Abstract
Diabetic muscle infarction is a rare complication of diabetes mellitus that is not clearly defined in the orthopaedic literature. This study is a descriptive case series of 7 new cases of diabetic muscle infarction and 55 previously reported cases in the literature. In the majority of patients, diabetic muscle infarction presents as a localized, exquisitely painful swelling and limited range of motion of the lower extremity. No cases affecting the muscles of the upper extremity have been observed. The onset is usually acute, persists for several weeks, and resolves spontaneously over several weeks to months without the need for intervention. Diabetic muscle infarction is a condition that should be considered in the differential diagnosis of any diabetic patient with lower extremity pain and swelling without systemic signs of infection. Magnetic resonance imaging is sensitive and specific enough to make the diagnosis. Muscle biopsy and surgical irrigation and debridement are not recommended since they are associated with complications. Pain management and activity restriction in the acute phase followed by gentle physical therapy is the treatment of choice. Recurrences in the same or opposite limb are common. Although the short-term prognosis is very good and the majority of cases resolve spontaneously, the long-term survival is uncertain in this patient population.
INTRODUCTION
Diabetes mellitus is a common disease with a total number of cases estimated at 14 million in the United States. The most common diabetic complications seen in orthopaedic practice include neuropathy and vasculopathy (60-80%), diabetic foot ulcers and infections (2-3%), and neuropathic arthropathy (0.1-2.5%). Diabetic muscle infarction is a rare complication of diabetes mellitus that is not included in most standard orthopaedic texts. While previous reports have illustrated some of the clinical and imaging characteristics of this condition, the scarcity and widespread report of cases throughout different medical and surgical disciplines makes it difficult to determine its natural history and the most appropriate method of diagnosis and treatment 1–14, 16–28 . This study is a descriptive case series of 7 new cases and 55 previously reported cases of diabetic muscle infarction. We reviewed several controversial aspects of the diagnosis and management of this condition. We wish to call the attention of the orthopaedic community to this condition, so that unnecessary invasive diagnostic testing, biopsy and surgical debridement which may lead to further complications, can be avoided.
MATERIAL AND METHODS
We retrospectively reviewed the charts and imaging studies of seven patients with the diagnosis of diabetic muscle infarction without gangrene that were evaluated and treated in the Department of Orthopaedics at the University of Iowa Hospitals and Clinics. We also reviewed all the articles published on diabetic muscle infarction (MEDLINE database search). Data from the charts and the cases reported, when available, were obtained including age, gender, type of diabetes, insulin use and years of use, glucose control and systemic effects of the disease (nephropathy, neuropathy, retinopathy). Clinical data recorded include presentation, location and duration of the symptoms; previous injury or injections at the site; presence of concurrent infection; and systemic symptoms. Physical exam data recorded include area affected; presence of a mass and characteristics; pain with range of motion; skin changes; joint effusion; compartment tenderness; presence of adenopathy or gangrene. Laboratory results recorded include WBC count; hematocrit; Erythrocyte Sedimentation Rate; CK/LDH enzymes; and other tests if reported. Additional laboratory tests were also recorded including knee aspiration and blood/urine cultures. Imaging studies recorded include plain radiographs; ultrasound; vascular studies including doppler/venogram; bone/gallium scans; CT scan; and MRI. Surgical pathology data included the use and type of biopsy, surgical irrigation and debridement, and tissue culture results. Treatment type, response to treatment, complications, recurrences, follow-up and survival were also recorded.
RESULTS
There are remarkable similarities in the clinical presentation of the seven patients from this series and the reported cases as summarized on Table 1. Diabetic muscle infarction occurs most commonly in insulin-dependent patients (85%) who have poorly controlled diabetes mellitus and concomitant end-organ complications (nephropathy in 58%, neuropathy in 50%, and retinopathy in 45% of the patients). The average time of insulin use prior to the diagnosis was 14 years (range, 1 to 50). It occurs in an equal male/female distribution (53% and 47%, respectively) and the average age at presentation was 44 years of age (range, 19 to 81). Average follow-up was 16 months (range, 1 to 48 months).
TABLE 1. CLINICAL CHARACTERISTICS OF PATIENTS WITH DIABETIC MUSCLE INFARCTION.
