Abstract
The recent World Health Organization (WHO) classification of hematopoietic and lymphoid tissue tumors represents the first worldwide consensus classification of these malignancies. However, the applicability of this classification to a representative number of hepatic lymphomas in liver biopsy specimens has not yet been investigated. The frequency and infiltration pattern of a series of 205 liver biopsies with lymphoma manifestations was analyzed with the aid of immunohistochemical and molecular pathological analyses. Diffuse large B-cell lymphoma (DLBCL) was by far the most frequent entity, comprising 45% of the cases analyzed. Using a previously published immunohistochemical algorithm, 35% of 80 DLBCL were assigned to a germinal center B-cell-like (GCB) and 65% to a non-GCB group. Most B-cell lymphoma entities involving the liver revealed a characteristic infiltration pattern. Diagnostically challenging entities were T-cell-rich B-cell lymphomas, anaplastic large cell lymphomas and peripheral T-cell lymphomas, which frequently required additional molecular clonality assessment. Overall, the percentage of T-cell lymphomas in the liver (12%) was higher as compared to other extranodal sites except for the skin and the small intestine. This study provides relevant data on the distribution of hepatic lymphomas and demonstrates the applicability of the WHO classification proposing a diagnostic algorithm for liver biopsies.
Keywords: Lymphoma, Liver, Differential diagnosis, Immunohistochemistry, PCR
Introduction
The liver is most commonly involved in non-Hodgkin lymphomas (NHL) next to lymph nodes, spleen, and bone marrow. In the vast majority of cases hepatic involvement reflects secondary dissemination in advanced disease [15, 18] rather than a primary site according to the definition of Caccamo and coworkers [5]. Histopathological analysis of liver biopsy may be required in patients with an established diagnosis of malignant lymphoma to differentiate lymphoma manifestation from other causes of hepatic dysfunction or to clarify elevated transaminases such as toxic damage because of chemotherapy or other medications. On the other hand, a biopsy may reveal a previously unknown lymphoma during the exploration of a solid hepatic mass or after measuring elevated liver enzyme serology [12]. Occasionally, a malignant lymphoma can be detectable in the setting of other liver diseases such as chronic hepatitis B [20, 22], chronic hepatitis C [2, 3, 7, 21, 25], or primary biliary cirrhosis (PBC) [24].
The goals of the present study were to test the feasibility of lymphoma subtyping according to the World Health Organisation (WHO) classification of tumors of hematopoietic and lymphoid tissues [16] in liver biopsy specimens and to describe the frequencies by which the different entities occur. For this purpose, a series of 205 liver biopsies with a diagnosis of malignant lymphoma were analyzed retrospectively with a specific focus on the histopathology, especially infiltration patterns, which may ultimately allow the use of diagnostic algorithms for subtyping of lymphomas in liver biopsies. This is an important addition in relation to previous studies, which have mainly described the frequency of liver involvement in autopsy material of patients with leukemia or lymphoma [8, 13, 26, 28, 29] differentiating only between low-grade and high-grade NHL [26] or having performed a categorization according to the outdated Kiel classification [29].
Overall, this study represents the largest series on hepatic lymphomas to date and due to its restriction to bioptic material reflects the primary diagnostic situation.
Materials and methods
All cases of hepatic lymphoma involvement diagnosed by liver biopsy during the years 1994–2003 were retrieved from the archives of the Institute of Pathology, Campus Benjamin Franklin, Charité-University Medicine Berlin and the Institute of Pathology, University of Cologne, Germany. The series comprised a total of 205 cases with 135 cases from Berlin and 70 cases from Cologne. All cases were reevaluated and reclassified independently by three pathologists (C.L., T.L., H.S.) according to the WHO classification [16]. For cases in which the diagnosis was not unanimous, a final consensus diagnosis was reached after further immunophenotyping, molecular analyses, and final consultation. In six cases initially considered “suspicious” for lymphoma, a final diagnosis of lymphoma was made after reevaluation and additional analyses. Nine liver biopsies with insufficient material for additional immunohistochemical or molecular analyses were excluded beforehand, as well as five cases where T-cell receptor (TCR) polymerase chain reaction (PCR) had revealed a polyclonal or oligoclonal rearrangement pattern without a reproducible dominant PCR product.
Overall, 32/205 (16%) cases were reclassified, including eight “low-grade” [5× B-CLL, 2× follicular lymphomas (FL), 1× marginal zone lymphoma (MZL)] and 20 “high-grade” [19× DLBCL, 1× Burkitt lymphoma (BL)] B-cell NHL that were assigned to a specific WHO lymphoma category as well as four cases with a change in the final diagnosis [BL, classical Hodgkin lymphoma (cHL), T-cell-rich B-cell lymphoma (TCRBCL), lymphoplasmacytic lymphoma (LPL) to DLBCL, anaplastic large cell lymphoma (ALCL), peripheral T-cell lymphoma (pTCL) and B-CLL, respectively].
The analyses of the infiltration pattern were based on the assessment of the hepatic architecture. Three main patterns were distinguished: Infiltrates for which an exclusive or predominant association to portal tracts was evident were recorded as portal infiltrates. The second pattern consisted of lymphoma infiltrates which showed a coherent growth pattern thus predominantly resulting in the replacement of the acinar structures. These infiltrates were designated as a nodular growth pattern. Finally, infiltrates that showed prominent intrasinusoidal dispersion of the lymphoma cells were recorded as a sinusoidal growth pattern. Additionally, the density of the infiltrate was semiquantitatively assessed. The presence of scattered neoplastic cells in a background rich in reactive bystander cells (e.g., nonneoplastic T-cells and/or macrophages in T-cell rich B-cell lymphoma) was defined as a loose infiltration pattern, whereas the appearance of coherently appearing neoplastic B- or T-cells was referred to as a dense infiltrate.
