Abstract
Background
Although one of the most important factors in predicting outcome of articular fracture, the comminution of the fracture is only subjectively assessed. To facilitate development of objective, quantitative measures of comminution phenomena, there is need for a bone fragmentation surrogate.
Methods
Laboratory investigation was undertaken to develop and characterize a novel synthetic material capable of emulating the fragmentation and radiographic behavior of human cortical bone.
Result
Screening tests performed with a drop tower apparatus identified high-density polyetherurethane foam as having suitable fragmentation properties. The material's impact behavior and its quasi-static mechanical properties are here described. Dispersal of barium sulfate (BaSO4) in the resin achieved radio-density closely resembling that of bone, without detectably altering mechanical behavior. The surrogate material's ultimate strength, elastic modulus, and quasi-static toughness are within an order of magnitude of those of mammalian cortical bone. The spectrum of comminution patterns produced by this material when impacted with varying amounts of energy is very comparable to the spectrum of bone fragment comminution seen clinically.
Conclusions
A novel high-density polyetherurethane foam, when subjected to impact loading, sustains comminuted fracture in a manner strikingly similar to cortical bone. Moreover, since the material also can be doped with radio-opacifier so as to closely emulate bone's radiographic signature, it opens many new possibilities for CT-based systematic study of comminution phenomena.
INTRODUCTION
Bone surrogates usefully serve a number of niche purposes in orthopaedics. Manufactured plastic anatomic replicas of skeletal members are widely used for student teaching, for motor skill development, and as visual aides for patient communication. They hold obvious advantages over natural bone in terms of durability, reproducibility, versatility (e.g., whole-joint models including ligaments, menisci, etc.), and economy. Recently, patient-specific stereolithographic plastic bone replicas generated from CT data have proven valuable in planning complex surgical procedures10. In laboratory settings, biomechanically realistic whole-bone models made from fiberglass laminates or from acrylic/epoxy are widely used for purposes of implant design and construct strength testing6. At the intrinsic tissue level, a large number of ad hoc research protocols18— and now even several consensus testing standards3— utilize surrogate materials specifically developed to mimic bone's local interaction with orthopaedic devices (e.g., screw purchase, suture holding strength,5,9,17 etc.)
Another potential use of surrogates is to help systematically investigate aspects of bone behavior which are otherwise difficult to study. One seemingly fertile area is the phenomenon of comminution in high-energy fractures. Complex fragmentation patterns in high-energy fractures typically defy classification by conventional measures11, despite recognition that comminution severity correlates with tissue-level energy absorption, and hence with treatment outcome. A surrogate material with bone-like radiographic properties, and which mimics bone's tendency to shatter into ever smaller pieces with increased impact energy, would be useful for developing objective digital image analysis techniques to quantify the morphology and displacement of comminuted fragments.
Taking this possibility a step further, it may even be feasible to quantify the energy responsible for a given fracture. A means to do so would be to draw upon the principles of engineering fracture mechanics, in that the energy absorbed in propagating a crack through a solid medium is equal to the medium's fracture energy per unit surface area (a measurable material property) times the total surface area liberated1. For a given comminuted fracture, in principle one could use image analysis of CT-apparent fragment margins to measure liberated surface area, and hence estimate energy delivery. Developing the necessary computer programs would be aided by having bone-fracture-mimicking surrogate material amenable to controlled-energy impacts. This paper describes the composition and characterization of a novel surrogate material that is being utilized to facilitate the development of objective, quantitative measures of comminution phenomena.
MATERIALS AND METHODS
Foam composition and radio-opacification
Various types of fracturable materials (acrylics, ceramics, brittle foams) were screened for fracture patterns consistent with those of bone, using a drop tower test. Of these materials, the most promising were dense, closed-cell foams created by mixing polyetherurethane resin with methyl diphenyl diisocyanate. That class of foams' cell size and texture can be controlled with a silicon surfactant. It was found that pulverization tendencies (i.e., the propensity to produce powder rather than discrete fragments) were less for glucose-based (versus sucrose-based) foams, and that powdering also tended to decrease with increased density. We judged that clinically realistic fragment morphology occurred for a density of about 640 kg/m3. To adjust the radiographic appearance of the material, finely sieved anhydrous BaSO4, a clinically familiar radio-opacifying agent, was dispersed throughout the foam in concentrations ranging from five to twenty-five weight-percent. Specimens were examined radiographically using a Toshiba Express/XS CT scanner (Toshiba, Tustin, CA) at 120 kVp and 250 mAs. The Hounsfield number (H) was sampled at five locations in each of four transverse CT slices ranging from 1 mm to 10 mm in thickness (1mm, 2mm, 5mm, 10mm). A best-fit curve was then determined for this data.