| Author / year | Age (years) /Gender | Years diabetes | Systemic Complications | Symptoms (weeks) | WBC | Diagnostic tests | Biopsy | Recurrences | Follow up (mo) | Complications | Deceased | Muscles involved |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angervall and Stener (1965) | 52 / M | 10 | K | 4 | NR | angiography | excisional | No | 36 | No | Yes | bcp |
| 23 / M | 30 | NR | 4 | NR | angiography | excisional | No | NR | No | No | vl / add | |
| Levinsohn and Bryan (1979) | 28 / F | NR | K | 1 | 8900 | radiographs / doppler / CT | excisional | Yes | 24 | No | Yes | quad |
| Reich et al. (1985) | 54 / M | 18 | K,R,N | 1 | 6700 | radiographs / ultrasound / Myelogram / Ga 6 7 / Tc99 / MRI | No | No | 3 | No | No | add |
| 29 / F | 19 | K,R,N | 1 | NR | CT / MRI | No | No | 3 | No | No | psoas / quad | |
| Chester and Banker (1973 / 1986) | 28 / F | 15 | K,R,N | 4 | 8800 | venogram | needle / excisional | Yes | 24 | Yes | Yes | quad x 2 |
| 50 / F | 10 | K,R,N | 4 | 11100 | radiographs | needle / excisional | Yes | 36 | Yes | Yes | quad x 2 | |
| 34 / F | 7 | K,R,N | 2 | 6100 | radiographs / venogram | needle / excisional | Yes | 36 | No | Yes | gs / quad | |
| 34 / F | 18 | K,R,N | 2 | 12000 | Tc 99 / CT | needle / excisional | Yes | 48 | Yes | Yes | quad x2 | |
| 35 / F | 6 | K,N | 4 | NR | radiographs | needle | NR | NR | NR | NR | quad x2 | |
| 29 / F | 19 | K,R,N | 3 | 11500 | CT / MRI | needle / excisional | Yes | 36 | No | Yes | quad | |
| Ratlif et al. (1986) | 57 / M | NR | NR | 4 | NR | radiographs / ultrasound | excisional | No | NR | NR | No | vm |
| Grau et al. (1988) | 75 / M | NR | NR | 4 | NR | CT | No | No | NR | No | NR | vm |
| Boluda et al. (1989) | 64 / M | 15 | None | NR | NR | doppler / EMG / CT | No | No | 12 | NR | No | gs |
| Becker et al. (1992) | 48 / F | 10 | NR | 4 | NR | CT / angiography | excisional | No | NR | No | NR | smb |
| Lauro et al. (1991) / Bahron and Kissel (1992) | 29 / F | 20 | K,R,N | 2 | 8000 | radiographs / venogram / Tc 9 9 / MRI / EMG | incisional | Yes | 12 | NR | Yes | smb / std |
| Barton and Palmer (1993) | 42 / M | 21 | K | 2 | 8000 | MRI | excisional | Yes | 47 | No | No | vl x2 |
| Nunez-Hoyo et al. (1993) | 28 / M | 16 | K,R,N | 2 | NR | doppler / CT / Tc99 / MRI | needle | Yes | 12 | No | No | vm / gs / vl x2 |
| Rocca et al. (1993) | 51 / M | 5 | NR | 2 | 14500 | doppler / CT | incisional | No | 5 | No | No | vl |
| Hinton et al. (1993) | 29 / M | 20 | NR | 7 | 9600 | radiographs / ultrasound / Tc 99/ MRI / EMG | incisional | No | 2 | No | No | add / vm |
| Bodner et al. (1994) | 46 / M | 5 | K,R,N | 2 | 6000 | doppler / EMG / MRI | excisional | Yes | 2 | No | No | gs x2 |
| Barohn et al. (1994) | 19 / M | NR | NR | 1 | NR | EMG / ultrasound / MRI / Tc 99 | No | No | 2 | No | No | add / vl |
| Van Slyke et al. (1995) | 25 / F | NR | NR | 8 | NR | MRI | incisional | No | 2 | No | No | pop / fhl / fdl |
| 55 / M | NR | K,R,N | 28 | 8000 | venogram / MRI / doppler | excisional | Yes | 12 | No | No | vl / bcp | |
| Kiers (1995) | 47 / F | 42 | K,R | 1 | 12600 | venogram / MRI | no | No | 7 | No | No | quad / sart |
| Bjornskov e t al. (1995) | 35 / F | 18 | NR | 2 | NR | MRI | incisional | yes | NR | No | No | gra / add / quad |
| 48 / F | 27 | K,R,N | 1 | NR | venogram / MRI / Tc 99 | incisional | yes | NR | No | no | gs / vm x 2 / vl x 2 / bcp / sart | |
| Chason et al. (1996) | 38 / F | NR | K,R | 1 | 10900 | radiographs / ultrasound | incisional | No | 3 | Yes | No | grac |
| 36 / M | NR | K,R | 1 | 6000 | radiographs / CT | incisional | No | 6 | Yes | No | rect fem | |
| 42 / M | NR | K,R | 3 | 12400 | radiographs / MRI | incisional | No | 24 | Yes | No | vm | |
| 43 / M | NR | K | 8 | 8800 | MRI | excisional | No | 36 | No | No | vl | |
| Vande Berg et al. (1996) | 28 / M | 21 | K,R,N | 4 | NR | radiographs/ doppler / CT / MRI | needle | No | 3 | No | No | gs |
| Umpierrez et al. (1996) | 26 / F | 20 | K,R,N | 6 | 5700 | doppler/ MRI | needle / incisional | No | 1 | No | No | vl x2 / vl / gs |