According to clinical information available, in 76/205 (37%) of the cases the diagnosis of a lymphoma had been previously established from extrahepatic biopsies or peripheral blood indicating secondary involvement of the liver. In four of these cases with known low-grade lymphoma (2× B-CLL, 2× FL) liver biopsy revealed transformation to a high-grade lymphoma (DLBCL). In the remaining cases, the biopsy obtained from the liver represented the site of primary diagnosis. In 26 (13%) patients, additional biopsies from extrahepatic sites were available (13 bone marrow biopsies, 10 lymph node biopsies, and three spleen biopsies). Furthermore, in a few cases clinical data regarding a potential predisposing condition for a primary hepatic lymphoma was available: chronic hepatitis C was reported in four patients (3× DLBCL, 1× B-CLL), HIV infection was present in three patients (2× DLBCL, 1× BL), and one marginal zone lymphoma occurred in a patient after liver transplantation.
The frequency of the various lymphoma entities diagnosed in liver biopsy specimen was compared to their frequency and distribution in other extranodal sites (cases from the Consultation and Reference Center for Lymph Node Pathology and Hematopathology, Berlin).
Immunohistochemistry
For immunostaining, 4 μm thick sections were cut, deparaffinized, and subjected to heat-induced epitope retrieval before incubation with antibodies. For this purpose, sections were immersed either in sodium citrate buffer at pH 6.0 or, alternatively, in ethylenediamintetraacetic acid (EDTA) at pH 8.0 and heated in a high-pressure cooker. After cooking, the slides were rinsed in running water, washed with Tris-buffered saline, pH 7.4, and incubated with the respective primary antibodies. All primary antibodies employed are listed in Table 1. With the exception of immunoglobulin detection, in which the streptavidin–biotin peroxidase method was applied [14], labeling was carried out using the alkaline–phosphatase/anti-alkaline–phosphatase complex method [6]. Alkaline phosphatase was developed using Fast Red as the chromogen, whereas peroxidase was visualized with diaminobenzidine chromogen as the substrate.
Table 1.
Antibodies used in this study
| Antibody | Clone | Antigen retrieval | Dilution | Source |
|---|---|---|---|---|
| ALK1 | ALK1 | Citrate | 1:20 | Dako |
| BCL2 | 124 | Citrate | 1:25 | Dako |
| BCL6 | 594 | Citrate | 1:25 | Dako |
| CD2 | AB75 | Citrate | 1:50 | Novocastra |
| CD3 | F7.2.38 | Citrate | 1:100 | Dako |
| CD4 | 1F6 | Citrate | 1:25 | Novocastra |
| CD5 | 4C7 | Citrate | 1:25 | Novocastra |
| CD7 | CD7-272 | EDTA | 1:50 | Novocastra |
| CD8 | C8/144B | Citrate | 1:100 | Dako |
| CD10 | 56C6 | Citrate | 1:25 | Novocastra |
| CD15 | C3D1 | Citrate | 1:20 | Dako |
| CD20 | L26 | Citrate | 1:50 | Dako |
| CD21 | 1F8 | Protease | 1:50 | Dako |
| CD23 | 1B12 | Citrate | 1:20 | Novocastra |
| CD27 | 137B4 | EDTA | 1:100 | Novocastra |
| CD30 | BerH2 | Citrate | 1:50 | Dako |
| CD43 | DF-T1 | Citrate | 1:50 | Dako |
| CD68 | PG-M1 | Citrate | 1:50 | Dako |
| CD79a | JCB117 | Citrate | 1:100 | Dako |
| CD138 | B-B4 | Citrate | 1:10 | Serotec |
| Cyclin D1 | P2D11F11 | Citrate | 1:50 | Novocastra |
| EBV-LMP | CS1-4 | Citrate | 1:100 | Dako |
| α-heavychain | Rabbit polyclonal | Citrate | 1:40 000 | Dako |
| γ-heavy chain | Rabbit polyclonal | Citrate | 1:30 000 | Dako |
| δ-heavy chain | Rabbit polyclonal | Citrate | 1:2000 | Dako |
| μ-heavy chain | Rabbit polyclonal | Citrate | 1:2000 | Dako |
| Ki-67 | MIB-1 | Citrate | 1:2000 | Dako |
| κ-light chain | Rabbit polyclonal | Citrate | 1:100 000 | Dako |
| λ-light chain | HP6054 | Citrate | 1:16 000 | Dako |
| MUM1/IRF4 | MUM1p | Citrate | 1:20 | Generously provided by Prof. B. Falini, Perugia, Italy |
| Pax-5 | 24 | Citrate | 1:10 | Transduction Laboratories |
| TIA-1 | 2G9 | Citrate | 1:500 | Coulter |
Molecular pathology analyses
Additional molecular pathology analyses were selectively performed for those cases in which immunoprofiling alone was not sufficient to establish a definite diagnosis of a malignant lymphoma.