Drop tower testing
Next, parametric drop tower testing was conducted at various input energies and with differing mass/velocity combinations. Specimens with twenty-percent BaSO4 concentration♣ were machined to uniform hollow cylinders (19.69 mm OD, 11.11 mm ID, 69.22 mm height). During testing, a flat platen was placed on top of test specimens to ensure even distribution of the impact load. For one set of test specimens (n=6 per group), five distinct energy levels (ranging from 2.73 x 106 to 9.63 x 106 J/m3) were delivered, all with the same impact velocity (6.24 m/s) but with different drop masses. The minimum energy level was based on a pilot test group which had shown that an input energy of 2.23 x 106 J/m3 reliably produced small cracks, but was insufficient to cleave the specimens into separate fragments. In a second set of drop tests (n=8 for each of five groups), all groups received the same energy input of approximately 4.66 x 106 J/m3, but impact velocities differed (3.46 to 6.92 m/s).
Specimen preparation
To further characterize the foam surrogate, its mechanical properties were compared to those of bovine cortical bone via quasistatic four-point bend testing and by pendulum impact testing. All bovine specimens were machined from three tibiae that had been obtained from a local slaughterhouse and fresh frozen to –20°C. During all phases of specimen preparation, the bones were continually hydrated with saline. Tibiae were first rough-cut into curved longitudinal beams using a bandsaw. The long axis of the specimens was made roughly parallel to the long axis of the bone. Bone samples were then milled to their final dimensions within 0.25 mm. Foam samples were also milled.
Four-point bend testing
An MTS Bionix 858 Servohydraulic Test Machine (MTS Corporation, Eden Prairie, MN) with a 2500 N load cell was used to perform four-point bending tests on the foam. Standard four-point bend fixtures (span separation of 38.1 mm (1.5 in) for the foam and 25.4 mm (1.0 in) for the bone) were used. The crosshead speed was set in such a manner as to produce a strain rate of 0.00017 s-1. Span-to-depth ratio (unsupported length to bone thickness) was 16:1, as recommended by ASTM standards.2 Flexure specimen dimensions were 139.3 x 12.7 x 7.14 mm for the foam (n=6), and 101.6 x 12.7 x 4.76 mm bone (n=5). From the recorded load-deflection curve, Young's modulus (E) was calculated according to:
where c = span distance between the inner contacts, I = cross-sectional area moment of inertia, and P/y = slope of the load-deflection curve.
Pendulum impact testing
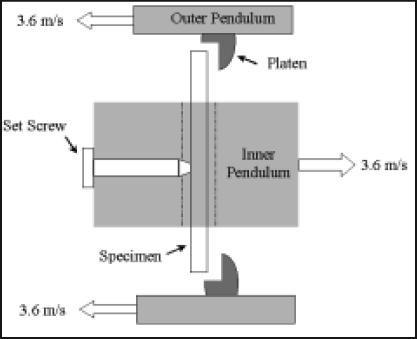
The test apparatus (Hounsfield balanced impact tester (Figure 1A)) consisted of two cast iron pendulums, simultaneously released from height. The test specimens traveled in a slot on one pendulum, and, at the lowest point in the swing (Figure 1B), percussed against two flat points on the other approaching pendulum. The energy lost to the specimen during this three-point bending impact loading event, manifest by reduced pendulum upswing, was registered on a dial scale. For our impact tests, the impact velocity was 7.2 m/s. Specimen dimensions were 45.72 x 13.72 x 5.97 mm (n=8 per group), and they were positioned such that impact was transverse to the (anatomic) longitudinal direction. Results for bone and foam were compared with an unpaired, homoscedastic t-test.
Figure 1a.
Hounsfield Balanced Impact Test Machine. The pendulum on the left (outer pendulum) is slotted in the middle (and has the impaction platens) such that the pendulum on the right (inner pendulum, with the specimen) can swing through it. The white dashed line indicates the location of the impact plane.