| 31 / F | 15 | K,R,N | 2 | 11200 | MRI | incisional | Yes | 4 | No | No | vl x2 / gs | |
| 65 / F | 50 | K,R,N | 2 | NR | radiographs/ doppler / MRI | incisional | Yes | 36 | No | No | vl | |
| Anonimus (1997) | 81 / M | 1 | K,N | NR | 6000 | MRI | incisional | NR | NR | NR | NR | psoas / pect /add / obt int |
| Khoury e al. (1997) | 28 / F | 13 | None | 12 | 6000 | doppler / MRI | No | Yes | 5 | No | No | quad x2 / bcp x 2 / sart / tens fascia |
| 63 / F | 15 | K,R | 12 | 6000 | CT / MRI | No | No | 2 | No | No | rect fem | |
| Damron etal. (1998) | 59 / M | 12 | R,N | 3 | 6000 | radiographs/ Tc 9 9 / MRI | incisional | No | 28 | No | No | quad / bcp |
| 31 / M | 0 | None | 3 | NR | radiographs / doppler / ultrasound / MRI | No | No | 42 | No | No | vm / sart | |
| 48 / M | 15 | K,R,N | 4 | 6000 | radiographs / MRI | No | No | 24 | No | No | vm /vl / bcp | |
| Aboulafia et al. (1999) | 45 / F | 8 | K,N | 3 | 12000 | MRI / Ga 6 7 | incisional | No | NR | No | NR | NR |
| 59 / F | 15 | N | 4 | 12000 | radiographs / MRI / Tc 99 | needle | No | NR | Yes | NR | NR | |
| 43 / M | 17 | N | 6 | 6000 | MRI | needle | No | NR | Yes | NR | NR | |
| 40 / M | 8 | N | 3 | 6000 | MRI | needle | No | NR | No | NR | quad x2 | |
| 41 / M | 5 | N | 8 | 6000 | MRI | needle | No | NR | No | NR | NR | |
| 32 / M | 9 | NR | 12 | NR | MRI | needle | No | NR | No | NR | gs | |
| 39 / F | 13 | NR | 7 | NR | CT / MRI / Tc 99 | incisional / excisional | No | NR | No | NR | NR | |
| 53 / F | 16 | NR | 6 | NR | CT / MRI / Tc 99 | needle / incisional | No | NR | No | NR | NR | |
| 35 / F | 1 | NR | 8 | NR | MRI | needle | No | NR | No | NR | NR | |
| 46 / F | 9 | NR | 4 | NR | CT / MRI | needle | No | NR | No | NR | NR | |
| 40 / M | 3 | NR | 4 | NR | MRI | needle | No | NR | No | NR | NR | |
| 43 / M | 1 | NR | 8 | NR | CT / MRI | needle/ excisional | No | NR | No | NR | vm | |
| 36 / M | 3 | NR | 3 | NR | CT / MRI | needle | No | NR | No | NR | NR | |
| 55 / M | 20 | NR | 36 | NR | CT / MRI | needle | No | NR | NR | NR | NR | |
| Current Series (2000) | 36 / F | 23 | K,R,N | 8 | 8800 | radiographs/ doppler / MRI | excisional | Yes | 6 | Yes | Yes | add /sat /rect fem / vm / vl /obt / glut med /glut min |
| 66 / M | 30 | K,R,N | 6 | 10200 | ultrasound / MRI | No | No | 12 | Yes | No | glut max / vm /add | |
| 58 / M | 7 | K,N | 4 | 9200 | radiographs / ultrasound/ MRI | excisional | Yes | 12 | No | No | bcp | |
| 45 / M | 20 | K,R,N | 5 | 8500 | Ultrasound / doppler / MRI | needle | Yes | 24 | No | No | vm / vm / sart x 2 / bcp | |
| 34 / F | 20 | K,R,N | 2 | 12700 | Ultrasound / doppler / MRI | No | No | 30 | No | Yes | add / ham /quad x 2 | |
| 33 / M | 17 | K,R,N | 2 | 14400 | radiographs / ultrasound / MRI | needle | Yes | 16 | No | No | gs / quad | |
| 45 / F | 14 | K,R,N | 5 | 9000 | radiographs / MRI | No | No | 56 | No | No | quad |
Abbreviations: Not reported, NR; nephropathy, K; retinopathy, R; neuropathy, N; quadriceps, quad; adductor, add; sartorious, sat; rectus femoris, rect fem; vastus medialis, vm; vastus lateralis, vl; obturators, obt; gluteus medius, glu med; gluteus maximus, glu max; gluteus minimus, glu min; biceps femoris, bcp; hamstrings, ham; gastrosoleus, gs; gracilis, grac; pectineus, pct; tensor fascia latta, tens fascia; popliteus, pop; flexor hallucis longus, fhl; flexor digitorum longus, fdl
The characteristic clinical presentation was a sudden onset of pain and swelling in the extremity with limitation of range of motion. The pain was usually excruciating, persisting during rest and increasing with activity. The most common anatomic locations were the thigh (75%), the calf (15%), or both (10%). No history of trauma or injections were observed in our cases or the reported cases. The presence of concurrent infections was reported in 2 cases (3%). Ninety-six percent of the patients had no systemic symptoms such as fever, chills, night sweats or weight loss. Two patients had low back pain and one patient had abdominal pain. The duration of the symptoms prior to clinical consultation was 4 weeks (range, 1 to 36 weeks).
