processing is not required for localization; however, the processing is essential for the function of MIG-17 to control the migration of DTCs. The presence of the prodomain thus seems to inhibit the ability of MIG-17 to promote DTC migration.
We examined whether other mutant constructs that localized or weakly localized to gonads but had very weak rescuing activity could be converted to the mature protease in vitro. MIG-17(D79N)-GFP, MIG-17(ΔDI)-GFP, MIG-17(ΔPLAC)-GFP and MIG-17(G292E)-GFP failed to be converted to the mature form (data not shown). Therefore, these mutations affect prodomain processing. In addition, we also assessed the effect of prodomain processing in the N-glycosylation mutants of MIG-17. MIG-17(ΔGly7–9)-GFP, which rescued mig-17, was processed normally (Figure 4F). The processing of MIG-17(ΔGly1–6)-GFP, which rescued mig-17 when expressed in DTCs but not when expressed in muscles, was very slow, but the mature form accumulated over time (Figure 4G). These results are consistent with the idea that prodomain processing is essential for MIG-17 activity in controlling DTC migration.
To examine whether prodomain processing is required for MIG-17 control of DTC migration in vivo, we tried to change the potential autocatalytic processing site of MIG-17 into the recognition site of furin, a Golgi enzyme that acts in prodomain processing of various proteases. The KLRK residues from 203 to 206 were substituted with RRRR (Figure 4B). When we expressed MIG-17(KLRK203RRRR)-GFP in the muscle cells, it successfully localized to the gonad but failed to rescue mig-17. Surprisingly, however, it efficiently rescued mig-17 when expressed in DTCs using the lag-2 promoter (Figure 4E). Western blot analysis revealed that prodomain processing of the mutant protein was much more efficient than autocatalytic processing although unprocessed proforms still existed (Supplementary Figure S4). We speculate that the prodomain of MIG-17(KLRK203RRRR)-GFP is processed by furin in the Golgi and that the mature MIG-17-GFP can function in DTC migration when it is secreted from DTCs but cannot when secreted from muscles. We therefore suggest that only unprocessed MIG-17-GFP secreted from muscles can localize to the gonad owing to the presence of the prodomain, which cannot be processed autocatalytically. To examine the activity of prodomain mutants, we expressed MIG-17(ΔPro)-GFP, MIG-17(D79N)-GFP and MIG-17(D79N, KLRK203RRRR)-GFP under the control of the lag-2 promoter. We found that all these constructs rescued mig-17 very weakly (Figure 4E). Western blot analysis revealed that MIG-17(D79N)-GFP was not processed, whereas MIG-17(D79N, KLRK203RRRR)-GFP was indeed processed (Supplementary Figure S4), suggesting that the mature form generated from MIG-17(D79N, KLRK203RRRR)-GFP is defective in controlling leader cell migration.
MIG-17 lacking N-glycosylation or the prodomain is secreted almost normally
To analyze quantitatively the secretion efficiencies of wild-type and mutant GFP-fusion proteins, we prepared primary cell cultures from transgenic embryos (Christensen et al, 2002). In the cell culture, muscle cells expressing MIG-17-GFP fusion proteins could be detected with fluorescence microscopy (Figure 5A). Sampling of culture medium after 24, 48 and 72 h in serum-free culture followed by Western blotting revealed gradual accumulation of secreted MIG-17-GFP in pro- and mature forms in the medium (Figure 5B). MIG-17(ΔGly1–9)-GFP similarly accumulated over time, although much more proform than mature form accumulated (Figure 5C). This is consistent with the slow processing observed in MIG-17(ΔGly1–6)-GFP (Figure 4G). MIG-17(ΔPro)-GFP also progressively accumulated in the medium (Figure 5D). Because it was possible that accumulation of MIG-17-GFP fusion proteins in the culture media could be due to leakage of these proteins from dead cells rather than secretion, we examined the primary culture from embryos expressing a membrane-bound construct, MIG-17-TM-GFP. We observed no accumulation of this protein in the medium (Figure 5E). It seems that MIG-17-TM-GFP can be efficiently processed into the mature form while retained at the cell surface. Therefore, the MIG-17-GFP proteins detected in the media were probably secreted. We assessed the secretion efficiency by determining the ratio of MIG-17-GFP proteins found in the medium to those retained in the cells. As shown in Figure 5F, the kinetics of secretion for these three constructs were almost similar except that a slight reduction in secretion was detected in cells expressing MIG-17(ΔGly1–9)-GFP or MIG-17(ΔPro)-GFP after 72 h. These results suggest that deletion of N-glycosylation sites or the prodomain does not significantly affect the secretion of MIG-17-GFP proteins.