Figure 1b.
Top view of "impact plane" just before the instant of specimen contact in the dual pendulum apparatus.
RESULTS
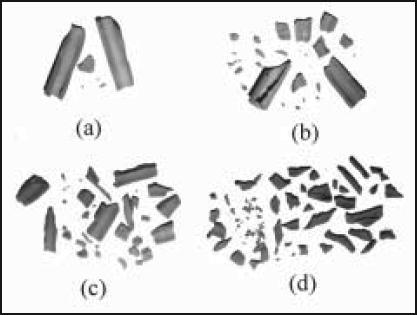
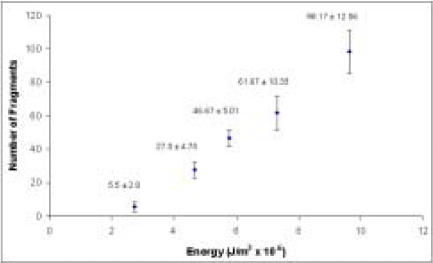
Fracture patterns obtained from drop tower tests confirmed that, as is seen in clinical bone fracture cases, polyetherurethane foam is fracturable into ever-larger numbers of irregular fragments with increasing energy input (Figure 2 and 3). As energy input increases, fragment size decreases, number of fragments increases, and 'sharpness' of fragments increases.
Figure 2a-d.
Foam surrogate fragmentation increases monotonically with input energy from drop tower tests. Input energy in this series ranges from 2.73 x 106 J/m3 to 9.63 x 106 J/m3.
Figure 3.
Fragment count for different energy levels in drop tower impacts to the surrogate material. (Constant velocity, varying drop mass).
The surrogate closely replicates the CT signature of natural bone at clinically relevant scan parameters. Hounsfield number varied in direct proportion to percentage of BaSO4. This relationship was very well characterized by a linear regression equation (R2 = 0.9999):
| H = 99.497 x (% BaSO4) – 427.6 |
Foam containing twenty percent BaSO4 appeared in the CT scans as 1555 ± 28 H, as averaged over all thicknesses.
In pendulum impact testing (Table I), the foam's energy absorption capacity was marginally higher than that of cortical bone. (The foam, as would be expected, exhibited far less variability than natural bone.) The foam material was an order of magnitude lower than cortical bone in terms of bending modulus and ultimate strength (Table I). The foam's quasi-static toughness (Table I), computed from a stress-strain curve, was halfway between the values reported for cortical bone and regressed for cancellous bone14.
Table I. Four-point bend and pendulum impact test results.
| Foam | Cortical Bone | |
|---|---|---|
| Absorbed Energy | 0.071 ± 0.009 J/mm2 | 0.106 ± 0.048 J/mm2 |
| Young's Modulus | 0.795 ± 0.030 GPa | 13.69 ± 4.25 GPa |
| Quasi-static Toughness | 4.00 ± 0.24 MPa | 21.17 ± 3.48 MPa |
| Ultimate Strength | 27.34 ± 1.74 MPa | 222.00 ± 39.53 MPa |
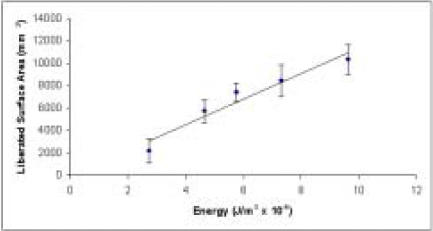
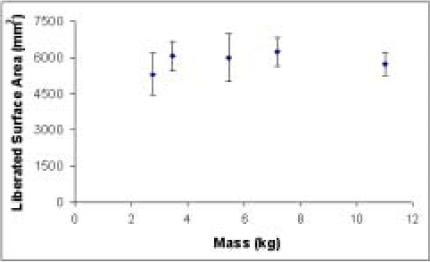
A first-order approximation of surface area can be obtained from fragment counts with the simplifying assumption of identical size and nominally spherical morphology for the foam fragments produced by each specimen. Plotted in that idealized manner, data in Figures 4 and 5 are reasonably consistent with the theoretically suggested linear proportionality between energy and interfragmentary surface area. Linear regression of the data from the constant velocity series yields the line of best fit depicted in Figure 4, with an R2 value of 0.937. In the series with constant energy input, analysis of variance demonstrates no significant dependence of liberated surface area solely on mass (p=0.240) or impact velocity (p=0.124).