On physical exam, ninety-eight per cent of the patients had a localized, tender area with swelling and induration of the surrounding tissue. A palpable, firm, well-demarcated mass was observed in 32 patients (50%). In 5 cases (8%), there was skin redness but no induration. Knee joint effusion was reported in 2 cases (3%). No associated adenopathy, compartment syndrome or gangrene has been reported.
Laboratory evaluation demonstrated normal white cell count (79%), erythrocyte sedimentation rate (72%), and creatine kinase (85%). Anemia was observed in 10 cases (16%). Blood and joint aspiration cultures were negative.
Diagnostic imaging studies included MRI (76%), radiographs (35 %), vascular studies (34%), CT scan (29%), Tc99 bone scan (18%), and affected-area ultrasound (18%). Other studies included EMG/NCV studies (5 cases), angiography (3 cases), Ga 67 scan (2 cases), and a myelogram (1 case). Many patients had several tests performed (Table 1). MRI features included T1-weighted images demonstrating uniform, low-intensity signal enhancement of the affected muscle(s) with high contrast provided by the intermuscular fat planes. There are usually minimal changes of the subcutaneous tissues. T2-weighted images demonstrate high-intensity signal changes in the intra-and perimuscular tissues secondary to edema and hemorrhage. There is involvement of non-contiguous compartments and there are no bone marrow abnormalities (Figure 1). Gadollinium-DTPA contrast demonstrated linear areas of decreased signal intensity surrounded by rim-enhancement (+/- fluid collections). The muscles affected are described in Table 1. No cases of upper extremity muscles have been observed.
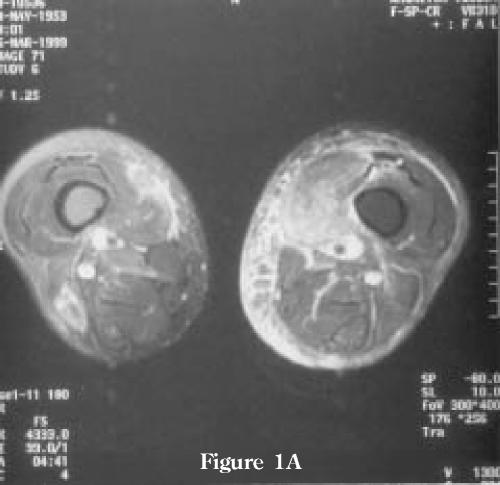
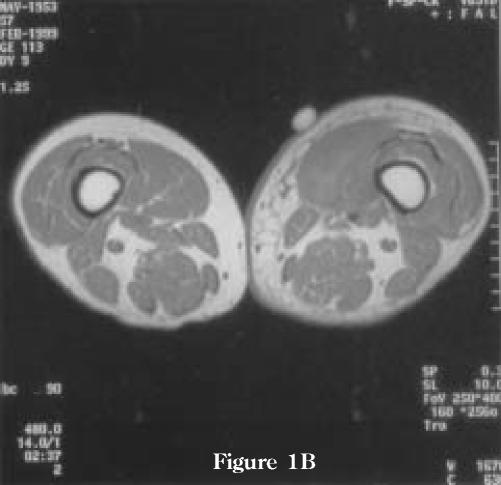
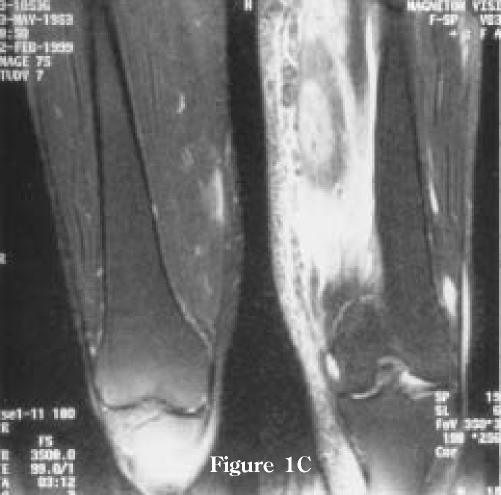
Figure 1.
