Abstract
A 27-year-old man developed an unruptured anterior-inferior cerebellar artery (AICA) feeding aneurysm from a transverse-sigmoid dural arteriovenous malformation. The patient, with a known history of left transverse and sigmoid sinus thrombosis, presented with pulse-synchronous tinnitus. Angiography revealed an extensive dural arteriovenous fistula (AVF), with feeders from both the extracranial and intracranial circulations, involving the right transverse sinus, the torcula, and the left transverse/sigmoid sinuses. Multimodal endovascular and open surgical therapy was used to manage the lesion. Before a planned second-stage treatment for the left sigmoid sinus component, the dural AVF improved significantly. During this interval, however, a small flow-related aneurysm developed on the left AICA feeding the petrous dural region. The aneurysm resolved after resection of the involved sigmoid sinus. This is the first reported case of an unruptured feeding-artery aneurysm in an intracranial dural AVF that resolved spontaneously with treatment of the dural AVF. Until more is known about the natural history, the decisions of when and whether to treat an unruptured dural AVF feeding-artery aneurysm must be made on an individual basis.
Keywords: Aneurysms, dural arteriovenous fistulas, endovascular therapy, surgery
Dural arteriovenous fistulas (AVFs) are vascular abnormalities of the dura mater that typically involve the dural sinuses, most commonly the transverse and sigmoid sinuses.1 Dural AVFs are acquired lesions, often developing after dural sinus thrombosis. Their presentation can range from pulse-synchronous tinnitus to intracerebral hemorrhage.
Unlike with arteriovenous malformations (AVMs), concurrent arterial aneurysms and intracranial dural AVFs are extremely rare.2,3,4 The two such patients detailed in the literature both presented with subarachnoid hemorrhage (SAH). We present a unique case of a patient who developed an unruptured anterior-inferior cerebellar artery (AICA) feeding-artery aneurysm from a transverse-sigmoid dural AVF. The aneurysm resolved spontaneously with treatment of the dural AVF. We discuss the unique issues surrounding the management of feeding-artery aneurysms associated with an intracranial dural AVF.
CASE STUDY
A 27-year-old man originally presented with an acute headache, vomiting, and meningismus in August 2003. The next day, he developed horizontal diplopia and was found to have bilateral sixth cranial nerve palsies and papilledema. Intracranial hypertension was diagnosed, and he was started on Diamox. The origin of the intracranial hypertension was initially thought to be related to doxycycline.5
Further evaluation with magnetic resonance venography (MRV) revealed thrombosis of the left transverse sinus and sigmoid sinus. The patient was started on Coumadin. His hypercoagulable work-up was remarkable for hyperhomocysteinemia. His diplopia and papilledema resolved with acetazolamide therapy. Several months later, despite maximal medical therapy, headaches and diplopia recurred and he developed visual field deficits associated with severe papilledema. As a result, he underwent fenestration of the right optic nerve sheath. Although the papilledema improved, the remainder of the patient's signs and symptoms of elevated pressure persisted.
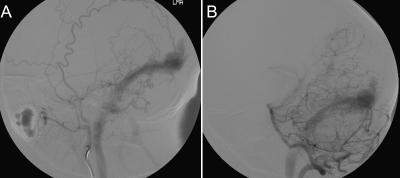
Ten months after his initial presentation, the patient developed a palpable and prominent left occipital artery with an audible bruit. Cerebral angiography showed an extensive dural AVF involving the proximal right transverse sinus, the torcula, the left transverse sinus, and the left sigmoid sinus. The AVF was supplied by the occipital arteries, the middle meningeal arteries, the left posterior meningeal artery, and a muscular branch of the left vertebral artery. The tentorial branches and a prominent left AICA also supplied the lesion (Fig. 1). No cortical reflux was seen.
Figure 1.
Cerebral angiogram of (A) left maxillary and (B) vertebral artery injections show an extensive dural AVF involving the torcula, left transverse sinus and sigmoid sinus with feeders from both the extracranial and intracranial circulation. AVF, arteriovenous fistula.
Because the patient's condition failed to improve after maximal medical therapy and optic nerve sheath fenestration and there was the potential for function of the left optic nerve to deteriorate, multimodal and staged therapy was planned. The patient underwent staged transarterial embolizations, followed by a bilateral occipital craniotomy for skeletonization and placement of interpositional dural xenograft (bovine pericardium) at the involved transverse dural sinuses and left sigmoid sinus. The goal of the operation was to disconnect all of the involved sinuses from aberrant arterial feeders and to prevent recurrences with the use of the interpositional dural xenograft, which has been described in this patient elsewhere.5a Postoperative angiography showed marked improvement of the dural AVF with small residual feeders to the transverse and sigmoid sinus components on the left.