Figure 4.
Estimated interfragmentary surface area increases in proportion to input energy in foam surrogate specimens.
Figure 5.
Approximate foam surface area liberated with constant energy input for various mass/velocity combinations. Analysis of variance shows no statistically significant effect of mass (p=0.240) on liberated surface area if energy is constant.
DISCUSSION
Predicting the force and energy necessary to cause bony fracture has been a topic of interest to biomechanists and orthopaedists for over a hundred years.13 With this new surrogate foam as a study vehicle, the reverse can now also be contemplated - examining a fractured bone and measuring the energy it absorbed.
Radio-opacification
There is appreciable variation in CT Hounsfield number for natural cortical bone, the reported range being from +1000 to +2000 H.8 When doped with twenty percent BaSO4 the foam falls at the midpoint of this range. If needed, the foam's radiodensity can be modulated to span the entire range from cortical to cancellous bone.
An added benefit of this novel surrogate material is that it is easily machinable. Thus, highly precise geometrical objects can be produced from the foam, for example for computer model validation purposes. Alternatively, as needed, the foam can be fabricated into irregular (e.g., anatomic) shapes by means of pressurization molding.
Mechanical testing
The present four-point ultimate bending strength of bovine cortical bone (222.00 ± 39.53 MPa) compares well with the results of Martin and Boardman12 for plexiform bovine tibial bone (230.5 ± 17.7 MPa). The present elastic modulus and ultimate strength are also similar to values previously reported by Behiri et al4. Compared to these typical cortical bone data, the polyetherurethane surrogate material is an order of magnitude lower in bending modulus and ultimate strength. The foam's quasi-static toughness is about halfway between cortical and cancellous bone fracture toughness14. In the four-point bend test, the foam exhibited about one-fifth the toughness of bovine cortical bone. However, it should be recognized that the present data for cortical bone stiffness, strength, and quasi-static toughness were collected for transversely loaded rectangular specimens, and therefore would tend to be overestimates of direction-averaged values that might more directly correspond to comminuted fractures.
Because the surface area to volume ratio is minimized for a sphere, estimates of liberated surface area based on spherical morphology are subject to a greater degree of underestimation as fragment number rises. This effect is consistent with the concave downward nature (apparent relative to the fitted regression line) of the trend in Figure 4.
In the pendulum impact test, there was much better repeatability in the values obtained for the polyetherurethane than in the values obtained for bone. Under these moderately high speed impact conditions, the surrogate exhibited a fracture toughness which was only slightly lower (p=0.065) from that of bone, unlike the order-of-magnitude difference prevailing quasi-statically. Obviously, both the bone and the polyetherurethane foam are viscoelastic materials, and therefore exhibit strain rate dependent mechanical properties. Establishing similarities and differences in strain rate dependency of the foam, versus that of bone, is an inviting subject for future study.
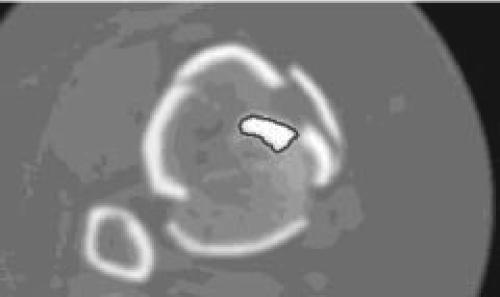
Our current use for the foam surrogate is as a gold standard (phantom) calibration material for development of CT-based image analysis routines for fragment perimeter identification (Figure 6). Besides aiding in classifying comminution for clinical injury description, we are working toward establishing an explicit linkage between interfragmentary surface area and energy absorption.
Figure 6.
Delineated perimeter, an index of interfragmentary surface area, is shown in (a) a foam surrogate CT slice and (b) a tibial pilon fracture CT slice.
Figure 6a.
Figure 6b.
ACKNOWLEDGEMENTS
Financial support provided by EBI Inc. and NIH grant # AR46601. Valuable technical assistance was provided by Alan VanBuskirk and David Edmundson of General Plastics Manufacturing Co.