MRI examination performed 4 weeks after the onset of pain in left thigh. (A) Axial T2-weighted images demonstrating high-intensity signal in the intra- and perimuscular tissues of the left vastus medialis muscle secondary to edema and hemorrhage. Note minimal associated edema of the subcutaneous tissue. There is some involvement of the non-contiguous compartments as well as the vastus medialis muscle on the non-symptomatic right side. (B) T1-weighted images demonstrating uniform, low-intensity signal enhancement of the left vastus medialis muscle. There are no bone marrow abnormalities. (C) Coronal T2 weighted image demonstrating the extension of the abnormalities in the left vastus medialis muscle and also minimal changes on the non-symptomatic right side.
Figure 1A.
Figure 1B.
Figure 1C.
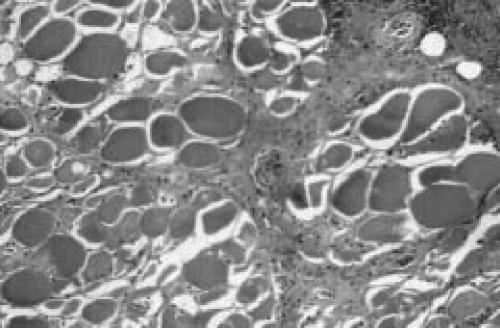
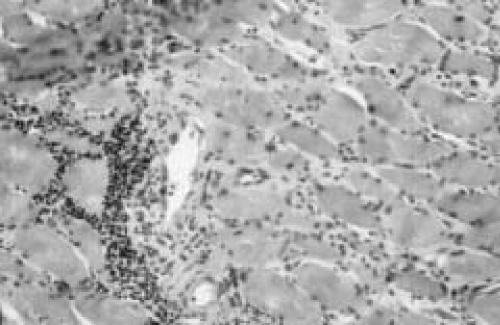
Needle biopsy was performed in 23 cases (45%), incisional biopsy in 17 cases (27%), and excisional biopsy 18 cases (29%). No biopsy was elected in 13 cases (21%). Four of the seven patients in our series had biopsies. The findings at the operation were consistent with what has been published in the literature. Briefly, there was edematous subcutaneous tissue and fascia. There was evidence of small pockets of edematous fluid deep to the fascia but no evidence of frank pus. The muscle was pale, felt woody and there was not responsiveness to pinching. Histologic examination showed skeletal muscle with areas of necrotic fibers surrounded by necrotic fibrous tissue, inflammatory tissue reaction, and hemorrhage (Figure 2). There were focal areas of intact muscle surrounded by abundant lymphocytic inflammation. Some of the areas showed collections of small atrophic fibers surrounded by fibrosis, and areas of muscle regeneration. Medium size arteries showed fibrinoid necrosis and others have a dense lymphocytic infiltrate in the media and intima. Occasionally, a superimposed infiltration of the subcutaneous tissues by polymorphonuclear leukocytes was also seen. Blood and tissues samples were examined for microorganisms. Gram stain demonstrated no white blood cells and no organisms. Aerobic, anaerobic, and fungus cultures were consistently negative.
Figure 2.
Representative histopathological findings of diabetic muscle infarction. (A) Skeletal muscle with areas of necrotic fibers surrounded by necrotic fibrous tissue and inflammatory tissue reaction. Scattered regenerating muscle fibers and marked endomysial edema are seen. Note thickening of the arteries and evidence of fibrinoid necrosis. (B) There are focal areas of abundant lymphocytic inflammation, but without evidence of concomitant vasculitis.
Treatment modalities reported include bed rest and analgesic in 94 percent of the patients, early ambulation in 25 per cent, and physical therapy in 43 percent. Other associated treatments included antibiotic therapy in 6 cases, anticoagulation in 3 cases and corticosteroids in 1 case. Clinical response demonstrated improvement of the symptoms in 82 percent of the patients at an average of 6 weeks (range, 3 to 14 weeks). There was recurrence of the condition in 21 per cent of the patients at an average of 20 weeks (range, 2 to 104 weeks). Ten patients (17%) were deceased.
Patients that had no biopsy or needle biopsy demonstrated no complications. Six patients that had aggressive physical therapy had recurrence of the symptoms. Patients that underwent excisional biopsy or surgical debridement had delayed wound healing (3 cases); a hematoma (2 cases); wound infection (1 case); nerve palsy (1 case); heterotopic ossification (1 case); and the need for blood transfusion (1 case).
DISCUSSION
Diabetic muscle infarction is an unusual complication of diabetes mellitus. The apparent rarity of this condition may make it difficult for clinicians and radiologists to become familiar with this entity, but diabetic muscle infarction is a distinctive illness that can be easily recognized. Angervall and Stener first described this condition as "tumoriform focal muscular degeneration" in two non-insulin dependent diabetic patients. Both patients were suspected of having a neoplasm prior to the biopsy. Pathologic examination of the muscle revealed no infection or tumor but a central area of hemorrhagic necrosis surrounded by muscle fibers in various stages of degeneration and regeneration, with hyalinosis and thickening of arterioles2.
