Abstract
Use of recombinant human erythropoietin (rhEPO) for treatment of pre-operative anemia in anticipation of orthopaedic surgical blood loss has become a routine practice. Use of rhEPO to help manage unanticipated blood loss from elective surgery or major orthopaedic trauma is limited by the rate and volume of erythropoiesis that is achievable with exogenously administered rhEPO. The rate and volume of erythropoiesis may be limited by the available population of cells responsive to EPO. Cytokines known to affect these early hematopoietic progenitors may potentiate the effects of rhEPO. In this study, mice were rendered anemic by loss of approximately one-third of their total blood volume. A control group received only iron supplementation. Mice in three experimental groups received three injections of rhEPO. Two of these groups also received either recombinant murine stem cell factor (rmSCF) or recombinant murine interleukin-3 (rmIL-3). Both were before and in conjunction with rhEPO. Animals were sacrificed for peripheral blood testing at baseline, after initiation of rmSCF and rmIL-3 prior to rhEPO administration, and at three time points after dosing of rhEPO. Additionally, the bone marrow was harvested and cultured to determine the concentration of erythroid progenitors after treatment with rmIL-3 or rmSCF, and after further treatment with rhEPO. Hematocrits were significantly higher in the first measurement point after administration of rhEPO in the groups receiving additional cytokines. The control and rhEPO-only groups were not different at this early time point. The maximal rate of erythropoiesis was also elevated in the groups receiving additional cytokines. The bone marrow of mice receiving SCF had a dramatically increased number of erythroid progenitors compared to all other groups. The population of EPO-responsive cells, dependent on cytokines not controlled by hypoxia, is a major rate-limiting and volume-limiting factor in the response to rhEPO during recovery from blood-loss anemia. Administration of earlier-acting cytokines has the potential to increase the rate and volume of exogenously stimulated erythropoiesis.
INTRODUCTION
Allogenic blood transfusions, though much safer than they were at times in the past, are not risk-free. While transmission of human immunodeficiency virus (HIV) or the hepatitis C virus via transfusion occurs only once per one-and-one-half million units transfused in the United States, other viruses such as hepatitis B and emerging pathogens cannot be as thoroughly screened for.1 In addition, transfusion reactions occur approximately once per fifty units transfused. Further, the immunomodulation resulting from blood transfusion has been suspected to increase post-operative infections and delay wound healing.2–6 The desire to minimize or eliminate patient exposure to transfusion risks has harnessed improving understanding of hematologic physiology and pathophysiology to engineer means by which anemia may be otherwise treated.
Erythropoietin (EPO), the most potent regulator of red blood cell development, or erythropoiesis, is a glycoprotein hormone secreted primarily by cells in the interstitium of the renal cortex. Its expression and secretion are very tightly linked to tissue hypoxia. EPO exerts its influence in the bone marrow, where it regulates the proliferation and differentiation of red blood cell precursors.
Recombinant human EPO (rhEPO) has been available for exogenous administration since the mid-1980s.7 By 1989, the U.S. Food and Drug Administration had approved its use for treating the anemia associated with chronic renal failure. Renal failure patients were the first obvious targets for treatment with the recombinant protein; their associated anemia is largely attributable to a deficiency of EPO secretion in their failing kidneys. From that point, use of exogenous rhEPO expanded to treating anemias of varied etiologies.
Blood-loss anemia from surgery and/or trauma remains a major indication for allogenic blood transfusion today.8 The acuity of erythrocyte mass reduction adds unique challenges to anemia from this etiology. Erythrocyte mass replacement must be rapid to avoid cardiovascular strain and physiologic devastation. It is not surprising, therefore, that most of the research with perioperative rhEPO has focused on the pre-operative period prior to elective orthopaedic surgery.9–15 Recently, the FDA approved use of pre-operative rhEPO for patients with moderate chronic anemia who are preparing for elective hip and knee surgeries, for which significant blood loss is expected.
One study is currently exploring the use of rhEPO in orthopaedic trauma, specifically with pelvis fracture patients,16 but use of rhEPO to deal with recovery from unanticipated blood loss has been limited.17 Victims of other major orthopaedic trauma and patients with unexpectedly large intraoperative blood loss from elective surgeries continue to have large transfusion requirements during their hospitalizations. Improved understanding of the physiologic regulation of erythropoiesis may lead to safer options, minimizing the need for allogenic transfusions.