Figure 5.
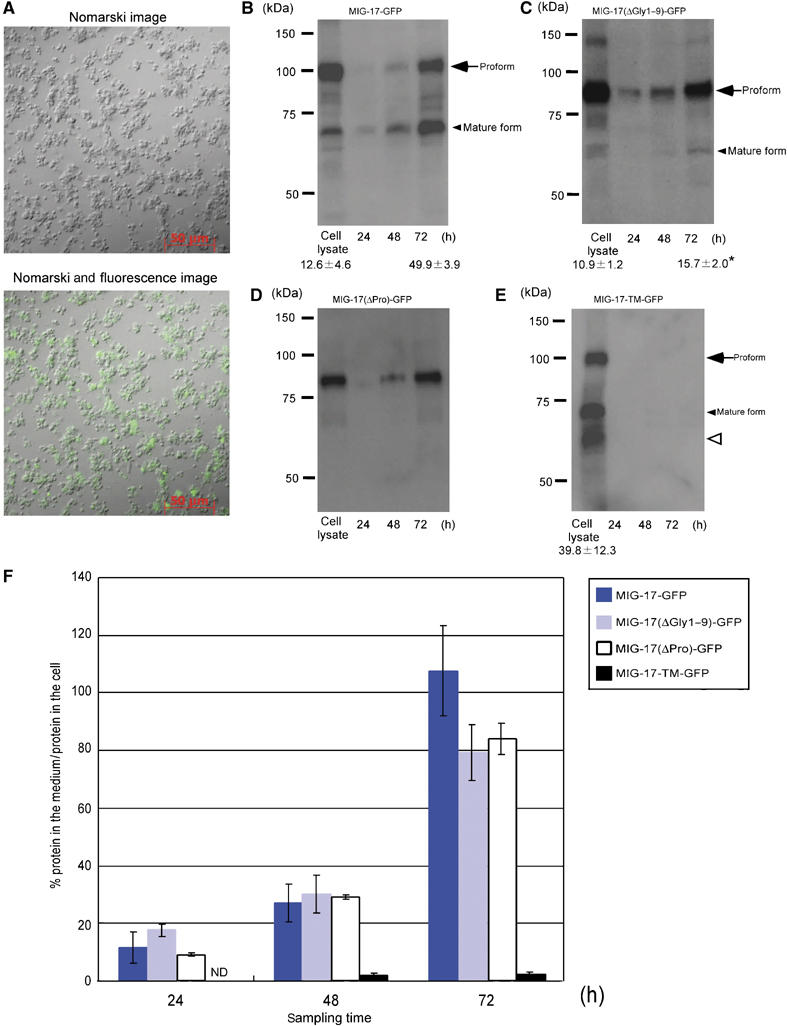
Secretion of MIG-17 from primary culture cells. (A) A typical culture of C. elegans embryonic cells 4 days after plating. Nomarski (upper) and combined Nomarski and fluorescence (lower) images of MIG-17-GFP-expressing primary culture cells. (B–E) Secretion of MIG-17-GFP, MIG-17(ΔGly1–9)-GFP, MIG-17(ΔPro)-GFP and MIG-17-TM-GFP into the media. The culture media were sampled at the indicated time points and cell lysates were prepared from 72-h cultures. The samples were immunoblotted with anti-GFP. The same set of experiments (from culture to immunoblot) was independently performed twice. The ratios of mature form to the sum of pro- and mature forms are shown for some lanes as the mean±s.e.m. The asterisk indicates that the rate of conversion from the proform to mature form was significantly slower in MIG-17(ΔGly1–9)-GFP-expressing cells compared with MIG-17-GFP-expressing cells (Student's t-test; P<0.05). The smeared band migrating slightly faster than the mature form (open arrowhead) in (E) appears to be partially degraded proteins. (F) Kinetics of secretion of MIG-17-GFP fusion proteins. The ratios of secreted MIG-17-GFP fusion protein to MIG-17-GFP fusion protein retained in the cell lysates were plotted against sampling times. For MIG-17-GFP, MIG-17(ΔGly1–9)-GFP and MIG-17-TM-GFP, the intensitiesy of the bands for the pro- and mature forms were summed and used for calculation. The error bars represent the mean±s.e.m.