In a diabetic patient with painful swelling in the extremity, the differential diagnosis should include the conditions summarized in Table 2. Typically, diabetic muscle infarction presents as a localized, exquisitely painful mass associated with swelling and limited range of motion of the extremity. The onset is usually acute, persists for a few weeks, and there are no systemic signs of infection.
TABLE 2. Differential Diagnosis of Diabetic Muscle Infarction.
|
Vascular conditions and infections are the most common conditions to consider in the differential diagnosis. Deep venous thrombosis has been considered in almost every case of diabetic muscle infarction. The localized nature of the condition and the absence of distal edema or involvement of the lower portions of the limb will help in the diagnosis. However, vascular studies may be necessary to differentiate between the two. Primary hemorrhage into the muscle presenting with a localized, painful mass may mimic diabetic muscle infarction and may require imaging studies for the differential diagnosis. Pseudothrombophlebitis may present as a painful, swollen limb, but there is a typical history of arthritis, trauma, or recent exertion, and many of the patients will have a palpable cyst at the time of presentation. In arterial occlusion, the major blood supply of the limb is compromised and there may be absence of distal pulses with skin changes. A history of blunt trauma or recent strenuous activity can differentiate contusion, muscle strain or posttraumatic false aneurysm.
In a diabetic patient, and particularly one who is receiving insulin injections, local infections are not uncommon. Cellulitis is usually easily recognized, as it is superficial in nature. Soft-tissue abscesses and pyomyositis do result in pain, swelling and the development of a mass in the extremity. Fulminant conditions, i.e., necrotizing fascitiis, may be more difficult to differentiate especially at the early stages. However, patients will become rapidly ill and there may be evidence on physical exam of diffuse undermining of the skin. Occasionally, an osteomyelitis with adjacent soft-tissue involvement may present as a mass within the muscle. However, there are accompanying systemic signs of infection and characteristic radiographic features. Parasitic infections are extremely rare and the diagnosis may require imaging studies and biopsy.
The acute onset of the symptoms and the initial degree of pain are inconsistent with a primary soft-tissue neoplasm. Inflammatory myositis may present as a painful swelling of the extremity but creatine kinase elevations and proximal muscle weakness usually indicate the appropriate diagnosis. Neurologic syndromes may begin with an abrupt onset of lower extremity pain that ultimately will involve the opposite side. However, the pain is usually localized to the low back and buttocks and patients develop dramatic weakness and atrophy of the muscles25.
Laboratory tests are helpful in the differential diagnosis, since the WBC, erythrocyte sedimentation rate, coagulation profile, and creatine kinase are usually normal, findings that help to distinguish diabetic muscle infarction from other entities. The failure of muscle enzymes to rise despite the presence of muscle necrosis is difficult to explain. It may depend upon when the CKs were obtained in the course of the disease (unclear for the cases reported in the literature) or to the limited amount of tissue involved. It is also possible that an elevated CK would be found more often if obtained within the first several days after the acute muscle infarct.
Different imaging modalities have been used to assess patients with painful swelling in the extremity. In the early reports, angiography was used and it showed atherosclerosis in the large and medium size arteries, but it has minimal value in the evaluation of these lesions today2. Standard radiographic films have been rarely helpful, except to exclude bony abnormalities or soft-tissue calcification. Radionucleotide studies using T99 or Ga67 demonstrate nonspecific accumulation of the tracer in the soft tissue5,6,24. Doppler ultrasound and venography of the lower extremity have been frequently performed, but they have been consistently negative. Ultrasonography has also been reported to show heterogenous, mass-like echogenic changes with loss of normal myofascial interfaces representing muscle swelling 12,28. However, most of these studies are nonspecific and are probably unnecessary in the diagnostic work-up of patients suspected to have diabetic muscle infarction.
CT scan will show increased muscle size and lower attenuation due to edema and may help to exclude localized abscess, tumor or bone destruction, but it is of limited help in the evaluation of muscle pathology11–13,17,21,24. MRI more sensitively evaluates pathologic changes in the muscle and offers additional advantages over other conventional imaging studies. The MRI provides better anatomic definition than radionucleotide imaging, and greater sensitivity to biochemical alterations than CT scanning. MRI also allows evaluation of bone and bone marrow to rule out osteomyelitis. An additional advantage of MR imaging is the ability to perform venograms, excluding the presence of deep venous thrombosis.
MR imaging shows an increase in T2 signal in the affected muscle, most likely secondary to increase in tissue water. On T1-weighted images, the involved areas are isointense or hypointense relative to normal muscle. Perifascial and subcutaneous edema is a rather uncommon finding and, when present, is usually minimal. The relative absence of subcutaneous edema in patients with diabetic muscle infarction is helpful to differentiate it from cellulitis, in which subcutaneous swelling is the rule. Rarely, early pyomyositis can present with similar findings to diabetic muscle infarction, i.e., diffuse muscle abnormality without fluid collection15,18. In this situation, if there are clinical and laboratory findings suggestive of infection and a rim-enhancing lesion on MRI, pyomyositis should be considered. Gadolinium-enhancement is not necessary for the diagnosis of diabetic muscle infarction and should only be used if pyomyositis or soft-tissue/ muscle abscess is considered in the differential diagnosis 18.