For this purpose, DNA was extracted after dewaxing 20-μm-thick paraffin sections with QIAEX (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. TCR-gamma rearrangements were analyzed using four different primer combinations (JGT1/2, JGT3, BioMed-2 Set A and Set B). The primer combinations JGT1/2 und JGT3 suitable to amplify the most frequent TCR-gamma rearrangements were used in a two-step seminested PCR. The BioMed-2 primer sets A and B able to detect all possible TCR-gamma rearrangements were performed as single round PCRs. The detailed cycling conditions (50 rounds of amplification for BioMed-2 set A and set B; two-time 35 cycles for primer combinations JGT1/2 and JGT3) for all PCRs have been described in detail elsewhere [9, 27].
Amplification of rearranged IgH genes was independently performed at least twice per case employing three different framework primer sets (BioMed-2 FR1, FR2, and FR3) separately in conjunction with a JH primer (JH22). PCR conditions consisted of 50 cycles of denaturation (95°C, 15 s), primer annealing (60°C, 40 s), and elongation (72°C, 45 s), and the reaction mixture contained 1.5 mM MgCl2, 0.8 mM deoxyribonucleotide triphosphates (dNTPs), 70 pmol VH primers, 30 pmol JH22 primer, and 2 U of AmpliTaq Gold polymerase (Applied Biosystems, Weiterstadt, Germany).
Results
Characteristics of hepatic lymphomas
Almost 90% (182/205) of the hepatic lymphomas belonged to the group of NHL, whereas only 23 of the 205 cases were cHL. Regarding NHL, 86% (157/182) were of B-cell and 14% (25/182) of T-cell origin. Overall, diffuse large B-cell lymphoma (DLBCL) was the most common type [51% (93/182) of all B-NHL analyzed] with TCRBCL—a morphologic variant of DLBCL—comprising 14% (13/93) of all DLBCL. According to the algorithm of Hans et al. [11] a predominance of DLBCL of non-GCB (germinal center B-cell-like) type (52/80; 65%) compared to the DLBCL of GCB type (28/80; 35%) was found (Fig. 1a–d,f). Only 4 out of 80 cases (5%) corresponded to the group of CD5-positive DLBCL (Fig. 1e).
Fig. 1.
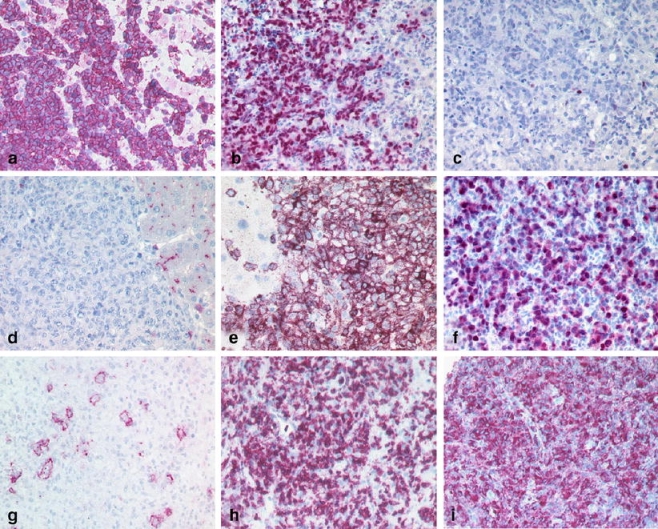
Nodular pattern: DLBCL of GCB group positive for CD10 (a) and BCL6 (b) and negative for IRF4/MUM1 (few scattered plasma cells serve as positive intrinsic control; c). CD5 positive DLBCL of ABC type negative for CD10 (positive bile canaliculi serve as intrinsic control; d), positive for CD5 (e) and IRF4/MUM1 (f). TCRBCL with less than 10% large CD20 positive neoplastic B-cells (g) associated with numerous CD3 positive T-cells (h) and CD68 positive histiocytes (i)
In all lymphoma subtypes, men were affected more often, except for FL, which occurred with equal frequencies in both sexes. The male predominance was most striking in lymphomas of T-cell origin, in which ALCL and hepatosplenic T-cell lymphoma (HSTCL) were encountered exclusively in men. Additional molecular analyses were essential to confirm the suspected diagnosis of a lymphoma in 5 out of 19 T-cell lymphomas (26%) and 3 out of 116 (3%) B-NHLs (Fig. 2).
Fig. 2.

Size fragment analyses (GeneScan) of the amplificates after TCR-gamma PCR (primer combination JGT1/2). Fluorescence-labelled PCR products (blue lines) were separated on capillary electrophoresis system (Applied Biosystems, Model 310A) in parallel to a size standard (red line). The dominant PCR product (arrow) indicating the presence of a clonal T-cell population was reproducibly detectable in addition to a moderate oligo-/polyclonal T-cell background
The relative frequencies of the lymphoma subtypes, the available patient data and the infiltration patterns are summarized in Table 2.
Table 2.
