ABSTRACT
Objectives: To present our experience of salvage surgery for recurrent nasopharyngeal carcinoma after primary treatment by radiotherapy. Patients and methods: Eleven of 25 patient treated for nasopharyngeal carcinoma between 1990 and 2003 with radiotherapy had either residual or recurrent disease and underwent salvage surgery. The type C infratemporal fossa approach was used to access residual tumor. The patients' progress was followed by clinical examination and interval magnetic resonance scans. Outcome measures and results: The results were analyzed in terms of morbidity and oncological outcome; patients were recorded as NED (no existing disease), AWD (alive with disease), and DOD (died of disease). A disease-free survival rate of 72% was achieved in the salvage surgery group of patients and an overall disease-free survival rate of 56% applied to the initial cohort of 25 patients, following both the single mode and combined treatment. Conclusion: Salvage surgery is feasible for patients with recurrent nasopharyngeal carcinoma and may be achieved with minimal morbidity using the type C infratemporal fossa approach.
Keywords: Salvage surgery, nasopharyngeal carcinoma, recurrent tumors of the skull base
The gold standard treatment for undifferentiated nasopharyngeal carcinoma (WHO type III) is radiotherapy. This tumor is usually radiosensitive and the alternative therapy, surgical resection, is both inappropriate and difficult to undertake. Local control rates of 80% and 5-year survival rates of 70% have been reported.1 The incidence of local recurrence varies from 18 to 54% in the first 5 years of follow-up.1,2 By the time recurrent nasopharyngeal carcinoma has been diagnosed, the disease is usually extensive. Not infrequently it will be found to have infiltrated the nasal vault, the pterygopalatine and infratemporal fossae, parapharyngeal space, the prevertebral musculature, soft palate, clivus, and sphenoid sinus. Furthermore, there is often perivascular and perineural spread that may involve the internal carotid artery in the foramen lacerum and the mandibular nerve in the foramen ovale. To complicate matters further, the precise extent of dural infiltration is extremely difficult to assess with current imaging techniques and it is as easy to overestimate the extent of disease as it is to underestimate it.
The diagnosis of recurrent disease is largely made by imaging. Fat-suppressed, gadolinium-enhanced magnetic resonance (MR) imaging is the best tool for evaluating the extent of infiltration. Once suspected, an endonasal-endoscopic biopsy usually confirms the diagnosis. Further treatment may be considered for some patients. Re-irradiation, stereotactic radiosurgery, and conventional surgery with or without postoperative irradiation have all been used at some time or another with variable results and consequences.
We report our experience over a 13-year period using salvage surgery for 11 patients with recurrent nasopharyngeal carcinoma derived from a cohort of 25 patients that we had treated for nasopharyngeal carcinoma at our institution.
MATERIAL AND METHODS
Between 1990 and 2003, 25 patients with undifferentiated nasopharyngeal carcinoma were treated at the ENT Department in Bergamo. There were 11 males and 14 females with a mean age of 45 years (range, 39 to 65 years; median, 45 years). None of these patients had received previous treatment and all were treated primarily with radiotherapy.
After the first course of radiotherapy, tumor either persisted or subsequently recurred in 16 of the 25 patients (64%). Salvage surgery was undertaken for 11 patients (44%) who either failed to respond completely to radiotherapy or subsequently developed recurrent disease. The diagnosis of residual or recurrent disease was made by both imaging and biopsy. A minimum period of 6 months had elapsed following radiotherapy before surgery was undertaken in all the patients.
Salvage surgery was performed using the type C infratemporal fossa. The mean period of follow-up was 5 years (range, 2 to 13 years; median, 4 years). MR scans were acquired annually for the first 5 years and every other year after that for 10 years.
RESULTS
All 25 patients were treated by primary radiotherapy. After this, 6 patients had a complete response, 3 died from distant metastases without apparent locoregional disease, and 16 patients failed to respond completely to radiotherapy or developed recurrent disease. In these patients, the presence of residual or recurrent tumor was confirmed by biopsy.
