ABSTRACT
Objective: To present the case of a rare tumor in the sphenoidoclival area and discuss potential pitfalls in diagnosis and management. Design: Case report with literature review. Setting: Tertiary referral center. Case Summary: Our patient presented with headache, vertigo, vision problems, and feeling of pressure in the central segment of the face. MR and CT showed a lesion in the body of the sphenoid, with signs of bone destruction and irregular borders. Differential diagnosis included intraosseous meningioma, chordoma, and inflammatory process. Results: Endoscopic/microscopic transnasal approach was performed to reach clival bone and to biopsy the tumor. Histopathological examination showed intraosseous lipoma. Conclusion: Intraosseous lipoma is a rare tumor, or more accurately a hamartoma, and is usually found in the calcaneus or in the proximal femur. It is even rarer in the skull base. Usually it does not present any symptoms and is an incidental finding during imaging for other symptoms. As a rule it runs an indolent course and does not require any treatment. Since no definitive diagnosis can be made only on the basis of imaging (CT and MRI), it requires an open biopsy that if possible should be made in accordance with the principles of minimally invasive surgery.
Keywords: Intraosseous lipoma, sphenoclival, skull base
Lipoma is a benign tumor that is frequently found in subcutaneous tissues but is extremely rare intracranially, representing only 0.34 to 0.46% of all brain tumors.1 Intraosseus lipoma is an even more rare variant, most commonly found in the appendicular skeleton and only rarely in the skull base. It is usually asymptomatic and is an incidental finding on the MR and/or CT scans. Imaging is not diagnostic in itself and the lesion can be mistaken for a variety of other tumors and inflammatory processes. Biopsy is necessary for a definitive diagnosis.
We present a case of an intraosseus lipoma in the sphenoidoclival area that was confirmed by biopsy. This site is an extremely rare location for such a tumor and to our knowledge only two other cases have been published previously.2
CASE PRESENTATION
A 37-year-old female was referred with a vague, unpleasant feeling and the sensation of pressure in her head and the central segment of her face. She also reported visual disturbance and vertigo. An MR scan revealed a hyperintensive lesion in both T1- and T2-weighted sequences. The lesion was poorly outlined and was situated in the body of the sphenoid bone. A narrow low-intensity signal on the outer rim of the lesion could be seen on T2-weighted images as could some hypointensive signal within the tumor (Figs. 1 and 2).
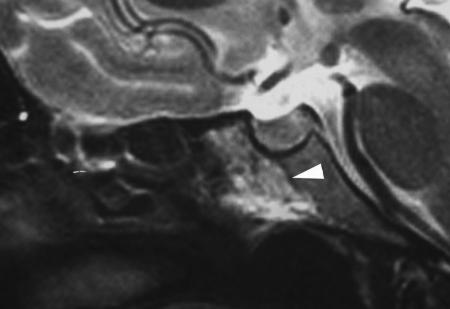
Figure 1.
T2-weighted image of the lesion shows a low-intensity signal on the outer rim of the lesions (white arrowhead), consistent with bone sclerosis.
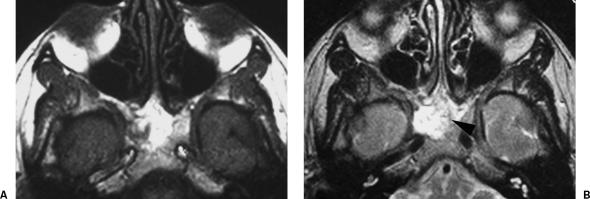
Figure 2.
(A) T1- and (B) T2-weighted images show a hyperintensive lesion in both sequences occupying the sphenoclival bone. In the T2-weighted image there are some regions of low-intensity signal (black arrowhead).
A high-resolution CT showed a defect in the bone of the sphenoclival region. The bony margins were highly irregular with no clear delineation between the lesion and the nasopharynx. The defect was filled with a hypodense soft-tissue mass. The right internal carotid artery in its vertical infraclinoid segment appeared to be dehiscent and in contact with the soft-tissue mass. The sphenoid sinuses were narrow and appeared to be normal (Fig. 3).
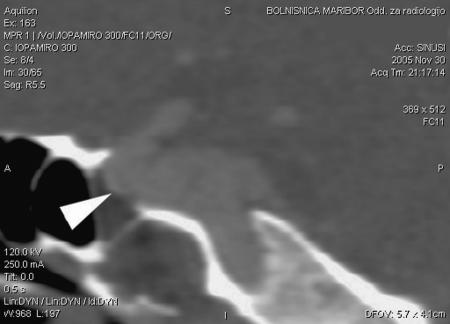
Figure 3.
CT scan shows dehiscent bony canal of the infraclinoid segment of the internal carotid artery (white arrowhead).
There were no neurological deficits on clinical examination. The patient's visual fields were also normal and there was no abnormality of either optic disc.
As no definitive conclusion could be drawn from imaging, we decided to biopsy the lesion.
We approached the lesion transnasally using a 0- and 30-degree telescope with a suction-irrigation system (Karl Storz GmbH & Co, Tuttlingen, Germany). A posterior ethmoidectomy and superior turbinate resection was made first and the sphenoid sinus was entered through the natural ostium which was subsequently enlarged. Mucosa of the sphenoid rostrum was removed and bone was drilled to remove the posterior aspect of the vomer and anterior wall of the sphenoid body. A soft, pulsative mass filled the whole of the sphenoid body. The opening was enlarged and the soft-tissue mass was carefully removed. The cavity was packed with Surgicel (Johnson & Johnson, New Brunswick, NJ, USA). Nasal packing was left in place for 24 hours. No postoperative complications occurred.
Histological examination of the operative specimen revealed fat cells and some dense connective tissue. We made the diagnosis of intraosseous lipoma (Fig. 4).