Use of rhEPO after blood loss is partly hampered by the biology by which it exerts its influence. For a cell to be effected by EPO, either recombinant or endogenous, it must express the EPO receptor on its surface. The earliest erythrocyte progenitor to express this receptor, and respond to stimulation by EPO, is the burst-forming unit erythrocyte (BFU-E). Proliferation of the early BFU-E and its precursors is dependent on cytokines other than EPO (Figure 1). Secretion of none of these other cytokines is directly linked with tissue hypoxia. The supply of early BFU-Es may therefore be the major production-limiting factor during the body's attempt to rapidly recover from blood-loss anemia. To evaluate this hypothesis, two factors known to support earlier non-erythroid-specific progenitors were selected to be used in conjunction with rhEPO in a mouse model of blood-loss anemia. The factors selected were stem cell factor (SCF) and interleukin-3 (IL-3).
Figure 1.
This schematic illustrates the effects of three hematopoietins on proliferation and differentiation in the myeloid lineage and its erythroid arm. The myeloid lineage initially includes cells with the ability to form colonies of precursors to granulocytes, erythrocytes, megakaryocytes and monocytes/macrophages (CFU-GEMM). The earliest cell to express the erythropoietin receptor and respond to stimulation with erythropoietin is the burst-forming unit-erythrocyte (BFU-E); it is also the earliest dedicated erythroid progenitor, ultimately proliferating/differentiating into a burst-like array of colony-forming units-erythrocytes (CFU-Es). These CFU-Es continue to respond to erythropoietin as they form a colony of erythroblasts, which lose the ability to proliferate and begin to terminally differentiate and build hemoglobin stores prior to release into the peripheral blood circulation and degradation of the nucleus.
Stem cell factor, also called steel factor, is the ligand for the c-kit receptor, found on pluripotent stem cells in the hematopoietic as well as other cell lineages. Its presence stimulates proliferation and early differentiation of hematopoietic progenitors, especially in the myeloid line. Interleukin-3 is a stimulator of proliferation more specific for the myeloid lineage.
METHODS
After obtaining approval from the Washington University Animal Care and Use Committee, blood-loss anemia was induced in sixty anaesthetized 20-gram female mice by microcapillary disruption of the retro-orbital plexus and drainage of 0.4 ml of blood, or one-third of the calculated total blood volume. Beginning two days later, all mice received daily subcutaneous injections of saline with albumin as a vehicle for administration of the various treatment regiments. All mice received a single injection of 10mg of iron dextran, 1 week after induction of blood-loss anemia, to remove iron deficiency as a confounding source of persistent anemia.
Treatment Groups
The control group received only vehicle injections, daily for one week, beginning at two days post-bleed. All three experimental groups received injections of 20 units of recombinant human erythropoietin (rhEPO) on the last three days of the week. The first of these experimental groups received only vehicle for the first four days. The second group received a single injection of 480 units of recombinant murine interleukin-3 (rmIL- 3), followed by six daily injections of 750 units of recombinant murine interleukin, in addition to the three rhEPO injections. The third experimental group received 0.2 micrograms (mcg) of recombinant murine stem cell factor on the first day and 0.3 micrograms on the subsequent six days, in addition to the three rhEPO injections.
Hematologic Testing
Two days after induction of blood-loss anemia, three mice were euthanized to test for baseline pretreatment anemia, applicable to all groups. Three mice from the IL-3 and SCF groups and three mice from the still-identical EPO/control group were euthanized for testing six days after anemia induction, or the day of the first intended EPO administration. Subsequently, three mice from the IL-3 and SCF groups and five mice from the EPO and control groups were euthanized for testing nine, 13, and 15 days post-anemia-induction.
On testing days, after humane euthanasia, blood was drawn via cardiac puncture, and placed in EDTA-coated microtubes. Automated complete blood counts were performed on a Serono-Baker Diagnostics System 9000 (Allentown, Pennsylvania). Blood for manual reticulocyte counts was stained using the new methylene blue Unopette Test 5821 (Becton-Dickinson, New Jersey) and smeared. Both manual reticulocyte count percentages and reticulocyte production indices (RPIs, corrected for an increased or lowered hematocrit) were calculated.