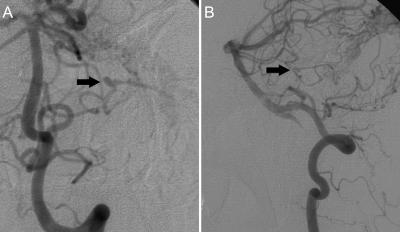
Follow-up angiography 4 months later showed that the feeders had become slightly more prominent. During the interval, a 3-mm left AICA feeding-artery aneurysm had developed beyond the floccular segment (Fig. 2). The patient underwent a planned second-stage transtemporal (presigmoid, retrolabyrinthine) approach for resection of the involved sigmoid sinus on the left. The sinus was removed from the entrance of the vein of Labbé to the jugular bulb. The left transverse sinus was then coil-embolized through a transvenous approach. A 1-month follow-up angiogram confirmed complete resolution of the feeding-artery AICA aneurysm (Fig. 3). The patient's symptoms, papilledema, sixth cranial nerve palsies, and bruit also resolved completely.
Figure 2.
(A) Anteroposterior and (B) lateral view follow-up angiograms, left vertebral artery injection, after the first-stage operation show the 3-mm left AICA feeding-artery aneurysm beyond the floccular segment (arrows) that developed during the interval. AICA, anterior-inferior cerebellar artery.
Figure 3.
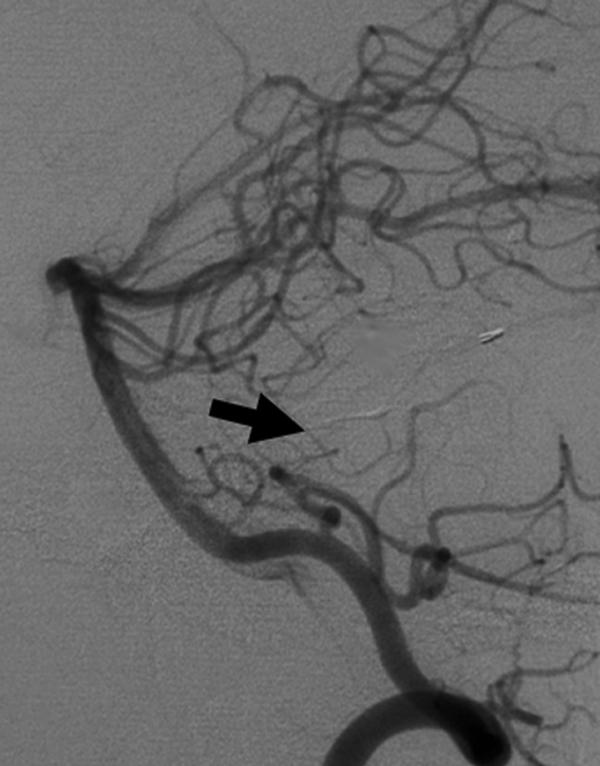
Follow-up angiogram, left vertebral artery injection, after the second-stage procedure confirms complete spontaneous resolution of the AICA feeding-artery aneurysm (arrow). AICA, anterior-inferior cerebellar artery.
DISCUSSION
Dural AVFs represent 10 to 15% of all intracranial vascular malformations. They consist of pathologic vascular channels within the dura mater and usually involve the walls of dural sinuses. Patients typically present between 40 and 60 years of age at various clinical stages, ranging from a pulse-synchronous bruit to a neurologic deficit to intracerebral hemorrhage.1
All dural AVFs are acquired, and many are preceded by dural sinus thrombosis.6,7,8 It is believed that venous hypertension from dural sinus thrombosis enlarges the normally present microscopic arteriovenous shunts in the walls of the sinus, leading to the formation of dural AVFs. Furthermore, venous hypertension induces ischemia, which also may play an important role in the pathogenesis of dural AVFs by stimulating angiogenic growth factors and angiogenesis.9
Several grading schemes10,11,12 have been proposed to predict the clinical course of dural AVFs. They all suggest that cortical venous drainage, a high-grade feature, is associated with a significant risk of intracerebral hemorrhage (33 to 42%).1,13
Dural AVFs involving the transverse and sigmoid sinuses are the most common, representing as many as 50% of cases. The epicenter is usually at the junction of the transverse and sigmoid sinuses. Only 10 to 15% of these lesions exhibit aggressive behavior.1 Typically, their arterial supply is from both the extracranial (branches of the occipital and middle meningeal arteries, posterior auricular artery, and ascending pharyngeal artery) and intracranial circulations (posterior meningeal branches of the vertebral artery and marginal tentorial branches of the meningohypophyseal trunk).