Footnotes
This specifically fabricated foam has been designated Grade FR7140 Last-A-Foam® (General Plastics Manufacturing Co., Tacoma, WA)
References
- 1.Anderson TL. Fracture Mechanics – Fundamentals and Applications. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- 2.ASTM D790M-86, Standard Test Methods for Flexural Properties of Unreinforced Plastics and Electrical Insulating Materials. 1992 Annual Book of ASTM Standards. 1992;3.01:279–287. [Google Scholar]
- 3.ASTM F1839-97, Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. 1992 Annual Book of ASTM Standards. 1998;13.01:1278–1283. [Google Scholar]
- 4.Behiri JC, Bonfield W. Fracture Mechanics Of Bone - The Effects Of Density, Specimen Thickness And Crack Velocity On Longitudinal Fracture. J Biomech. 1984;17(1):25–34. doi: 10.1016/0021-9290(84)90076-9. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JR, Harrington RM, Lee KM, Anderson PA, Tencer AF, Kowalski D. Factors affecting the pullout strength of cancellous bone screws. J Biomech Eng. 1996;118:391–398. doi: 10.1115/1.2796022. [DOI] [PubMed] [Google Scholar]
- 6.Cristofolini L, Viceconti M, Cappello A, Toni A. Mechanical Validation of Whole Bone Composite Femur Models. J Biomech. 1996;29(4):525–535. doi: 10.1016/0021-9290(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 7.Evans FG. Mechanical Properties of Bone. Vol. 17. Springfield, IL: Charles C Thomas Publisher; 1973. [Google Scholar]
- 8.Greensfield GB. Radiology of Bone Diseases. Philadelphia: JB Lippincott; 1986. [Google Scholar]
- 9.Hale JE, Anderson DD, Johnson GA. A Polyurethane Foam Model for Characterizing Suture Pull-through Properties in Bone. Proc Amer Soc Biomech. 1999;23:288–289. [Google Scholar]
- 10.Lopponen H, Holma T, Sorri M, Jyrkinen L, Karhula V, Koivula A, Ilkko E, Laitinen J, Koivukangas J, Oikarinen J, Alamaki O. Computed Tomography Data Based Rapid Prototyping Model of the Temporal Bone Before Cochlear Implant Surgery. Acta Octo-Laryngologica. 1997;529(Supplement):47–49. doi: 10.3109/00016489709124077. [DOI] [PubMed] [Google Scholar]
- 11.Martin JS, Marsh JL. Current Classification of Fractures. Radiologic Clinics of North America. 1997;35(3):491–506. [PubMed] [Google Scholar]
- 12.Martin RB, Boardman DL. The Effects of Collagen Fiber Orientation, Porosity, Density, and Mineralization on bovine Cortical Bone Bending Properties. J Biomech. 1993;26(9):1047–1054. doi: 10.1016/s0021-9290(05)80004-1. [DOI] [PubMed] [Google Scholar]
- 13.Melvin JW. Fracture Mechanics of Bone. J of Biomech Eng. 1993;115:549–554. doi: 10.1115/1.2895538. [DOI] [PubMed] [Google Scholar]
- 14.Norman TL, Vashishth D, Burr DB. Fracture Toughness Of Human Bone Under Tension. J Biomech. 1995;28(3):309–320. doi: 10.1016/0021-9290(94)00069-g. [DOI] [PubMed] [Google Scholar]
- 15.Reilly DT, Burstein AH. Elastic And Ultimate Properties Of Compact Bone Tissue. J Biomech. 1975;8(6):393–405. doi: 10.1016/0021-9290(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 16.Sedlin ED, Hirsch C. Factors Affecting the Determination of the Physical Properties of Femoral Cortical Bone. Acta Orthop Scand. 1966;37:29–48. doi: 10.3109/17453676608989401. [DOI] [PubMed] [Google Scholar]
- 17.Simonian PT, Sussmann PS, Baldini TH, Crockett HC, Wickiewicz TL. Interference Screw Postion And Hamstring Graft Location for Anterior Cruciate Ligament Reconstruction. Arthroscopy. 1998;14(5):459–464. doi: 10.1016/s0749-8063(98)70072-6. [DOI] [PubMed] [Google Scholar]
- 18.Szivek JA, Thompson JD, Benjamin JB. Characterization of Three Formulations of a Synthetic Foam as Models for a Range of Human Cancellous Bone Types. J of Applied Biomaterials. 1995;6(2):125–128. doi: 10.1002/jab.770060207. [DOI] [PubMed] [Google Scholar]