The 6 patients who had a complete response to radiotherapy were alive with no existing disease at a median follow-up period of 5 years. The crude disease-free survival rate for primary radiotherapy in these patients was 24% (Table 1).
Table 1.
First Course Radiotherapy
| Patients | DOD | AWD | DOC | NED | Recurrences | Total |
|---|---|---|---|---|---|---|
| DOD, died of disease; AWD, alive with disease; DOC, died of other causes; NED, no existing disease. | ||||||
| 25 | 3 | – | – | 6 | 16 | 25 |
Of the 16 patients (64%) who either had residual or subsequently developed recurrent disease, 11 underwent salvage surgery. The remaining 5 patients were not suitable for surgery, were given palliative care, and died from their disease.
Data on the clinical stage and the outcome of the 11 patients treated by salvage surgery are summarized in Table 2. Eight of these 11 patients have remained alive with no existing disease, 2 are alive with distant metastasis, and 1 patient has died from local disease.
Table 2.
Salvage Surgery
| Patients | pT Staging | DOD | AWD | DOC | NED | Total |
|---|---|---|---|---|---|---|
| DOD, died of disease; AWD, alive with disease; DOC, died of other causes; NED, no existing disease. | ||||||
| T1 | – | – | – | 2 | 2 | |
| T2 | – | 1 | – | 5 | 6 | |
| T3 | – | 1 | – | 1 | 2 | |
| T4 | 1 | – | – | – | 1 | |
| Total | 1 | 2 | 0 | 8 | 11 | |
The disease-free survival rate after salvage surgery is 72% at a minimum follow-up period of 2 years. Overall, 14 of the original cohort of 25 patients treated by either primary radiotherapy (RT) or a combination of radiotherapy and salvage surgery (RT + salvage surgery) have remained alive with no evidence of disease. The overall disease-free survival rate for the entire cohort of patients is 56% (Table 3).
Table 3.
Overall Outcome
| Patients | DOD | AWD | DOC | NED | Total |
|---|---|---|---|---|---|
| DOD, died of disease; AWD, alive with disease; DOC, died of other causes; NED, no existing disease; RT, radiotherapy. | |||||
| RT Alone | 3 | – | – | 6 | 9 |
| RT + Salvage Surgery | 1 | 2 | – | 8 | 11 |
| RT + Palliative Therapy | 5 | – | – | – | 5 |
| Total | 9 | 2 | 0 | 14 | 25 |
Postoperative morbidity was limited to a weakness of the frontal branch of the facial nerve, which usually recovered after a short time. Three patients' wounds became infected and three others were troubled by trismus that developed as a result of the surgery and previous radiotherapy. Most complained of mucosal dryness that affected both the mouth and nasal passages. There were no other complications recorded.
DISCUSSION
This article is based on a cohort of 25 patients with undifferentiated carcinoma of the nasopharynx that was treated primarily with radiotherapy. Six of these patients had a complete response to radiotherapy, 3 died from distant metastasis, and 16 either failed to respond completely and had residual disease or subsequently developed recurrence. Eleven of these 16 patients underwent resection of their residual or recurrent tumor through the infratemporal type C approach (Figs. 1 and 2). The remaining 5 patients were given palliative care and died from their disease.
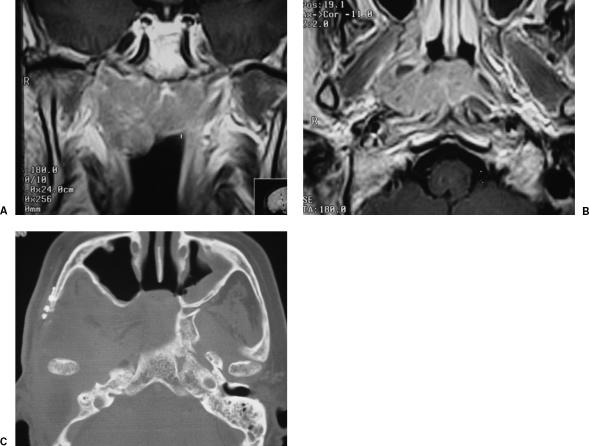
Figure 1.