The relationship between MRI abnormalities and symptoms is still controversial. MRI changes can resolve with the patient's clinical improvement and reappear with recurrence of symptoms6,9,18,20,22,27. However, abnormal MRI changes in muscle that do not appear to be clinically involved and that preceded clinical symptoms by up to 6 months have been observed5,6,17. Hence, while it has been suggested that MRI changes be closely related to the duration of the symptoms, larger series are needed to correlate these changes27.
Biopsy may be needed in cases of diabetic muscle infarction in which the clinical presentation or imaging findings is atypical, or in which the recovery is delayed. MRI may obviate biopsy by excluding pyomyositis, abscess, osteomyelitis or tumor. If confirmatory biopsy is needed, needle or incisional biopsy has been recommended since open biopsy has been associated with an increased risk of postoperative complications4,13.
The treatment of choice of diabetic muscle infarction is analgesics and activity restriction in the acute phase, followed by gentle physical therapy until the resolution of the symptoms. In most cases, symptoms will resolve spontaneously without the need for surgical debridement. In very rare occasions, patients might not improve with this regimen and will benefit from surgical resection1. However, the complications reported in the literature appear to occur only when attempts have been made to excise the involved muscle and when physical therapy was begun early in the postoperative period. Banker and Chester described two patients who had excisional biopsy and early mobilization4. They developed hemorrhage and one required blood transfusion. In addition, the recovery period was prolonged because of repeated episodes of pain and swelling. In addition, when physical therapy has begun within the first few weeks after the diagnosis had been made, the symptoms were exacerbated4,13,14,27. Therefore, not only open biopsy, but zealous postoperative physical therapy should be discouraged.
Recurrences involving the original or contralateral limb have been observed in 21 percent of the patients. In all instances, the second event resolved rapidly. Although the short-term prognosis is very good and the majority of cases resolve spontaneously, the long-term survival is uncertain in this patient population. As many as half of the patients with diabetic muscle infarction have systemic end-organ complications. Seventeen per cent of the patients died between one and four years after the diagnosis had been made.
In summary, diabetic muscle infarction is a condition that should be considered in the differential diagnosis of any diabetic patient with lower extremity pain and swelling without systemic signs of infection. Magnetic Resonance Imaging is sensitive and specific for making the diagnosis. Muscle biopsy and surgical irrigation and debridement are not recommended, since they are associated with further complications. Pain management and activity restriction in the acute phase followed by gentle physical therapy are the recommended treatments.
References
- 1.Aboulafia AJ, Monson DK, Kennon RE. Clinical and radiological aspects of idiopathic diabetic muscle infarction. Rational approach to diagnosis and treatment. J Bone Joint Surg. (B) 1999;81:323–326. doi: 10.1302/0301-620x.81b2.9205. [DOI] [PubMed] [Google Scholar]
- 2.Angervall L, Stener B. Tumoriform focal muscle degeneration in two diabetic patients. Diabetologica. 1965;1:39–42. [Google Scholar]
- 3.Image interpretation session:1996. Diabetic muscle infarction (DMI) Radiographics. 1997;17(1):246–248. doi: 10.1148/radiographics.17.1.9017817. Anonymous. [DOI] [PubMed] [Google Scholar]
- 4.Banker BQ, Chester CS. Infarction of thigh muscle in the diabatic patient. Neurology. 1973;23:667–677. doi: 10.1212/wnl.23.7.667. [DOI] [PubMed] [Google Scholar]
- 5.Barohn RJ, Kissel JT. Case of the month: painful thigh mass in a young woman: diabetic muscle infarction. Muscle & Nerve. 1992;15:850–855. doi: 10.1002/mus.880150715. [DOI] [PubMed] [Google Scholar]
- 6.Barohn RJ, Bazan C, Timmons JH, Tegeler C. Bilateral diabetic thigh muscle infarction. J Neuroimaging. 1994;4:43–44. doi: 10.1111/jon19944143. [DOI] [PubMed] [Google Scholar]