Frequency, age distribution and pattern of infiltration in the different lymphoma entities presenting in the liver (Berlin/Cologne 1994–2003)
| Berlin | Cologne | Berlin + Cologne, n (%) | Age (range) | Age (mean) | Male/female | Pattern | Density of infiltrate | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sinusoidal | Portal | Nodular | Dense | Loose | |||||||
| Diffuse large B-cell lymphoma (DLBCL)a | 52 | 28 | 80(39) | 16–87 | 64 | 50/30 | 6 | 19 | 72 | 75 | 5 |
| T-cell rich B-cell lymphoma (TCRBCL) | 9 | 4 | 13(6) | 35–85 | 59 | 9/4 | 0 | 11 | 5 | 0 | 13 |
| B-cell chronic lymphocytic leukemia (B-CLL) | 13 | 13 | 26(13) | 44–82 | 66 | 16/10 | 7 | 25 | 2 | 25 | 1 |
| Classical Hodgkin lymphoma (cHL) | 10 | 13 | 23(11) | 21–91 | 54 | 14/9 | 1 | 21 | 10 | 3 | 20 |
| Follicular lymphoma (FL) | 11 | 3 | 14(7) | 38–83 | 62 | 7/7 | 0 | 11 | 4 | 14 | 0 |
| Marginal zone lymphoma (MZL) | 6 | 1 | 7(3) | 49–80 | 70 | 6/1 | 2 | 7 | 0 | 6 | 1 |
| Leukemic Plasmacytoma (PL) | 4 | 2 | 6(3) | 61–88 | 70 | 4/2 | 4 | 1 | 2 | 5 | 1 |
| Burkitt lymphoma (BL) | 5 | – | 5(2) | 30–70 | 47 | 3/2 | 0 | 0 | 5 | 5 | 0 |
| Mantle cell lymphoma (MCL) | 3 | – | 3(1) | 60–66 | 63 | 2/1 | 1 | 3 | 1 | 3 | 0 |
| B-lymphoblastic leukaemia (B-ALL) | 2 | – | 2(1) | 18–28 | 22 | 1/1 | 2 | 0 | 0 | 2 | 0 |
| Hairy cell leukaemia (HCL) | 1 | – | 1 | 42 | – | 0/1 | 1 | 0 | 0 | 1 | 0 |
| Peripheral T-cell lymphoma, unspecified (pTCL) | 13 | 5 | 18(9) | 38–84 | 62 | 14/4 | 6 | 6 | 8 | 11 | 7 |
| Anaplastic large cell lymphoma (ALCL)b | 5 | – | 5(2) | 24–76 | 58 | 5/0 | 3 | 3 | 2 | 2 | 3 |
| Hepatosplenic T-cell lymphoma (HSTCL) | 1 | 1 | 2(1) | 38–51 | 45 | 2/0 | 2 | 0 | 0 | 2 | 0 |
| Total | 135 | 70 | 205 | ||||||||
aIncluding centroblastic (n = 69), immunoblastic (n = 3) and anaplastic variant (n = 8)
bIncluding one anaplastic large cell lymphoma kinase (ALK) protein positive case
Infiltration patterns of the various lymphoma types
Most of the cases showed the predominance of a certain growth pattern, although some cases exhibited more than one growth pattern. Almost all DLBCL and BL showed a nodular infiltration pattern (91%) composed of a dense infiltrate (94%; Table 3), except for the TCRBCL variant, which was located predominantly within the portal tracts (85%) and exhibited the characteristic scattered infiltrate of fewer than 10% large neoplastic B-cells admixed with many reactive T-cells and histiocytes (Fig. 1g–i). Lymphomas showing a dense portal infiltration pattern were chronic lymphocytic leukemia/small lymphocytic lymphoma (96%; Fig. 3a–c), FL (79%; Fig. 3d–f), MZL (100%; Fig. 3g–i), and mantle cell lymphoma (100%). In contrast, in precursor B lymphoblastic leukemia/lymphoblastic lymphoma (B-ALL/B-LBL; Fig. 4a–c), HSTCL (Fig. 4d–f), leukemic plasmacytoma (Fig. 4g–i), and hairy cell leukemia, a sinusoidal infiltration pattern was observed in the majority of cases. The cHL displayed a predominantly portal infiltration pattern (Fig. 5a–c). Peripheral T-cell lymphoma and ALCL showed a spectrum of portal, sinusoidal, nodular, or mixed patterns with a varying density of infiltration, and thus did not allow for pattern-based selection of additional analyses (Fig. 5d–f). The infiltration patterns, differential diagnoses, and discriminative immunohistochemical markers are summarized in Table 3.
Table 3.
Infiltration pattern, differential diagnosis and characteristic immunohistochemical markers
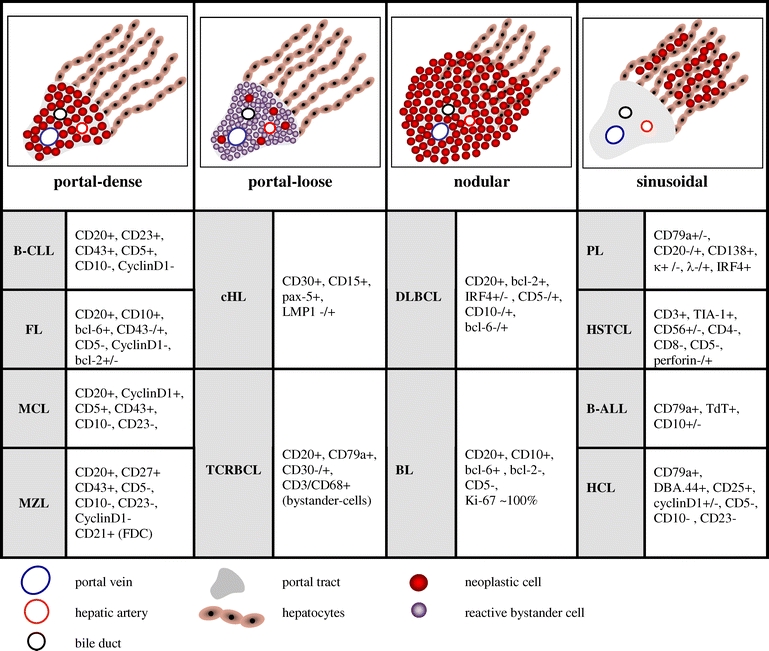
Fig. 3.