MIG-17 is secreted and localizes as a proform
To understand the distribution of endogenous MIG-17, we generated an antibody against its prodomain. This anti-MIG-17 prodomain antibody recognized the proform but not the mature form of MIG-17-GFP in a Western blot (Figure 6A and B). When we immunostained cross-sections of wild-type and mig-17 mutant animals, we detected specific signals only in the wild-type specimens. The signal was detected on the surface of the gonad and within the gonad. In addition, we detected somewhat weaker signals at the surfaces of the intestine and hypodermis, as well as in the intestinal lumen (Figure 6C, D and G). Because MIG-17-GFP is secreted from the muscle cells and localized to the gonad surface (Nishiwaki et al, 2000), these results strongly suggest that MIG-17 is secreted as a proform and diffuses to various tissues. It is likely that a portion of the MIG-17 population is internalized in these tissues. The localization of the signal at the intestinal lumen and the apical surface of the hypodermis suggests that some MIG-17 is internalized and transported to these sites. To confirm the observed tissue distribution of wild-type and mutant MIG-17-GFP proteins, we also immunostained cross-sections of transgenic animals using anti-GFP. We found that wild-type MIG-17-GFP localized to the surface of the gonad and the intestine, whereas MIG-17(D79N)-GFP failed to do so (Figure 6E and F).
Figure 6.
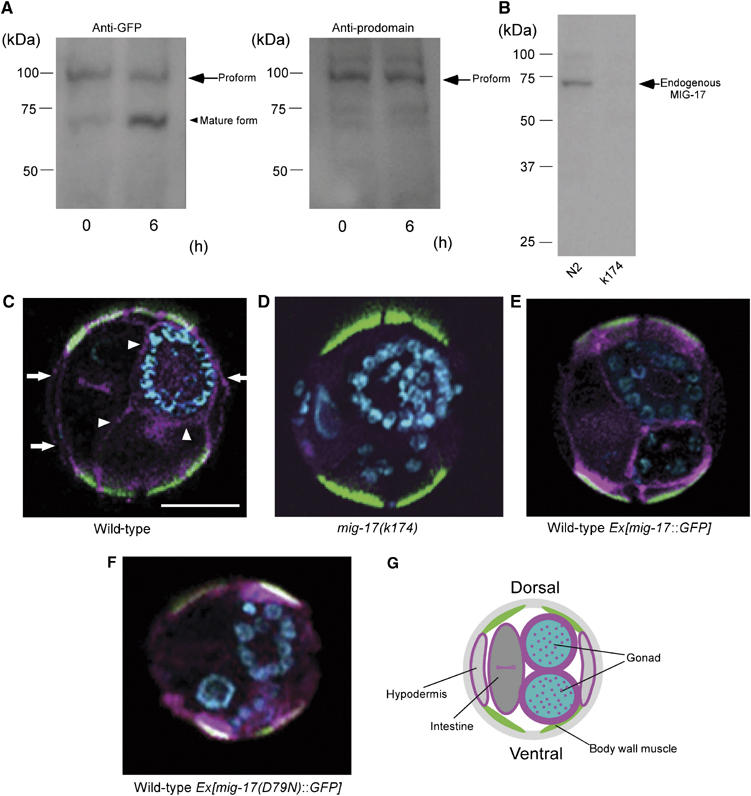
MIG-17 is secreted and localizes to the gonad as a proform. (A) The antibody against the MIG-17 prodomain recognizes the MIG-17 proform but not the mature form. Extracts from wild-type worms expressing MIG-17-GFP were incubated for 0 and 6 h at room temperature and analyzed. The proform and the mature form were recognized by anti-GFP (left panel), but only the proform was detected by anti-MIG-17 prodomain (right panel). The faint bands in the right panel seem to be nonspecific binding of anti-prodomain because these also appeared in the immunoblot of the mig-17 mutant extract after long exposure. (B) Detection of endogenous MIG-17. About 200 μg of TCA-precipitated lysate from wild-type or mig-17 mutant animals was immunoblotted with the anti-MIG-17 prodomain. (C–F) Immunohistochemistry using anti-MIG-17 prodomain (C, D) and anti-GFP (E, F). Cross-sections of hermaphrodites for wild-type (C), mig-17 mutant (D) and wild-type carrying mig-17∷GFP (E) or mig-17(D79N)∷GFP (F) were stained. Merged images of the specimens stained with antibodies (pink), fluorescein–phalloidin (green) and DAPI (blue) are shown (dorsal to the top). Fluorescein–phalloidin stains actin filaments in dorsal and ventral body wall muscles. Arrowheads indicate prodomain localization in the gonadal basement, whereas arrows indicate localization in the hypodermal basement. Scale bar, 20 μm. (G) Schematic presentation of a wild-type cross-section depicting anti-prodomain signals shown in pink.