- 7.Barton KL, Palmer BF. Bilateral infarction of the vastus lateralis muscle in a diabetic patient: a case report and review of the literature. J Diab Comp. 1993;7:221–223. [PubMed] [Google Scholar]
- 8.Becker BN, Otley CC, McNeill DB, Weintraub ID, Harrelson JM. Microangiopathic ischemic myopathy of semimembranosus muscle with diabetes mellitus. Diabetes Care. 1992;15:586–587. doi: 10.2337/diacare.15.4.586. [DOI] [PubMed] [Google Scholar]
- 9.Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5:39–45. doi: 10.1016/0960-8966(94)e0027-6. [DOI] [PubMed] [Google Scholar]
- 10.Bodner RA, Younger DS, Rosoklija G. Diabetic muscle infarction. Muscle and Nerve. 1994;17:949–950. doi: 10.1002/mus.880170817. [DOI] [PubMed] [Google Scholar]
- 11.Boluda B, Mesa J, Obiols G, Simo R. Focal muscle infarction in a diabetic. Diabete Metab. 1989;15:269–270. letter. [PubMed] [Google Scholar]
- 12.Chason DP, Fleckenstein JL, Burns DK, Rojas G. Diabetic muscle infarction: radiologic evaluation. Skeletal Radiol. 1996;25:127–132. doi: 10.1007/s002560050048. [DOI] [PubMed] [Google Scholar]
- 13.Chester CS, Banker BQ. Focal infarction of muscle in diabetics. Diabetes Care. 1986;9:623–630. doi: 10.2337/diacare.9.6.623. [DOI] [PubMed] [Google Scholar]
- 14.Damron TA, Levinshon EM, McQuail TM, Stadnick M, Rooney M. Idiopathic necrosis of skeletal muscle in patients who have diabetes. J Bone Joint Surg. (A) 1998;80:262–267. doi: 10.2106/00004623-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Gordon BA, Martinez S, Collins AJ. Pyomyositis: characteristics at CT and MR imaging. Radiology. 1995;197:279–286. doi: 10.1148/radiology.197.1.7568838. [DOI] [PubMed] [Google Scholar]
- 16.Grau A, Ricart W, Sola P. Infarto muscular espontaneo en la diabetes mellitus. Med Clin. 1988;91:37. [PubMed] [Google Scholar]
- 17.Hinton A, Heinrich SD, Craver R. Idiopathic diabetic muscular infarction: the role of ultrasound, CT, MRI, and biopsy. Orthopaedics. 1993;16:623–625. doi: 10.3928/0147-7447-19930501-19. [DOI] [PubMed] [Google Scholar]
- 18.Khoury NJ, El-Khoury GY, Kathol MH. MRI diagnosis of diabetic muscle infarction: report of two cases. Skeletal Radiol. 1997;26:122–127. doi: 10.1007/s002560050205. [DOI] [PubMed] [Google Scholar]
- 19.Kiers L. Diabetic muscle infarction: magnetic resonance imaging (MRI) avoids the need for biopsy. Muscle Nerve. 1995;18:129–130. letter. [PubMed] [Google Scholar]
- 20.Lauro GR, Kiessel JT, Simon SR. Idiopathic muscle infarction in a diabetic pattient. Report of a case. J Bone Joint Surg. (A) 1991;73:301–304. 2. [PubMed] [Google Scholar]
- 21.Levinsohn EM, Brian PJ. Computed tomography in unilateral extremity sweling of unusual cause. J Comput Assit Tomogr. 1979;3:67–70. doi: 10.1097/00004728-197902000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Nuñez-Hoyo M, Gardner CL, Motta AO, Ashmead JW. Skeletal muscle infarction in diabetes: MR findings. J Comp Assit Tomog. 1993;17(6):986–988. doi: 10.1097/00004728-199311000-00027. [DOI] [PubMed] [Google Scholar]
- 23.Ratliff JL, Matthews J, Blalock JC, Kasin JV. Infarction of th equadriceps muscle: a complication of diabetic vasculopathy. South Med J. 1986;79:1595. doi: 10.1097/00007611-198612000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Reich S, Weiner S, Chester C, Ruff R. Clinical and radiological features of spontaneus muscle infarction in the diabetic. Clin Nucl Med. 1985;10:876–879. doi: 10.1097/00003072-198512000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Rocca PV, Alloway JA, Nashel DJ. Diabetic muscular infarction. Sem Arth Rheum. 1993;22(4):280–287. doi: 10.1016/0049-0172(93)80076-r. [DOI] [PubMed] [Google Scholar]
- 26.Umpierrez GE, Stiles RG, Kleinbart J, Krendel DA, Watts NB. Diabetic Muscle Infarction. Am J Med. 1996;101:245–250. doi: 10.1016/S0002-9343(96)00156-8. [DOI] [PubMed] [Google Scholar]
- 27.Van Slyke MA, Ostrov BE. MRI evaluation of diabetic muscle infarction. Magnetic Resonance Imaging. 1995;12:32–329. doi: 10.1016/0730-725x(94)00102-9. [DOI] [PubMed] [Google Scholar]
- 28.Vande Berg B, Malghem J, Puttemans T, Vandeleene B, Lagneau G, Maldague B. Idiopathic muscular infarction in a diabetic patient. Skeletal Radiol. 1996;25:183–185. doi: 10.1007/s002560050059. [DOI] [PubMed] [Google Scholar]