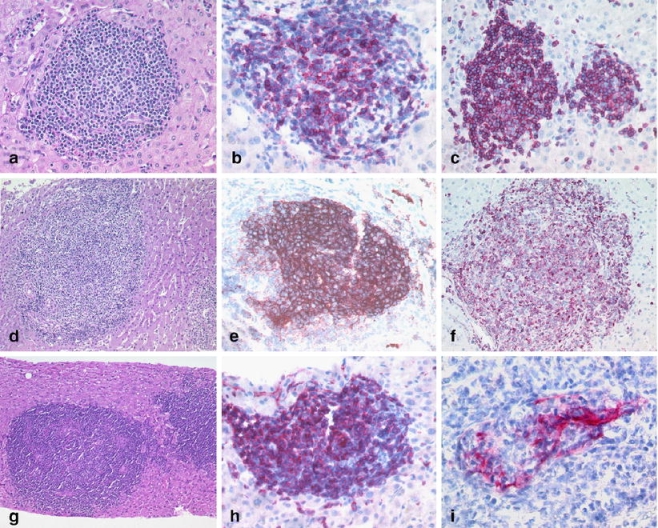
Portal pattern: chronic lymphocytic leukemia (B-CLL) with a predominantly portal infiltrate of small lymphocytes (a) with expression of CD23 (b) and CD5 (c). FL (d) with expression of CD10 (e) and BCL2 (f). Marginal zone B-cell lymphoma with portal involvement (g), expression of memory B-cell marker CD27 (h) and a lymphoepithelial lesion of a CK7 positive bile duct (i)
Fig. 4.
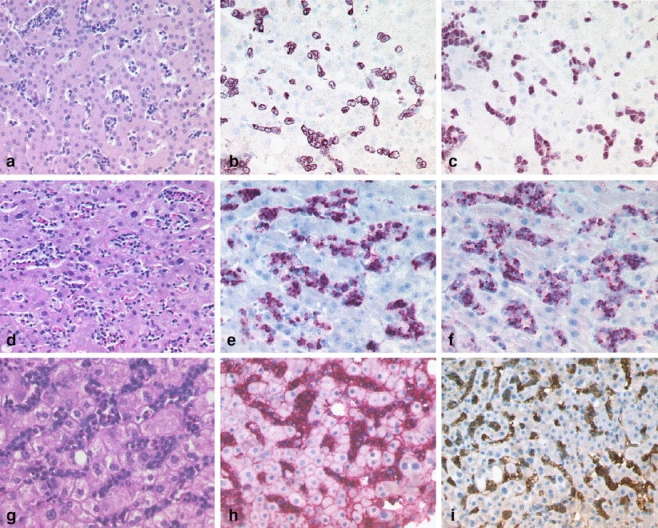
Sinusoidal pattern: precursor B lymphoblastic lymphoma (B-LBL) with a sinusoidal infiltrate of small blasts (a) with cytoplasmic expression of CD79a (b) and nuclear expression of terminal deoxynucleotidyl transferase (TdT; c). HSTCL with a sinusoidal infiltrate of monotonous neoplastic cells (d) with expression of CD3 (e) and the cytotoxic granule associated protein TIA-1 (f). Peripheral blood involvement (plasma cell leukemia) in a plasmacytoma with a sinusoidal infiltrate of plasma cells (g) with expression of CD138 (h) and IgG (i)
Fig. 5.
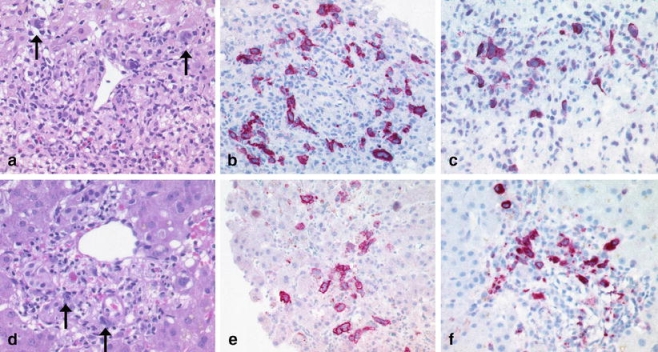
Classical Hodgkin lymphoma vs. anaplastic large cell lymphoma: cHL with portal infiltrates of Hodgkin and Reed-Sternberg HRS cells (arrows) in a background rich in eosinophils (a). The HRS cells strongly express CD30 (b) and the EBV encoded latent membrane protein 1 (LMP1) (c). Anaplastic large cell lymphoma (ALCL) with portal infiltrates of pleomorphic large cells resembling HRS cells (arrows) (d) and strong positivity for CD30 (e) and the cytotoxic molecule perforin (f)
Comparison of hepatic lymphomas with other extranodal lymphomas
The percentage of T-cell lymphomas was considerably higher in the liver (12%, 25/205) when compared to other extranodal sites (5%, 165/3252) except for the skin (50%, mainly mycosis fungoides/Sézary syndrome) and small intestine (34%, mainly enteropathy-type T-cell lymphomas).