Discussion
Using various mutant constructs, we analyzed N-glycosylation, protein domains and specific amino-acid residues of MIG-17 to understand their functions in MIG-17 gonadal localization and prodomain processing. These results are summarized in Figure 7A. N-glycosylation of the prodomain is essential for gonadal localization, but is not essential for processing, as a prodomain lacking N-glycosylation was slowly processed in vitro. Residue D79, which is in the prodomain, is essential for both localization and processing. The DI domain and the active site residue E303 are essential for processing but not for localization, as the corresponding mutant proteins localized weakly to the gonad. The PLAC domain, K202, K203 and G292 are also essential for processing but dispensable for localization.
Figure 7.
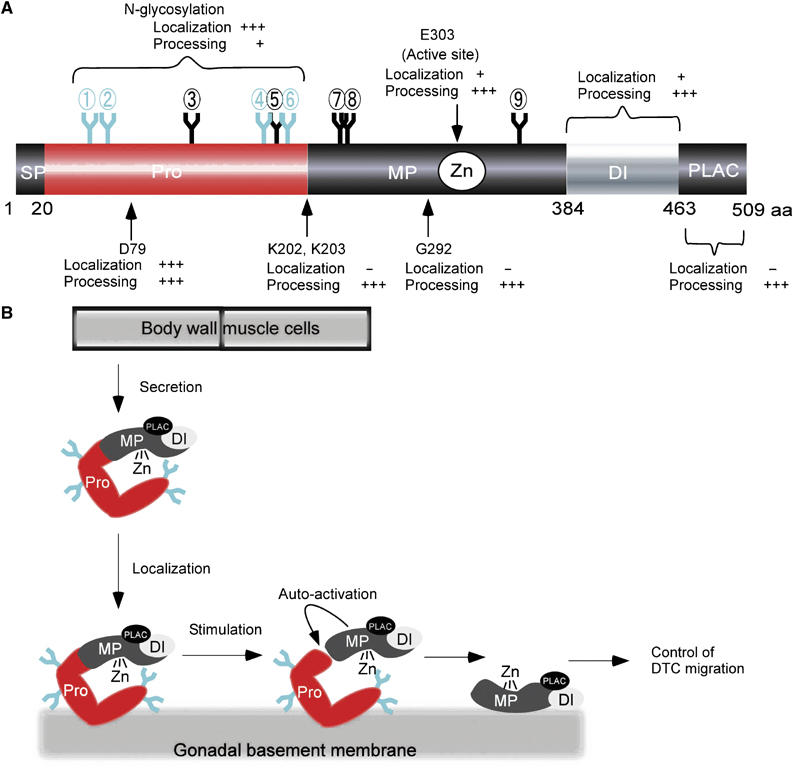
Expected function of the MIG-17 modules and a model of MIG-17 action in gonad development. (A) Summary of experiments using various mutant constructs. Requirement of each module for localization and processing of MIG-17 is indicated: +++, + and − indicate essential, partial and dispensable, respectively. N-glycosylations that are especially important for prodomain-dependent targeting are shown in light blue. (B) Model for MIG-17 action in gonad development. MIG-17 is secreted from muscle cells as a proform and then localizes to the gonadal basement membrane. The glycosylated prodomain plays a critical role in localization. Responding to an unknown stimulus, MIG-17 is probably activated by its autocatalytic activity. The MIG-17 mature form is anchored by an unknown receptor in the gonadal basement membrane and acts in DTC migration. The MP or DI domain might be involved in this anchoring function.
Prodomain targeting of MIG-17
We found that the prodomain of MIG-17 plays an important role in targeting MIG-17 to the gonad. This was unexpected because the current paradigm is that the prodomains of ADAMTS proteases are required for maintenance of enzymatic latency, polypeptide folding or secretion. Although the prodomain of MIG-17 is important for the targeting function, the prodomain alone, even if fully glycosylated, cannot localize GFP to the gonad surface. Thus, the prodomain appears to act together with one or more other domains of MIG-17 to achieve proper localization. The observation that mutations in the MP domain (E303Q) or in the DI domain (ΔDI) also partially affected targeting further suggests this possibility. The prodomain is thought to fold back toward the MP domain and inhibit its catalytic activity. The prodomain of MIG-17 may fold back and interact with both MP and DI domains, and such an interaction may be necessary for the prodomain to achieve a conformation compatible with adherence to the gonadal basement membrane. The N-glycans of the MIG-17 prodomain likely contribute to this interdomain interaction. Intriguingly, 14 out of the 19 human ADAMTS proteins have potential N-glycosylation sites in their prodomains (unpublished data), which may also act in proper folding of the prodomain and/or interaction with the MP and DI domains.