Spinal dural AVFs with associated feeding-artery aneurysms have been reported.14,15 However, there are only a few reported cases of feeding-artery aneurysms associated with intracranial dural AVFs.2,3,4 The two cases detailed in the literature involved an AICA and a posterior-inferior cerebellar artery feeding-artery aneurysm. Both aneurysms presented with SAH and were treated surgically with good outcomes.2,3 In contrast, our patient developed an asymptomatic AICA feeding-artery aneurysm from a transverse-sigmoid dural AVF that resolved spontaneously with treatment of the dural AVF.
The origin of feeding-artery aneurysms is controversial, but the main theory focuses on flow-related stress on the arterial feeders.16,17 Based on the morphology of the feeding vessel, we believe that the feeding-artery aneurysm in our patient was also flow-related and a result of the hemodynamic stress on the AICA caused by the high-flow dural AVF.
Intracranial hemorrhage resulting from venous hypertension is a main cause of morbidity and mortality related to dural AVF. Cortical venous drainage, as mentioned, is the major risk factor. At present, the additional effect of concurrent arterial aneurysms on the hemorrhagic risk of dural AVFs is unknown, although clearly these feeding aneurysms can hemorrhage.2,3 The literature on AVMs and concurrent aneurysms may shed some light on the issue. Aneurysms associated with AVMs have been reported in 5 to 7% of cases.18,19 Several retrospective and prospective studies have suggested a higher risk of hemorrhage in patients with an AVM and concurrent feeding-artery and intranidal aneurysm.18,19,20,21 Brown and colleagues22 reported an increased annual risk of 7% for intracranial hemorrhage in the setting of an unruptured AVM associated with a concurrent intranidal or feeding-artery aneurysm, creating an annual hemorrhage rate of 10%.
The treatment of feeding-artery aneurysms at the same time as an AVM is controversial. Some authors have recommended a conservative approach because they believe that these lesions are flow-related and may regress or remain stable once the AVM is removed.11 Others have proposed that such aneurysms should be treated immediately because of their risk of hemorrhage.18,23 At present, there is no treatment guideline for a concurrent arterial aneurysm and dural AVF.
In our case, we elected to follow the aneurysm because it was small and asymptomatic. We believed that it was flow related and would regress once the dural AVF was obliterated. In fact, the aneurysm regressed spontaneously after the staged, multimodal treatment of the dural AVF. Until more data are available regarding concurrent aneurysms and dural AVFs, however, the decisions of when and whether to treat an unruptured dural AVF feeding-artery aneurysm must be made on an individual basis.
ACKNOWLEDGMENT
We thank Kristin Kraus for her editorial support during the preparation of this article.
References
- Malek A M, Halbach V V, Higashida R T, Phatouros C C, Meyers P M, Dowd C F. Treatment of dural arteriovenous malformations and fistulas. Neurosurg Clin N Am. 2000;11:147–166. [PubMed] [Google Scholar]
- Kaech D, de Tribolet N, Lasjaunias P. Anterior inferior cerebellar artery aneurysm, carotid bifurcation aneurysm, and dural arteriovenous malformation of the tentorium in the same patient. Neurosurgery. 1987;21:575–582. doi: 10.1227/00006123-198710000-00027. [DOI] [PubMed] [Google Scholar]
- Gacs G, Vinuela F, Fox A J, Drake C G. Peripheral aneurysms of the cerebellar arteries: review of 16 cases. J Neurosurg. 1983;58:63–68. doi: 10.3171/jns.1983.58.1.0063. [DOI] [PubMed] [Google Scholar]
- Houser O W, Baker H L, Jr, Rhoton A L, Jr, Okazaki H. Intracranial dural arteriovenous malformations. Radiology. 1972;105:55–64. doi: 10.1148/105.1.55. [DOI] [PubMed] [Google Scholar]
- Friedman D I, Gordon L K, Egan R A, et al. Doxycycline and intracranial hypertension. Neurology. 2004;62:2297–2299. doi: 10.1212/wnl.62.12.2297. [DOI] [PubMed] [Google Scholar]