The high proportion of DLBCL in lymphomas of the liver (45%) was only surpassed by the small intestine (64%), the brain (83%), and the testis (97%). Of note, TCRBCL was extremely rare in other extranodal sites (0,3%) with only a few cases occurring in the bone marrow and spleen. Interestingly, one case fulfilled the criteria of TCRBCL in the liver, and an additional lymph node biopsy showed the picture of a typical DLBCL, whereas in another case, both liver and spleen biopsy showed the characteristic features of TCRBCL. The frequencies of other extranodal lymphomas compared to hepatic lymphomas are summarized in Table 4.
Table 4.
Frequency of extranodal lymphoma at different sites (Berlin 1994–2003)
| Brain (n = 31) | Testis (n = 61) | Lung (n = 79) | Bone marrow (n = 1996) | Stomach (n = 855) | Small intestine (n = 71) | Large intestine (n = 102) | Spleen (n = 128) | Skin (n = 427) | Liver (n = 135) | |
|---|---|---|---|---|---|---|---|---|---|---|
| DLBCL | 25 | 57 | 22 | 66 | 238 | 30 | 32 | 21 | 78 | 52 |
| TCRBCL | – | – | – | 7 | – | – | – | 3 | – | 9 |
| FL | 1 | – | 2 | 168 | 16 | 3 | 3 | 20 | 91 | 11 |
| B-CLL | 1 | – | 2 | 656 | 12 | – | 4 | 10 | 7 | 13 |
| PL | 3 | – | – | 473 | – | – | – | 1 | 2 | 4 |
| MZL | – | – | 40 | 17 | 577 | 4 | 12 | 18* | 12 | 6 |
| MCL | – | – | 3 | 107 | 7 | 7 | 41 | 19 | 6 | 3 |
| LPL | – | – | 3 | 122 | – | – | 2 | 13 | 5 | – |
| HCL | – | – | – | 112 | – | – | – | 6 | – | 1 |
| B-ALL | – | 1 | – | 78 | – | – | – | 1 | – | 2 |
| BL | – | 1 | – | 4 | – | 3 | 2 | – | 2 | 5 |
| cHL | – | – | 3 | 52 | – | – | – | 3 | 10 | 10 |
| B-NHL total (%) | 30/31 (97%) | 59/61 (97%) | 75/79 (95%) | 1862/1996 (93%) | 850/855 (99%) | 47/71 (66%) | 96/102 (94%) | 115/128 (90%) | 213/427 (50%) | 116/135 (86%) |
| pTCL | 1 | – | 3 | 93 | 4 | 6 | 6 | 12 | 94 | 13 |
| NK/T | – | 1 | – | – | – | 2 | – | – | 2 | – |
| T-ALL | – | 1 | – | 29 | – | – | – | – | 9 | – |
| ALCL | – | – | 1 | 8 | 1 | 6 | – | – | 26 | 5 |
| Enteropathy-type TCL | – | – | – | – | – | 10 | – | – | – | – |
| HSTCL | – | – | – | 5 | – | – | – | 1 | – | 1 |
| MF | – | – | – | – | – | – | – | – | 83 | – |
| T-NHL total (%) | 1/31 (3%) | 2/61 (3%) | 4/79 (5%) | 134/1996 (7%) | 5/855 (1%) | 24/71 (34%) | 6/102 (6%) | 13/128 (10%) | 214/427 (50%) | 19/135 (14%) |
*Including nine cases of splenic marginal zone lymphoma (SMZL)
Discussion
To the best of our knowledge, this study represents the largest series focusing on the histopathology of hepatic lymphoma involvement and the first application of the WHO classification of tumors of hematopoietic and lymphoid tissues for this purpose [16]. Our data demonstrate the feasibility of in vivo lymphoma subtyping in liver biopsy with the aid of infiltration pattern analysis, the selection of differentiating immunophenotypic markers and additional molecular methods.
Many B-cell-derived lymphomas involving the liver revealed a characteristic infiltration pattern, which facilitated the use of a restricted panel of immunohistochemical markers to reach a final diagnosis. This approach is critical in those liver biopsies in which a lymphoma diagnosis has previously not been established, as accurate subtyping of lymphomas is fundamental for the initiation of adequate treatment.
Diffuse large B-cell lymphomas (DLBCL) accounted for 51% of all B-NHL cases. DLBCL was recently subdivided into different prognostic groups designated GCB, activated B-cell-like (ABC or non-GCB) and Type 3, according to their gene expression profiles [1]. The GCB group is described to have a better survival than the ABC group and the heterogeneous Type 3 group. Hans et al. [11] have proposed a simple immunohistochemical algorithm to assign cases to a GCB and a non-GCB group. In the present study, the frequency of either group in 80 liver biopsies with DLBCL was investigated, excluding the T-cell/histiocyte rich variant (n = 13). DLBCL of non-GCB type (65%) were more frequent in the liver than the GCB type (35%), whereas these two groups are described in other sites at about equal frequency [23]. As liver involvement with malignant lymphomas occurs secondary in advanced disease [15, 18], this observation may reflect the biological behavior of ABC-group DLBCLs through the selection of more aggressive cases by liver biopsy.
Approximately 10% of the de novo DLBCL express CD5 [4] and may have a poor outcome [31]. They tend to be associated with extranodal sites, especially bone marrow and spleen [19]. In the present series of hepatic lymphoma involvement, the number of CD5 positive DLBCL (4/80 or 5%) was not increased.