Although many ADAMTSs are activated by proteolytic removal of the prodomain within the secretory pathway by serine proteases such as furin (Cal et al, 2001; Longpre and Leduc, 2004; Somerville et al, 2004b), there are cases of ADAMTS enzymes secreted as proforms. For example, ADAMTS-1 and ADAMTS-10 are secreted as both pro- and mature forms. Although both forms of ADAMTS-1 localize to the ECM, only the ADAMTS-10 proform is able to do so (Rodriguez-Manzaneque et al, 2000; Somerville et al, 2004a). The processing of ADAMTS-9 and at least part of the final processing event of ADAMTS-7B occur at the cell surface (Somerville et al, 2004b; Koo et al, 2006). It may be possible that, in addition to MIG-17, some other ADAMTSs have prodomains that function in tissue-specific targeting.
Activation of MIG-17
Transgenic MIG-17-GFP molecules were mostly detected as proforms in Western blots. However, mature forms were detected in cell extracts in vitro. Although most of the mammalian ADAMTS proteases are activated by prodomain removal by other processing enzymes, it was recently shown that ADAMTS-4 can also be activated by an autocatalytic activity (Tortorella et al, 2005). MIG-17 is similar in this respect. Although the processing site remains to be determined, processing at or near Lys202Lys203 is consistent with the molecular size of the mature form of MIG-17. The amino-acid substitution of Lys202Lys203 to Leu202Leu203 perturbed prodomain processing without affecting targeting function. A similar effect was also detected in the G292E mutant and upon deletion of the PLAC domain, suggesting that these mutations specifically affect removal of the prodomain.
The substitution of a small, uncharged glycine with a large, charged glutamate in the G292E mutant may affect the conformation of the MP domain. One possibility is that the PLAC domain may normally interact with the region containing G292 to activate or specify the processing activity. Our data show that the D79N mutation strongly affects both the localization and processing of MIG-17. Also the mature protein generated from MIG-17(D79N, KLRK203RRRR)-GFP was not functional even when expressed in DTCs. Thus, this mutation may alter the conformation of the prodomain so severely that it loses its affinity for the gonad and concomitantly blocks proper folding of the MIG-17 polypeptide, resulting in loss of enzymatic activity. We also found that MIG-17 lacking prodomain glycosylation is processed very slowly compared to the wild type. This may suggest the involvement of the glycans of the prodomain in protein folding.
Model for MIG-17 action in the control of DTC migration
Based on our findings, we propose a model for MIG-17-mediated control of DTC migration (Figure 7B). MIG-17 is secreted as a proform from the body wall muscle cells to the body cavity. The prodomain probably masks the active site to maintain enzymatic latency. Pro-MIG-17 localizes to the gonadal basement membrane in a prodomain-dependent manner. N-glycosylation at residues 52, 65, 172 and 189 is especially important for proper localization of the protein. After localization, MIG-17 may be converted to the mature form by autocatalytic removal of its prodomain. Because MIG-17 functions on the surface of DTCs, it follows that activated MIG-17 must have affinity for the gonadal basement membrane even without the prodomain. The MP or DI domain may confer such affinity. Activated mature MIG-17 probably proteolyzes its substrate, which may be in the gonadal or body wall basement membrane, to direct DTC migration.
MIG-17 localizes to the basement membranes of various tissues
Using an anti-MIG-17 prodomain antibody, we detected MIG-17 signal not only in the gonadal basement membrane, but also in basement membranes of the intestine and hypodermis. These results suggest that MIG-17 accumulates as the proform in various basement membranes. Although the signal was somewhat stronger in the gonadal basement membrane than in other basement membranes, these observations contrast with what we observed using anti-GFP. We observed that MIG-17-GFP localizes much more strongly to the gonadal basement membrane than other basement membranes (Figure 6E; Kubota et al, 2006). It may be possible that the GFP tag partially inhibited sequestration of MIG-17-GFP to tissues other than the gonad. Alternatively, the capacity to accommodate MIG-17-GFP accumulation might be higher in the gonadal basement membrane than in other tissues.