Bone Marrow Culture
From mice sacrificed on days six and nine post bleed, or just before and just after the three doses of rhEPO, bilateral tibiae and femora were harvested and the marrow collected. This was then cultured in a semi-solid culture, Methocult M3230 (Stemcell Technologies, British Columbia, Canada). Cultures were supported by a standard milieu of multiple hematopoietic cytokines, at 20% carbon dioxide at 37 degrees Celsius. Marrow from one mouse from each group at six days post bleed and two mice from each group at nine days post bleed was cultured. Cultures were scored for the concentration of colony-forming unit erythrocytes (CFU-Es) on day four of culture, and burst-forming unit erythrocytes (BFU-Es) on day eight of culture. These scorings provided a percentage of cells, representative of the time they were obtained from the marrow, that were dedicated to erythrocyte lineages, or a measure of how much the marrow was directed toward erythropoiesis, specifically.
Statistics
One-way analysis of variance was performed initially. Any testing day noted to have significant variance was further tested with a Fisher's pair-wise comparison using individual group-to-group comparison t-tests. Alpha was set at 0.05. No statistical comparisons were performed on the bone marrow culture results, due to the small numbers tested.
RESULTS
Hematologic Data
The hematocrit at the pre-treatment baseline, two days post bleed, was 31.6 ± 2.5. By post-bleed day six, before any rhEPO treatments but after initial treatments with IL-3 and SCF, the groups did not differ significantly. By day nine, immediately after completion of all injection regimens, the EPO-only and control groups did not differ significantly. The SCF group had a significantly higher hematocrit than any other group. The IL-3 group had a significantly higher hematocrit than the control and EPO-only groups. By days 13 and 15, the EPO-only group was no longer superceded by the IL-3 and SCF groups, but all three were still increased over the control group (Figure 2).
Figure 2. Hematocrit Recovery after Blood Loss Anemia.
This chart demonstrates the change in hematocrit over time for different cytokine treatment regimens for blood-loss anemia. The groups receiving recombinant murine stem cell factor (rmSCF) or recombinant murine interleukin-3 (rmIL-3) in addition to recombinant human erythropoietin (rhEPO) demonstrate higher and earlier peaks in hematocrit. Recombinant human erythropoietin (rhEPO) alone is not significantly different than the control at day nine. The asterisks denote statistically significant differences from all other groups at the same time point (p>0.05). NS denotes no significant difference. Error bars represent standard deviations from the mean.
The reticulocyte counts and production indices demonstrated higher rates of erythropoiesis in the IL-3 and SCF groups at day nine compared to the EPO-only and control groups (Figure 3). The EPO-only group did have an elevated rate of erythropoiesis over the control by day nine, but the lack of elevation in hematocrit by that point demonstrates that it was newly elevated. Rates of erythropoiesis dropped to below-normal levels in all groups but the control by day 15, reflecting the supranormal hematocrit, which prevents baseline hypoxia and endogenous EPO secretion.
Figure 3. Rate of Erythrocyte Production after Blood Loss Anemia.
This chart demonstrates the change in reticulocyte production index over time for different cytokine treatment regimens for blood-loss anemia. The reticulocyte production index is a corrected (for hematocrit) percentage of reticulocytes, or recently released, immature red blood cells, among total erythrocytes in the peripheral blood. The index is a measure of the number of red blood cells that have completed the bone marrow stages of erythropoeisis in approximately the last 24 hours, and therefore a measure of the rate of red blood cell production. The groups receiving recombinant murine stem cell factor (rmSCF) or recombinant murine interleukin-3 (rmIL-3) in addition to recombinant human erythropoietin (rhEPO) demonstrate a higher peak rate of erythropoiesis. These groups drop to subnormal rates of red cell production when exogenous cytokines are no longer circulating, as the endogenous secretion of EPO is suppressed by the lack of hypoxia resulting from the supranormal hematocrit. Asterisks denote statistically significant dif ferences from control groups (p<0.05). Error bars represent standard deviations from the mean.
Bone Marrow Culture Assay
Cultures demonstrated that the mice that received SCF had dramatically higher concentrations of CFU-Es and BFU-Es in their bone marrow both before (Figure 4) and after (Figure 5) exogenous rhEPO administration.