T1-enhanced magnetic resonance image of a recurrent carcinoma of the nasopharynx, in (A) coronal and (B) axial views. (C) Postoperative imaging.
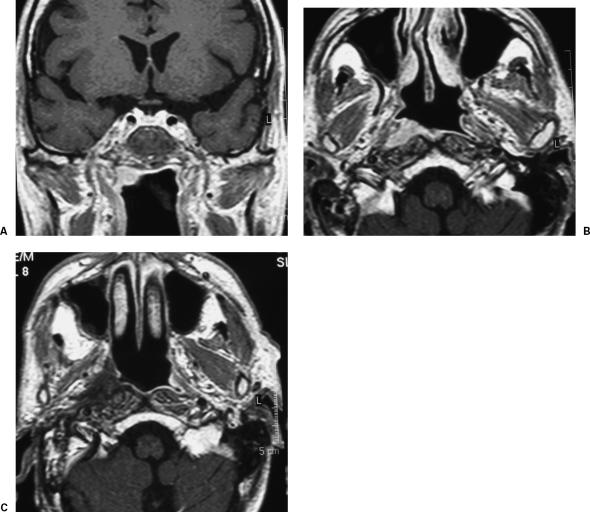
Figure 2.
T1-enhanced magnetic resonance image of a recurrent carcinoma of the nasopharynx, in (A) coronal and (B) axial views. (C) Postoperative image shows the filling of the surgical cavity with the temporal muscle.
The outcome for the salvage surgery group showed that 8 of 11 were alive with no sign of disease at a minimum follow-up of 2 years. Two were alive with distant metastases and one had died from disease. The 2-year disease-free survival rate for this group of patients was 72%. The 5-year disease-free survival rate for the group of patients who were treated by radiotherapy alone and had complete response was 24%. By combining salvage surgery with primary radiotherapy, an overall disease-free survival rate of 56% was achieved at 2 years.
In our cohort of patients, the likelihood of developing recurrence increased with the initial T stage of the tumor. Those cured by radiotherapy were mainly patients with T1 and T2 disease, while those who achieved a partial response to radiotherapy had T2 and T3 tumors. In this series of salvage surgery the only patient that died had a T4 tumor and subsequently an extensive recurrence. Surgery for such patients should be considered as palliative care. Not surprisingly, it would seem that increasing T stage was related to a worse prognosis, though this was just a trend and did not reach significance.
In our opinion, the main indications for salvage surgery are recurrent T1 and T2 tumors and selected cases of T3 nasopharyngeal carcinoma that have limited erosion of the skull base. T4 tumors are generally considered to be unresectable (Fig. 3), though in some patients the tumor had only been afforded T4 status because of invasion of the infratemporal fossa. This feature alone does not represent a contraindication to surgery. The role of palliative surgery is certainly questionable, particularly for large T3 and T4 tumors. Some would even question the value of any salvage surgery for this condition. However, in the context of this study, it contrasts usefully with the natural history of untreated recurrence in the skull base and neck. Our solitary patient with a T4 nasopharyngeal carcinoma died from local recurrence. In other words, salvage surgery in this case failed.
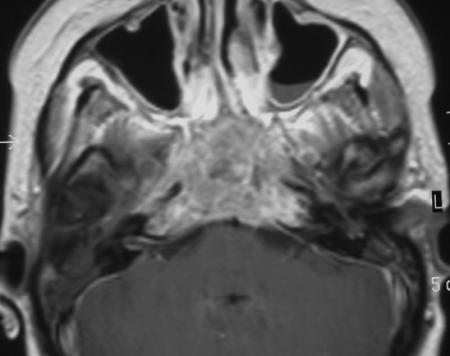
Figure 3.
T1-enhanced magnetic resonance image of an unresectable case.