The TCRBCL, a variant of DLBCL in which the majority of cells are nonneoplastic T-cells and histiocytes, are often misdiagnosed as a reactive inflammatory condition or as a T-cell lymphoma infiltration of the liver [8, 17]. TCRBCL is relatively rare in lymph node biopsies [(23/976) or 2% of the DLBCL in the Berlin Reference Centre for Lymph Node Pathology from 1994–2003]. However, in the present study, 13/93 (14%) of the DLBCL in the liver were found to fulfill the WHO criteria for TCRBCL thus confirming previous data from Dargent and coworkers in their series of 62 liver specimens [8]. Additionally, one case with TCRBCL characteristics in the liver showed the typical morphology of a conventional DLBCL in an additional lymph node biopsy. This finding indicates that the composition of the reactive coinfiltrate may be influenced by the tissue-specific microenvironment. Therefore, the specific environment of a certain organ may influence the intensity of the T-cell response and subsequently explain the higher frequency of the TCRBCL variant of DLBLC presenting in the liver [10]. Limitations were encountered in the grading of follicular lymphoma and the subtyping of the cHL, which is generally not possible with sufficient certainty in needle biopsies.
In contrast to B-cell lymphomas, T-cell lymphomas infiltrating the liver generally lack a typical infiltration pattern, making their diagnosis more challenging. In particular, ALCL may mimic cHL due to the possible resemblance of Hodgkin and Reed-Sternberg cells and expression of CD30. Thus, additional markers against pan-T and pan-B cell antigens (including pax-5), CD15, ALK-1, cytotoxic molecules, e.g., TIA-1, granzyme B, and perforin) are required when confronted with this differential diagnosis (Fig. 5).
Similarly, the distinction between a T-cell lymphoma and a drug-induced or viral hepatitis (e.g., due to Epstein-Barr virus) can be very difficult in biopsies containing an increased number of sinusoidal T-cells. Therefore, additional clonality analyses by TCR or IgH PCR were necessary in 5 out of 19 (26%) T-cell lymphomas compared to only 3 out of 116 (3%) B-cell lymphomas to establish a diagnosis.
The reason for the relatively high frequency of liver involvement by T-cell lymphomas is not known. As T-cells play an important role in the regulation of the hepatic immune responses, especially in chronic viral and autoimmune hepatitis [30], it is tempting to speculate whether this may influence the relatively high incidence of T-cell lymphomas in the liver compared to other extranodal sites. In the present study, 25 of the 205 (12%) cases were found to represent lymphomas derived from T-cells, whereas lymphomas of the testis, brain, lung, and stomach were B-cell lymphomas in nearly all instances (Table 4).
In summary, the present study demonstrates the feasibility of subtyping lymphoma infiltrates in liver biopsies according to the WHO classification. The large number of 205 cases provides reliable information regarding the relative frequencies of the different lymphoma entities encountered in the liver and demonstrates the usefulness of infiltration pattern analysis for diagnostic purposes.
Acknowledgement
We would like to thank Erika Berg, Constanze Cieluch, and Hans-Henning Müller for their excellent technical assistance and Harald Krosch for graphical assistance. This work was supported by grants from the German Cancer Aid.
Footnotes
Christoph Loddenkemper and Thomas Longerich contributed equally to the work.
References
- 1.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511 [DOI] [PubMed]
- 2.Ascoli V, Lo Coco F, Artini M, Levrero M, Martelli M, Negro F (1998) Extranodal lymphomas associated with hepatitis C virus infection. Am J Clin Pathol 109:600–609 [DOI] [PubMed]
- 3.Bronowicki JP, Bineau C, Feugier P, Hermine O, Brousse N, Oberti F, Rousselet MC, Dharancy S, Gaulard P, Flejou JF, Cazals-Hatem D, Labouyrie E (2003) Primary lymphoma of the liver: clinical-pathological features and relationship with HCV infection in French patients. Hepatology 37:781–787 [DOI] [PubMed]
- 4.Burns BF, Warnke RA, Doggett RS, Rouse RV (1983) Expression of a T-cell antigen (Leu-1) by B-cell lymphomas. Am J Pathol 113:165–171 [PMC free article] [PubMed]
- 5.Caccamo D, Pervez NK, Marchevsky A (1986) Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med 110:553–555 [PubMed]
- 6.Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, MacDonald S, Pulford KA, Stein H, Mason DY (1984) Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 32:219–229 [DOI] [PubMed]
- 7.Dammacco F, Gatti P, Sansonno D (1998) Hepatitis C virus infection, mixed cryoglobulinemia, and non-Hodgkin’s lymphoma: an emerging picture. Leuk Lymphoma 31:463–476 [DOI] [PubMed]