Although MIG-17 expression in DTCs (either in secreted or membrane-bound forms) is sufficient to promote normal DTC migration in mig-17 mutants, we sometimes detected small bulges in the gonad arms in mig-17 mutants regardless of whether the MIG-17 construct was expressed in the DTCs (unpublished data). Thus, it is possible that MIG-17 activity is also required in the gonad or other tissues to fine-tune gonad arm development. We generally observed that mutant constructs rescued the DTC migration defects of the mig-17(k174) null mutant more strongly in the anterior DTCs than in the posterior DTCs despite the fact that MIG-17-GFP fusion proteins accumulated similarly. This is probably because the level of MIG-17 activity required for correct DTC migration is higher in the posterior than in the anterior DTCs. The fact that the weak mig-17(k113) allele has a stronger effect on posterior DTC migration also suggests this possibility (Nishiwaki, 1999).
Conclusions
The ADAMTS proteins were first identified only 10 years ago (Kuno et al, 1997). Nevertheless, they have received considerable attention because defects in some of these proteins have been linked to hereditary diseases related to disorders in ECMs (Levy et al, 2001; Colige et al, 2004; Dagoneau et al, 2004). ADAMTSs play important roles in promoting development and homeostasis. However, how and when these ADAMTSs are recruited to their target tissues, as well as when and where they are activated, remain to be elucidated. In this study, using C. elegans gonadogenesis as a model system, we showed the spatiotemporal action of the MIG-17 ADAMTS in its control of DTC migration. Our findings present the first clear picture of the molecular behavior of an ADAMTS protease in vivo and further our understanding of ADAMTS proteases that function in organ morphogenesis during animal development.
Materials and methods
Strains and culture conditions
Culture and handling of C. elegans were as described (Brenner, 1974). The following strains were used: N2 (wild-type), mig-17(k174) (Nishiwaki et al, 2000) and unc-119(e2498) (Maduro and Pilgrim, 1995). mig-17(k174) is a nonsense mutation that changes the codon for amino acid 111 in the prodomain from a glutamine (CAA) to a stop codon (TAA).
Microscopy
Nomarski and fluorescence microscopy were performed as described (Kubota et al, 2006). The localization of wild-type and mutant MIG-17-GFP proteins was analyzed with a laser-scanning confocal microscope (LSM5 PASCAL ver. 3.2; Zeiss).
Plasmid construction
The plasmid constructs mig-17∷GFP, mig-17(ΔPro)∷GFP, mig-17(ΔDI)∷GFP, mig-17(ΔPLAC)∷GFP and mig-17(ΔGly1–9)∷GFP were described previously (Nishiwaki et al, 2000, 2004). The mig-17∷GFP construct contains a 1142-bp region upstream of the initiation codon and the 2533-bp coding region of mig-17. The mig-17∷GFP construct was used for site-directed and deletion mutagenesis. To create site-directed mutations, the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used with primers containing appropriate nucleotide changes. To create deletion mutants, the fragments of mig-17∷GFP were amplified with appropriate primers using pfu Turbo polymerase (Stratagene), and the resulting fragments were ligated. All mutant constructs were confirmed by DNA sequencing. For construction of lag-2p∷mig-17∷GFP, the mig-17∷GFP coding region was placed downstream of the lag-2 promoter (Henderson et al, 1994). To create mig-17∷TM∷GFP, an oligonucleotide encoding the transmembrane region of INA-1 (residues 1086–1110) was inserted into the mig-17∷GFP plasmid.
Production of transgenic animals
Young adult hermaphrodites were used for microinjection, and heritable transgenic lines were obtained from offspring (Mello et al, 1991). The DNA mixtures were first injected into unc-119(e2498) animals, and the transgenic array was transferred to mig-17(k174); unc-119(e2498) animals by mating. Wild-type and mutant mig-17∷GFP plasmid constructs were injected at 100 μg/ml with 25 μg/ml pBSII KS(−) and 25 μg/ml unc-119 plasmid, pDP#MM016B (Maduro and Pilgrim, 1995). Wild-type and mutant lag-2p∷mig-17∷GFP constructs were injected at 20 μg/ml with 105 μg/ml pBSII KS(−) and 25 μg/ml pDP#MM016B. non-Unc transgenic animals were scored. For each experiment, more than two independent transgenic lines with similar phenotypes were obtained. The data for DTC migration of representative lines are shown in figures. We also produced transgenic lines by injecting mig-17∷GFP or lag-2p∷mig-17∷GFP at 1 μg/ml with 124 μg/ml pBSII KS(−) and 25 μg/ml pDP#MM016B. These low-copy transgenic arrays rescued mig-17 with an efficiency similar to that observed in this work; 92 and 95% of anterior and 88 and 93% of posterior DTCs (n=60 for each experiment) showed normal migration in mig-17∷GFP and lag-2p∷mig-17∷GFP transgenics, respectively. Therefore, it seems that difference in the levels of MIG-17-GFP fusion proteins expressed from the arrays does not affect the rescuing activity, although GFP expression could not be detected in low-copy arrays.