Figure 4. Prevalence of Erythroid Progenitors on Day 6 of Blood Loss Anemia.
This chart illustrates the concentration of dedicated, early erythrocyte progenitors cultured from the bone marrow of mice after four days of injections with recombinant murine stem cell factor (rmSCF), recombinant murine interleukin-3 (rmIL-3), or vehicle alone. These cells are the earliest cells that will respond to stimulation by erythropoietin. The strength of this population of cells appears to be a major rate-limiting factor in the effectiveness of erythropoietin administration.
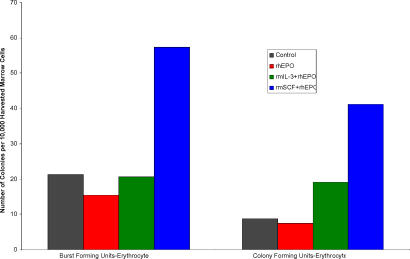
Figure 5. Prevalence of Erythroid Progenitors on Day 9 of Blood Loss Anemia.
This chart illustrates the concentration of dedicated, early erythrocyte progenitors cultured from the bone marrow of mice after treatment with vehicle alone, recombinant human erythropoietin (rhEPO) alone, or rhEPO with recombinant murine stem cell factor (rmSCF), or with recombinant murine interleukin-3 (rmIL-3). The rhEPO-only group appears to have a lower-than-normal presence of these cells, suggesting that the combination of exogenous and high endogenous levels of EPO, without prior priming to build up the early progenitor population, has depleted it, explaining the lag in red blood cell production in this group.
DISCUSSION
A major limiting factor to the effectiveness of exogenously administered rhEPO is the provision of a population of cells able to respond to its stimulation. In these mice, young and healthy, other than their blood-loss anemia, no statistically significant effect on blood restoration over the control was afforded by exogenous administration of EPO alone, until 13 days after induction of anemia, and seven days after initiation of EPO treatments. This argues that endogenous EPO secretion, up-regulated from the hypoxia due to blood loss, may have already saturated the receptors available on EPO-responsive cells. This is in stark contrast to previous experiments in mice without blood loss, which demonstrated a statistically significant and dramatic increase in hematocrit over control mice (60.0% compared to 54.5%) on the first day following three consecutive rhEPO administrations identical to this experiment. The presence of blood loss in an otherwise healthy mouse may have induced sufficient endogenous EPO secretion to render the exogenous rhEPO initially extraneous.
Administration of cytokines known to affect earlier stages of hematopoiesis potentiated the effects of rhEPO on erythropoiesis. Both IL-3 and SCF demonstrated statistically significant increases in hematocrit after administration of exogenous rhEPO. This experiment did not test whether these factors administered without exogenous EPO may have stimulated similarly rapid blood restoration by potentiating the hypoxia-induced elevation in endogenous EPO. While the peripheral blood measures of erythropoiesis were not elevated after the initial doses of SCF and IL-3, but before EPO administration, it cannot be concluded that the exogenous EPO was necessary for the subsequent increase in blood mass over the control. The cells affected by these factors require from four to eightdays to mature into peripheral blood erythrocytes.
That the bone marrow cultured from the SCF treatment group demonstrated markedly increased concentration of erythrocyte-specific progenitors over the control mice, argues that the increased endogenous level of EPO was already directing the proliferation of these early progenitors toward the erythroid lineage. While the factors used were expected to increase the number of available cells for EPO to stimulate, they should not have individually stimulated the marrow to focus on erythropoiesis. Perhaps more interesting was that the group receiving rhEPO treatments without other cytokines had a somewhat reduced presence of erythroid progenitors compared to the control. This is important because the increased demand for these cells by the presence of additional EPO did not stimulate a replenishment of their population.
The cytokines which regulate the proliferation of cells leading up to the EPO-responsive BFU-E are not linked to tissue hypoxia as endogenous EPO secretion is. This probably results in a physiologic lag in the erythropoietic response to the endogenous EPO surge induced by tissue hypoxia. Once the available population of BFU-Es is throughput by EPO to mature erythrocytes, the marrow may be slow to replace it. The possibility of stimulating its replacement or even initial build-up with exogenous cytokines holds promise for enabling the body to recover blood volume in a much more rapid sequence.