- 8.Dargent JL, De Wolf-Peeters C (1998) Liver involvement by lymphoma: identification of a distinctive pattern of infiltration related to T-cell/histiocyte-rich B-cell lymphoma. Ann Diagn Pathol 2:363–369 [DOI] [PubMed]
- 9.Dippel E, Assaf C, Hummel M, Schrag HJ, Stein H, Goerdt S, Orfanos CE (1999) Clonal T-cell receptor gamma-chain gene rearrangement by PCR-based GeneScan analysis in advanced cutaneous T-cell lymphoma: a critical evaluation. J Pathol 188:146–154 [DOI] [PubMed]
- 10.Dogan A, Burke JS, Goteri G, Stitson RN, Wotherspoon AC, Isaacson PG (2003) Micronodular T-cell/histiocyte-rich large B-cell lymphoma of the spleen: histology, immunophenotype, and differential diagnosis. Am J Surg Pathol 27:903–911 [DOI] [PubMed]
- 11.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282 [DOI] [PubMed]
- 12.Harris AC, Ben-Ezra JM, Contos MJ, Kornstein MJ (1996) Malignant lymphoma can present as hepatobiliary disease. Cancer 78:2011–2019 [DOI] [PubMed]
- 13.Harris AC, Kornstein MJ (1993) Malignant lymphoma imitating hepatitis. Cancer 71:2639–2646 [DOI] [PubMed]
- 14.Hsu SM, Raine L, Fanger H (1981) The use of antiavidin antibody and avidin–biotin–peroxidase complex in immunoperoxidase technics. Am J Clin Pathol 75:816–821 [DOI] [PubMed]
- 15.Jaffe ES (1987) Malignant lymphomas: pathology of hepatic involvement. Semin Liver Dis 7:257–268 [DOI] [PubMed]
- 16.Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) (2001) World Health Organisation of Tumours. Pathology World Health Organisation of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC, Lyon
- 17.Khan SM, Cottrell BJ, Millward-Sadler GH, Wright DH (1993) T-cell-rich B-cell lymphoma presenting as liver disease. Histopathology 23:217–224 [DOI] [PubMed]
- 18.Kim H, Dorfman RF (1974) Morphological studies of 84 untreated patients subjected to laparotomy for the staging of non-Hodgkin’s lymphomas. Cancer 33:657–674 [DOI] [PubMed]
- 19.Kroft SH, Howard MS, Picker LJ, Ansari MQ, Aquino DB, McKenna RW (2000) De novo CD5+diffuse large B-cell lymphomas. A heterogeneous group containing an unusual form of splenic lymphoma. Am J Clin Pathol 114:523–533 [DOI] [PubMed]
- 20.Matano S, Nakamura S, Annen Y, Hattori N, Kiyohara K, Kakuta K, Kyoda K, Sugimoto T (1998) Primary hepatic lymphoma in a patient with chronic hepatitis B. Am J Gastroenterol 93:2301–2302 [DOI] [PubMed]
- 21.Mohler M, Gutzler F, Kallinowski B, Goeser T, Stremmel W (1997) Primary hepatic high-grade non-Hodgkin’s lymphoma and chronic hepatitis C infection. Dig Dis Sci 42:2241–2245 [DOI] [PubMed]
- 22.Ozaki S, Ogasahara K, Kosaka M, Inoshita T, Wakatsuki S, Uehara H, Matsumoto T (1998) Hepatosplenic gamma delta T-cell lymphoma associated with hepatitis B virus infection. J Med Investig 44:215–217 [PubMed]
- 23.Poulsen CB, Borup R, Nielsen FC, Borregaard N, Hansen M, Gronbaek K, Moller MB, Ralfkiaer E (2005) Microarray-based classification of diffuse large B-cell lymphoma. Eur J Haematol 74:453–465 [DOI] [PubMed]
- 24.Prabhu RM, Medeiros LJ, Kumar D, Drachenberg CI, Papadimitriou JC, Appelman HD, Johnson LB, Laurin J, Heyman M, Abruzzo LV (1998) Primary hepatic low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) associated with primary biliary cirrhosis. Mod Pathol 11:404–410 [PubMed]
- 25.Rubbia-Brandt L, Brundler MA, Kerl K, Negro F, Nador RG, Scherrer A, Kurt AM, Mentha G, Borisch B (1999) Primary hepatic diffuse large B-cell lymphoma in a patient with chronic hepatitis C. Am J Surg Pathol 23:1124–1130 [DOI] [PubMed]
- 26.Scheimberg IB, Pollock DJ, Collins PW, Doran HM, Newland AC, van der Walt JD (1995) Pathology of the liver in leukaemia and lymphoma. A study of 110 autopsies. Histopathology 26:311–321 [DOI] [PubMed]
- 27.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, Garcia-Sanz R, van Krieken JH, Droese J, Gonzalez D, Bastard C, White HE, Spaargaren M, Gonzalez M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 17:2257–2317 [DOI] [PubMed]
- 28.Voigt JJ, Vinel JP, Caveriviere P, Pradere B, Chittal S, al Saati T, Cales P, Delsol G. (1989) [Immunochemical diagnosis of hepatic localizations in malignant lymphoid hematologic diseases. Study of 80 cases]. Gastroenterol Clin Biol 13:343–352 [PubMed]
- 29.Walz-Mattmuller R, Horny HP, Ruck P, Kaiserling E (1998) Incidence and pattern of liver involvement in haematological malignancies. Pathol Res Pract 194:781–789 [DOI] [PubMed]
- 30.Wick MJ, Leithauser F, Reimann J (2002) The hepatic immune system. Crit Rev Immunol 22:47–103 [PubMed]
- 31.Yamaguchi M, Seto M, Okamoto M, Ichinohasama R, Nakamura N, Yoshino T, Suzumiya J, Murase T, Miura I, Akasaka T, Tamaru J, Suzuki R, Kagami Y, Hirano M, Morishima Y, Ueda R, Shiku H, Nakamura S (2002) De novo CD5+ diffuse large B-cell lymphoma: a clinicopathologic study of 109 patients. Blood 99:815–821 [DOI] [PubMed]