Preparation of anti-MIG-17 prodomain antibody
To produce a MIG-17 prodomain tagged with histidines, the coding sequence for residues 21–164 of MIG-17 was inserted into the pET-19b fusion protein vector (Novagen). The tagged protein was isolated from Escherichia coli. The rabbit antiserum against the prodomain was purified on a column fixed with the histidine-tagged prodomain. The appropriate concentrations of the anti-prodomain antibody used in Western blots and in situ staining were determined by serial dilution experiments.
Western blot analysis
Mixed populations of worms grown at 20°C were collected and disrupted by glass beads using Micro Smash MS-100 (Tomy) in 100 mM Tris–HCl, pH 7.4; 150 mM NaCl and 1% (w/v) Triton X-100. After disruption, the lysates were rotated at 4°C for 30 min. The lysates were centrifuged at 15 000 r.p.m. (17 400 g) for 20 min at 4°C. The supernatants were boiled in SDS–PAGE sample buffer, separated on 7.5% SDS–polyacrylamide gels and then transferred to nitrocellulose membranes. The membranes were immunoblotted with rabbit anti-GFP (2 μg/ml; Molecular Probes) or with anti-prodomain (1.4 μg/ml) at room temperature for 1 h followed by incubation with peroxidase-conjugated anti-rabbit IgG (0.4 μg/ml; Amersham) at room temperature for 1 h.
In situ staining
Frozen sections were prepared as described (Kubota et al, 2006). After blocking the sections with 1% BSA in PBS, samples were incubated with rabbit anti-prodomain (2 μg/ml) or rabbit anti-GFP (Molecular Probes) for 2 h, TRITC donkey anti-rabbit IgG (Jackson) for 1 h, fluorescein–phalloidin (10 U/ml; Molecular Probes) for 1 h and DAPI (2 μg/ml; Wako) for 10 min at room temperature.
Quantitative analysis of secretion of MIG-17-GFP fusions
The method for primary culture of C. elegans embryonic cells was described by Christensen et al (2002). Embryos transgenic for mig-17∷GFP, mig-17(ΔGly1–9)∷GFP, mig-17(ΔPro)∷GFP and mig-17∷TM∷GFP were used. Briefly, cells were seeded at a density of 2352±216 × 102 (n=10) cells/cm2 in poly-lysine-coated one-well chamber slides (Iwaki) each containing 3 ml of L-15 medium supplemented with 10% fetal bovine serum and antibiotics. Cultures were maintained at 20°C in a humidified incubator. Twenty-four hours later, the culture medium was changed to serum-free L-15 medium with antibiotics and the incubation was continued at 20°C. Each 0.5 ml of medium was sampled 24, 48 and 72 h after culturing in serum-free medium and proteins were precipitated by TCA. Lysates of cells were prepared from 72-h cultures. Twenty micrograms of protein samples was subjected to Western blot with anti-GFP. The intensity of bands was quantified using a lumino-image analyzer LAS-1000 mini equipped with software Image Gauge Ver. 3.46 (Fuji Film).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Data
Acknowledgments
We thank Andy Fire for GFP fusion vectors and Yukihiko Kubota, Katsuyuki Tamai and Norio Suzuki for critical reading of the manuscript.