A 72% 2-year disease-free survival rate3 with salvage surgery is certainly an encouraging result, particularly in the context of a disease for which a control rate of 80% and 5-year survival of 70% has been reported for treatment with primary radiotherapy. However, many problems remain for those who attempt to treat recurrent tumor.4 There are significant difficulties performing surgery in what is a very complex anatomical area. It is important to be radical without being excessively destructive, otherwise surgery could be associated with significant morbidity. In this situation, the main therapeutic options are re-irradiation; salvage surgery, and, more recently, radiosurgery. Re-irradiation with high-dose external radiotherapy2,3,4,5,6,7 induces severe and potentially unbearable complications. Local control rates of between 18% and 35% have been reported2,4,5 with 5-year overall survival rates varying from 9.4 to 21%. The most frequent late postirradiation sequelae are brain and soft-tissue necrosis, skull base necrosis, severe pain, dysphagia, trismus, hearing impairment, and cranial nerves deficits. Morbidity from re-irradiation is usually very significant with a cumulative incidence varying from 22 to 29% of the patients.2,4,5,6,7 These complications seem to overwhelm any oncological benefit derived from control of the disease. Gamma knife radiosurgery has been suggested as an option for those patients with early recurrence that is not suitable for either external beam therapy or surgery; an example is recurrence that infiltrates the skull base or has an intracranial transdural extension. There are not sufficient data to assess the efficacy of this form of treatment. Better results have been reported2,8 for stereotactic radiotherapy used as boost adjuvant treatment after salvage re-irradiation. The 3-year follow-up statistics show local control of the disease in 56% of patients and overall survival rates of 54%, though stereotactic radiotherapy does not prevent all of the late post re-irradiation complications. Moreover, the long-term efficacy and morbidity of this adjuvant therapy is not established.2,8
In view of the drawbacks of external beam re-irradiation, salvage surgery is an alternative that should be considered. It has an evidence base that would suggest that it can provide long-term local control and good overall survival.2,6,7,9 The main difficulties are first, surgical access, and second, safe control of the intracranial internal carotid artery and cranial nerves. Several approaches have been described to overcome these issues and we unhesitatingly recommend surgery if there is still radiological evidence of residual tumor 6 months after irradiation or if recurrence develops at a later date. Biopsy of the mass is advocated for residual or recurrent disease, and an endonasal-endoscopic approach is recommended to minimize the morbidity of the procedure. The contraindications for surgery are extensive transdural infiltration, multiple cranial nerve deficits, distant metastasis, extensive destruction of the skull base, and total encasement of the internal carotid artery. Clinical staging must be the initial step in planning an adequate resection, as the T classification of nasopharyngeal recurrent carcinoma identifies the degree of involvement of the nasopharynx and its surrounding structures.
Extracranial anterior approaches to the nasopharynx include transfacial procedures (transoral, endonasal, lateral rhinotomy with or without lip split and nasal rotation for bilateral access, midface degloving, and the maxillary swing).2,6,9,10,11 The midface degloving approach is suitable for midline recurrences that extend into the pterygopalatine fossa. Further access laterally can be achieved by a mandibular swing, a lateral facial split, temporary removal of the zygoma, maxillary mobilization, or a Le Fort 1 osteotomy if appropriate. The overriding limitation of the midface degloving approach is its inability to provide access that offers good identification and protection of the internal carotid artery. The maxillary swing, a more aggressive approach, gives wider exposure but fails to solve the problem of poor control of the internal carotid artery. Anterior widening of the surgical route cannot be justified as it is associated with higher morbidity and visible scars and does not allow the surgeon to be more radical. Facial translocation, as modified by Janecka,12 can be combined with neurosurgical approaches and has been proposed for the management of tumors in the nasopharynx that extend intracranially through the skull base.13 This approach, like all the other anterior routes, gives limited access for control of the internal carotid artery. Although these approaches can be refined by endoscopic techniques, all leave the surgeon in a quandary over the posterior margin. This margin is not exposed until the mass has been completely removed and this makes an adequate en bloc resection extremely difficult if not impossible.