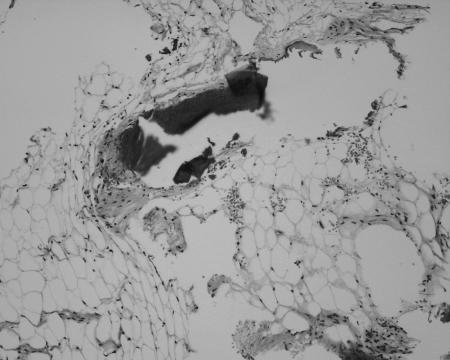
Figure 4.
Microscopic appearance of the tumor, showing some mature fat cells intermixed with dense fibrous tissue and trabecular bone.
The patient has been asymptomatic since the endoscopic resection of the intraosseous sphenoclival lipoma and is scheduled for a review MR scan every year.
DISCUSSION
Intraosseous lipomas are extremely rare and represent proliferation of fat tissue within the marrow of normal trabecular bone. They are benign neoplasms, but malignant transformation as well as its occurrence alongside meningiomas has been described.3,4 Intraosseus lipomas represent around 0.08% of all bone tumors. The incidence in the skull base is even rarer.2 A PubMed search using the terms “lipoma skull base” yields only 19 results. The query “lipoma sphenoid” yields 14 results, while “intraosessous lipoma sphenoid” produces only two. It is therefore an extremely rare tumor and is usually an incidental finding during the imaging of the head and skull base for other symptoms.
Intraosseus lipomas are far more frequent in other parts of the body. The high prevalence of the intraosseous lipoma in the calcaneus and intertrochanteric portion of the femur, where trabecular bone is scarce, has led to the postulation that intraosseous lipoma is an “overshoot” phenomenon that develops during the transition of hematopoetic to fatty marrow, and therefore that intraosseous lipomas in these sites might more correctly be considered hamartomas rather than neoplasms.5
Milgram6 classified intraosseous lipomas into three types. In the first type, the tumor contains viable fat cells; in the second, viable fat cells are partially replaced by necrosis and calcification; in the third type, fat necrosis, calcification, and cyst formation are found. According to Milgram, intraosseus lipomas develop predominantly in the appendicular and axial skeleton. In his series of 66 patients only two lipomas were found in the skull.
MR imaging shows viable fat in type 1 intraosseous lipomas. The fat is isointense to subcutaneous fat on T1-weighted images and exhibits low signal intensity with fat suppression on T2-weighted images. A thin rim of low-intensity signal is usually visible at the margin of the lesion, consistent with reactive sclerosis in the surrounding bone. In type 2 lesions one can again identify fat and the circumferential rim of decreased signal on T1- and T2-weighted images. Low signal intensity areas within the central portion of the lesion on T1- and T2-weighted images are consistent with calcifications. Type 3 lesions show a thin peripheral rim of fat that can be identified on MR imaging. Central calcification and a thick rim of surrounding sclerosis have low signal intensity on T1- and T2-weighted sequences. Areas of fat necrosis have a variable signal on T1-weighted and increased signal on T2-weighted images.7 In our case a retrospective review of the MR scans revealed an outer rim of the hypointensive signal and some low signal pattern in the tumor itself, consistent with calcification. According to Milgram this corresponds to the type 2 intraosseous lipoma.
With a symptomatic patient and a rare lesion, a broad differential diagnosis and treatment options must be considered. The differential diagnoses in this case included chordoma, fibrous dysplasia, inflammation, or intraosseous meningioma. Intraosseous lipoma is one of the rarest lesions in the skull base and was therefore not included in the differential diagnosis list. Due to differences in treatment of these diseases, a reasonable decision to obtain a biopsy was made. In most cases, biopsy of the sphenoid body can be achieved by minimally invasive surgery and is thus associated with low morbidity.
Complete removal of intraosseous lipoma is seldom indicated. Usually these lipomas don't present any symptoms, run an indolent course, and can be managed by a “wait and scan” policy. Exceptions to that rule are rare cases of lipomas that involve surrounding brain tissue and present with various compression symptoms. MacFarlane and colleagues2 reported two cases of intraosessous lipoma in the sphenoid bone that were the cause of visual disturbance and hypopituitarism and had to be surgically removed.
REFERENCES
- Kazner E, Stochdorph O, Wende S, Grumme T. Intracranial lipoma: diagnostic and therapeutic considerations. J Neurosurg. 1980;52:234–245. doi: 10.3171/jns.1980.52.2.0234. [DOI] [PubMed] [Google Scholar]
- MacFarlane M R, Soule S S, Hunt P J. Intraosseous lipoma of the body of the sphenoid bone. J Clin Neurosci. 2005;12:105–108. doi: 10.1016/j.jocn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Milgram J W. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19:347–352. doi: 10.1007/BF00193088. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Kimura M, Kinoshita A, Hasegawa M, Yamashita J. Meningioma associated with intraosseous lipoma. Clin Neurol Neurosurg. 2003;105:221–224. doi: 10.1016/s0303-8467(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Murphey M D, Carroll J F, Flemming D J, Pope T L, Gannon F H, Kransdorf M J. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24:1433–1466. doi: 10.1148/rg.245045120. [DOI] [PubMed] [Google Scholar]
- Milgram J W. Intraosseous lipomas. A clinicopathologic study of 66 cases. Clin Orthop Relat Res. 1988 Jun;(231):277–302. [PubMed] [Google Scholar]
- Propeck T, Bullard M A, Lin J, Doi K, Martel W. Radiologic-pathologic correlation of intraosseous lipomas. AJR Am J Roentgenol. 2000;175:673–678. doi: 10.2214/ajr.175.3.1750673. [DOI] [PubMed] [Google Scholar]