References
- Baum PD, Garriga G (1997) Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron 19: 51–62 [DOI] [PubMed] [Google Scholar]
- Blelloch R, Anna-Arriola SS, Gao D, Li Y, Hodgkin J, Kimble J (1999) The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev Biol 216: 382–393 [DOI] [PubMed] [Google Scholar]
- Blelloch R, Kimble J (1999) Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature 399: 586–590 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cal S, Arguelles JM, Fernandez PL, Lopez-Otin C (2001) Identification, characterization, and intracellular processing of ADAM-TS12, a novel human disintegrin with a complex structural organization involving multiple thrombospondin-1 repeats. J Biol Chem 276: 17932–17940 [DOI] [PubMed] [Google Scholar]
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM III, Strange K (2002) A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron 33: 503–514 [DOI] [PubMed] [Google Scholar]
- Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM (1997) cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci USA 94: 2374–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colige A, Nuytinck L, Hausser I, van Essen AJ, Thiry M, Herens C, Ades LC, Malfait F, Paepe AD, Franck P, Wolff G, Oosterwijk JC, Smitt JH, Lapiere CM, Nusgens BV (2004) Novel types of mutation responsible for the dermatosparactic type of Ehlers–Danlos syndrome (type VIIC) and common polymorphisms in the ADAMTS2 gene. J Invest Dermatol 123: 656–663 [DOI] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V (2004) ADAMTS10 mutations in autosomal recessive Weill–Marchesani syndrome. Am J Hum Genet 75: 801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Greenwald I (2001a) Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics 159: 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares H, Greenwald I (2001b) Regulation of endocytosis by CUP-5, the Caenorhabditis elegans mucolipin-1 homolog. Nat Genet 28: 64–68 [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS (2001) Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem 276: 31502–31509 [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Lambie EJ, Kimble J (1994) lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development 120: 2913–2924 [DOI] [PubMed] [Google Scholar]
- Ihara S, Miyoshi E, Nakahara S, Sakiyama H, Ihara H, Akinaga A, Honke K, Dickson RB, Lin CY, Taniguchi N (2004) Addition of β1–6 GlcNAc branching to the oligosaccharide attached to Asn 772 in the serine protease domain of matriptase plays a pivotal role in its stability and resistance against trypsin. Glycobiology 14: 139–146 [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG (1981) On the control of germ cell development in Caenorhabditis elegans. Dev Biol 81: 208–219 [DOI] [PubMed] [Google Scholar]
- Koo BH, Longpre JM, Somerville RP, Alexander JP, Leduc R, Apte SS (2006) Cell-surface processing of pro-ADAMTS9 by furin. J Biol Chem 281: 12485–12494 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sano M, Goda S, Suzuki N, Nishiwaki K (2006) The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development 133: 263–273 [DOI] [PubMed] [Google Scholar]
- Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K (1997) Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem 272: 556–562 [DOI] [PubMed] [Google Scholar]
- Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K (2000) ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett 478: 241–245 [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD Jr, Ginsburg D, Tsai HM (2001) Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413: 488–494 [DOI] [PubMed] [Google Scholar]
- Longpre JM, Leduc R (2004) Identification of prodomain determinants involved in ADAMTS-1 biosynthesis. J Biol Chem 279: 33237–33245 [DOI] [PubMed] [Google Scholar]
- Maduro M, Pilgrim D (1995) Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, Hockfield S (2000) Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem 275: 22695–22703 [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K (1999) Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics 152: 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K, Hisamoto N, Matsumoto K (2000) A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288: 2205–2208 [DOI] [PubMed] [Google Scholar]
- Nishiwaki K, Kubota Y, Chigira Y, Roy SK, Suzuki M, Schvarzstein M, Jigami Y, Hisamoto N, Matsumoto K (2004) An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat Cell Biol 6: 31–37 [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR (2005) The ADAMTS metalloproteinases. Biochem J 386: 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML (2000) Characterization of METH-1/ADAMTS1 processing reveals two distinct active forms. J Biol Chem 275: 33471–33479 [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW (2001) Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem 276: 13372–13378 [DOI] [PubMed] [Google Scholar]
- Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M, Skelton L, Jockusch H, Bartsch JW (2002) The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem 277: 48210–48219 [DOI] [PubMed] [Google Scholar]
- Somerville RP, Jungers KA, Apte SS (2004a) Discovery and characterization of a novel, widely expressed metalloprotease, ADAMTS10, and its proteolytic activation. J Biol Chem 279: 51208–51217 [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Apel ED, Lewis RM, Wang LW, Sanes JR, Leduc R, Apte SS (2004b) ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem 279: 35159–35175 [DOI] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS (2003) Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem 278: 9503–9513 [DOI] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E (2000) The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem 275: 25791–25797 [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Arner EC, Hills R, Gormley J, Fok K, Pegg L, Munie G, Malfait AM (2005) ADAMTS-4 (aggrecanase-1): N-terminal activation mechanisms. Arch Biochem Biophys 444: 34–44 [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC Jr, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC (1999) Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science 284: 1664–1666 [DOI] [PubMed] [Google Scholar]
- Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS (2003) Transforming growth factor-β induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem 278: 19549–19557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Data