When the tumor has infiltrated bone or surrounding soft tissues, lateral approaches like the infratemporal fossa B and C are the most appropriate. The infratemporal fossa type C14,15 (IT-C) is tailored for the nasopharynx and has the advantage of early and safe control of the internal carotid artery and of wide exposure of the surgical field. This approach encompasses the infratemporal fossa and parapharyngeal space to expose the foramen ovale, foramen spinosum, the mandibular nerve, Eustachian tube, the root of the pterygoid plates, and the lateral wall of the nasopharynx. In this way, the tumor can be exposed and removed radically while controlling all vital structures.
The IT-C approach is suitable for cases of recurrent tumor of the nasopharynx that extend laterally and posterolaterally to the infratemporal fossa and parapharyngeal space and superiorly to the skull base. This access has the advantage of enabling en bloc resection of the soft tissue around the nasopharynx. Instead of requiring the surgeon to work in a blind space, this access progressively exposes the inferior aspect of the lateral skull base and all the neurovascular structures coming out from the bone as landmarks.
Surgical Technique
To summarize the important aspects of this approach, first a mastoidectomy is performed, the seventh cranial nerve is identified in its vertical portion, and the Eustachian tube is followed anteriorly to expose the sphenoid spine, foramen ovale and spinosum, the middle meningeal artery, and mandibular nerve, which is usually sectioned. The vertical and horizontal segments of the internal carotid artery are skeletonized. The root of the pterygoid plates marks the lateral extent of the nasopharyngeal wall. Further extension beyond the pterygoid muscles to the infratemporal fossa or posteriorly to the parapharyngeal space is feasible. This approach offers little danger to the internal carotid artery or facial nerve. It is not actually necessary to expose either structure, but having then under direct vision prevents inappropriate retraction. The dura is exposed easily and lifted with safety throughout the surgical field. The access afforded allows an en bloc resection of the superior, posterior, and lateral walls of a surgical box that contains the tumor, peritubal space, and surrounding soft tissues. The anterior wall of the resection is the posterior wall of the nasopharynx and can be extended inferiorly beyond the level of the soft palate. There is little difficulty in reconstructing the surgical defect because the temporalis muscle can be rotated to obliterate the cavity and fill the mucosal defect of the missing nasopharyngeal wall. In general, distant flaps are not required. A small depression in the area of rotation of the muscle, a conductive hearing loss due to the external auditory canal closure, and mandibular nerve deficit are the price paid for the exposure. There should be no facial nerve damage. If performed with care, retraction of the mandibular condyle does not add further fibrosis or ankylosis to that acquired as an unavoidable consequence of previous irradiation.
The IT-C approach is at its best when used for the salvage of T1 and T2 recurrences after failed irradiation. The poor prognosis of T3 and T4 recurrences is not because of a lack of local control but more because of regional and distant metastasis. In these advanced cases, surgery can be considered for palliation only. There remains the question whether this form of surgical procedure is justified, but each case must be judged on its own merits and the patient's wishes. Nevertheless, the IT-C approach can deliver enough exposure without adding much morbidity in these difficult cases.
CONCLUSION
Recurrent carcinoma of the nasopharynx has been considered by many to be incurable and either untreatable or not worth resection. Previously, surgery was thought to be unsafe and technically difficult, so re-irradiation and palliative therapy have been proposed by others to be the only feasible treatment. Our experience with 11 cases of recurrent carcinoma of the nasopharynx, reinforced by some data from the literature,2,6,7,9 shows that in addition to being technically feasible with low morbidity, surgery provides good oncological results. We have achieved a disease-free survival rate of 72% for patients treated with salvage surgery and an overall disease-free survival rate of 56% in the whole cohort of 25 patients affected by rhinopharyngeal carcinoma, at an average follow-up of 5 years. There have been no serious postoperative sequelae.
The surgical technique is not difficult to perform, but requires good knowledge of the surgical anatomy of the skull base and the application of the basic principles of microsurgery.