- Finn M, Klimo P, Jr, Couldwell W T. Interpositional dural graft technique for the treatment of dural arteriovenous fistulas. Neurosurg Focus. 2007;22(3):E10. doi: 10.3171/foc.2007.22.3.12. [DOI] [PubMed] [Google Scholar]
- Kutluk K, Schumacher M, Mironov A. The role of sinus thrombosis in occipital dural arteriovenous malformations: development and spontaneous closure. Neurochirurgia (Stuttg) 1991;34:144–147. doi: 10.1055/s-2008-1052075. [DOI] [PubMed] [Google Scholar]
- Chaudhary M Y, Sachdev V P, Cho S H, Weitzner I, Jr, Puljic S, Huang Y P. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol. 1982;3:13–19. [PMC free article] [PubMed] [Google Scholar]
- Touho H, Ohnishi H, Komatsu T, Furuoka N, Karasawa J. Dural arteriovenous fistula caused by sinus thrombosis: case report. Neurol Med Chir (Tokyo) 1994;34:543–546. doi: 10.2176/nmc.34.543. [DOI] [PubMed] [Google Scholar]
- Lawton M T, Jacobowitz R, Spetzler R F. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267–274. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- Djindjian R, Cophignon J, Theron J, Merland J J, Houdart R. Embolization by superselective arteriography from the femoral route in neuroradiology: review of 60 cases. 1: Technique, indications, complications. Neuroradiology. 1973;6:20–26. doi: 10.1007/BF00338854. [DOI] [PubMed] [Google Scholar]
- Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539–546. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- Lalwani A K, Dowd C F, Halbach V V. Grading venous restrictive disease in patients with dural arteriovenous fistulas of the transverse/sigmoid sinus. J Neurosurg. 1993;79:11–15. doi: 10.3171/jns.1993.79.1.0011. [DOI] [PubMed] [Google Scholar]
- Castaigne P, Bories J, Brunet P, Cassan J L, Meininger V, Merland J J. Arteriovenous fistulae of the dura mater: clinical and radiological study of 13 cases [in French] Ann Med Interne (Paris) 1975;126:813–817. [PubMed] [Google Scholar]
- Koch C, Gottschalk S, Giese A. Dural arteriovenous fistula of the lumbar spine presenting with subarachnoid hemorrhage: case report and review of the literature. J Neurosurg. 2004;100:385–391. doi: 10.3171/spi.2004.100.4.0385. [DOI] [PubMed] [Google Scholar]
- Malek A M, Halbach V V, Phatouros C C, et al. Spinal dural arteriovenous fistula with an associated feeding artery aneurysm: case report. Neurosurgery. 1999;44:877–880. doi: 10.1097/00006123-199904000-00114. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Handa H, Hashimoto N. Location of intracranial aneurysms associated with cerebral arteriovenous malformation: statistical analysis. Surg Neurol. 1984;22:335–340. doi: 10.1016/0090-3019(84)90135-6. [DOI] [PubMed] [Google Scholar]
- Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467–490. doi: 10.3171/jns.1966.25.4.0467. [DOI] [PubMed] [Google Scholar]
- Batjer H, Suss R A, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986;18:29–35. doi: 10.1227/00006123-198601000-00006. [DOI] [PubMed] [Google Scholar]
- Lasjaunias P, Piske R, Terbrugge K, Willinsky R. Cerebral arteriovenous malformations (C. AVM) and associated arterial aneurysms (AA): analysis of 101 C. AVM cases, with 37 AA in 23 patients. Acta Neurochir (Wien) 1988;91:29–36. doi: 10.1007/BF01400524. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mohr J P, Pile-Spellman J, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73:294–298. doi: 10.1136/jnnp.73.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Grzyska U. Clinical significance of pedicle aneurysms on feeding vessels, especially those located in infratentorial arteriovenous malformations. J Neurosurg. 2000;92:995–1001. doi: 10.3171/jns.2000.92.6.0995. [DOI] [PubMed] [Google Scholar]
- Brown R D, Jr, Wiebers D O, Forbes G S. Unruptured intracranial aneurysms and arteriovenous malformations: frequency of intracranial hemorrhage and relationship of lesions. J Neurosurg. 1990;73:859–863. doi: 10.3171/jns.1990.73.6.0859. [DOI] [PubMed] [Google Scholar]
- Thompson R C, Steinberg G K, Levy R P, Marks M P. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998;43:202–211. disc 211–212. doi: 10.1097/00006123-199808000-00006. [DOI] [PubMed] [Google Scholar]