REFERENCES
- Hsu M M, Hong R L, Ting L L, et al. Factors affecting the overall survival after salvage surgery in patients with recurrent nasopharyngeal carcinoma at the primary site. Arch Otolaryngol Head Neck Surg. 2001;127:798–802. [PubMed] [Google Scholar]
- Chang K P, Hao S P, Tsang N M, Ueng S H. Salvage surgery for locally recurrent nasopharyngeal carcinoma: a 10-year experience. Otolaryngol Head Neck Surg. 2004;131:497–502. doi: 10.1016/j.otohns.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Huang S C, Lui L T, Lynn T C. Nasopharyngeal cancer: study III. A review of 1206 patients treated with combined modalities. Int J Radiat Oncol Biol Phys. 1985;11:1789–1793. doi: 10.1016/0360-3016(85)90033-1. [DOI] [PubMed] [Google Scholar]
- Pryzant R M, Wendt C D, Delclos L, Peters L J. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys. 1992;22:941–947. doi: 10.1016/0360-3016(92)90792-g. [DOI] [PubMed] [Google Scholar]
- Lee A W, Foo W, Law S C. Reirradiation for recurrent nasopharyngeal carcinoma: factors affecting the therapeutic ratio and ways for improvement. Int J Radiat Oncol Biol Phys. 1997;38:43–52. doi: 10.1016/s0360-3016(97)00244-7. [DOI] [PubMed] [Google Scholar]
- Hsu M M, Ko J Y, Sheen T S, Chang Y L. Salvage surgery for recurrent nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:305–309. doi: 10.1001/archotol.1997.01900030087011. [DOI] [PubMed] [Google Scholar]
- Shu C H, Cheng H, Lirng J F, et al. Salvage surgery for recurrent nasopharyngeal carcinoma. Laryngoscope. 2000;110:1483–1488. doi: 10.1097/00005537-200009000-00014. [DOI] [PubMed] [Google Scholar]
- Pai P C, Chuang C C, Wei K C. Stereotactic radiosurgery for locally recurrent nasopharyngeal carcinoma. Head Neck. 2002;24:748–753. doi: 10.1002/hed.10116. [DOI] [PubMed] [Google Scholar]
- Shu C H, Shiau C Y, Chi K H, Yen S H, Li W Y. Salvage surgery for recurrent nasopharyngeal carcinoma in anterior marginal miss after radiotherapy. Otolaryngol Head Neck Surg. 1999;121:622–666. doi: 10.1016/S0194-5998(99)70069-0. [DOI] [PubMed] [Google Scholar]
- Cocke E W, Robertson J H, Crook J P. The extended maxillotomy and subtotal maxillectomy for excision of skull base tumors. Arch Otolaryngol Head Neck Surg. 1990;116:92–104. doi: 10.1001/archotol.1990.01870010096026. [DOI] [PubMed] [Google Scholar]
- Wei W I, Lam K H, Sham J ST. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13:200–207. doi: 10.1002/hed.2880130306. [DOI] [PubMed] [Google Scholar]
- Janecka I P, Sen C N, Sekhar L N, Arriaga M. Facial translocation: new approach to cranial base. Otolaryngol Head Neck Surg. 1990;103:413–419. doi: 10.1177/019459989010300312. [DOI] [PubMed] [Google Scholar]
- Hao S P. Facial translocation approach to the skull base: the viability of translocated facial bone graft. Otolaryngol Head Neck Surg. 2001;124:292–296. doi: 10.1067/mhn.2001.112308. [DOI] [PubMed] [Google Scholar]
- Fisch U. The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope. 1983;93:36–44. doi: 10.1288/00005537-198301000-00007. [DOI] [PubMed] [Google Scholar]
- Fisch U, Fagan P, Valavanis A. The infratemporal fossa approach for the lateral skull base. Otolaryngol Clin North Am. 1984;17:513–552. [PubMed] [Google Scholar]