The higher hematocrits, observed in the groups receiving early-acting cytokines, are explained by the elevated rate of erythropoiesis, which is directly measured with the reticulocyte count and production index. By stimulating the proliferation of progenitors to EPO-responsive cells, the maximal rate of erythropoiesis was both higher and reached earlier. The rate of erythropoiesis was elevated on the first testing after administration of rhEPO alone, but the lack of elevation in hematocrit at this time point argues that this was a very newly elevated rate of production. Further, the rhEPO-only group's reticulocyte production indices were markedly lower than those at the same time point after an identical EPO regiment, outside the setting of blood-loss anemia (15.4 compared to 7.5). The week of endogenously stimulated increased erythropoiesis after the anemia induction had likely depleted the population of EPO-responsive cells. This, along with the suggestion from the bone marrow culture data that the rhEPO group had fewer erythroid progenitors than the control, strengthens the argument that there is a physiologic lag in replenishment of EPO-responsive cells, due to the fact that the factors stimulating the progenitors to that cell population are not up-regulated by tissue hypoxia.
The data demonstrate that it is possible to potentiate the effects of exogenous EPO by administration of cytokines that stimulate proliferation of earlier erythroid progenitors. This information is yet far from being clinically applicable. Recombinant human IL-3 is currently only under FDA-approved investigation for oncologic treatments and recombinant human SCF is not even approved yet for human research. However, these data identify one of the major rate-limiting and volume-limiting factors in the effects of rhEPO on blood restoration: The population of cells responsive to EPO. Expediting and increasing the magnitude of the response to exogenous rhEPO may improve its utility for recovery from blood loss, as opposed to only preparation for blood loss. As science moves forward, clinical methods of acutely increasing the EPO-responsive cell population may improve the ability to use recombinant cytokines in the management of post-operative and traumatic blood-loss anemia.
References
- 1.Dodd RY. Current safety of the blood supply in the United States. Int J Hematol. 2004;80(4):301–305. doi: 10.1532/ijh97.04123. [DOI] [PubMed] [Google Scholar]
- 2.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg. (Am) 1999;81(1):2–10. doi: 10.2106/00004623-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol. 1997;34(3 Suppl 2):34–40. [PubMed] [Google Scholar]
- 4.Borghi B, Casati A. Incidence and risk factors for allogenic blood transfusion during major joint replacement using an integrated autotransfusion regimen. Eur J Anaesthesiol. 2000;17(7):411–417. doi: 10.1046/j.1365-2346.2000.00693.x. The Rizzoli Study Group on Orthopaedic Anaesthesia. [DOI] [PubMed] [Google Scholar]
- 5.Innerhofer P, Walleczek C, Luz G, Hobisch-Hagen P, Benzer A, Stockl B, Hessenberger G, Nussbaumer W, Schobersberger W. Transfusion of buffy coat-depleted blood components and risk of postoperative infection in orthopedic patients. Transfusion. 1999;39(6):625–632. doi: 10.1046/j.1537-2995.1999.39060625.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212–217. doi: 10.1046/j.1537-2995.1991.31391165169.x. [DOI] [PubMed] [Google Scholar]
- 7.Groopman JE, Molina JM, Scadden DT. Hematopoietic growth factors. Biology and clinical applications. N Engl J Med. 1989;321(21):1449–1459. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- 8.Goodnough LT, Riddell JT, Verbrugge D, Marcus RE. Blood transfusions in hip fracture patients: implications for blood conservation programs. J Orthop Trauma. 1993;7(1):47–51. doi: 10.1097/00005131-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Canadian Orthopedic Perioperative Erythropoietin Study Group. Effectiveness of perioperative recombinant human erythropoietin in elective hip replacement. Lancet. 1993;341(8855):1227–1232. [PubMed] [Google Scholar]
- 10.Adamson J. Perisurgical use of epoetin alfa in orthopedic surgery patients. Semin Hematol. 1996;33(2 Suppl 2):55–58. discussion 59. [PubMed] [Google Scholar]
- 11.de Andrade JR, Jove M, Landon G, Frei D, Guilfoyle M, Young DC. Baseline hemoglobin as a predictor of risk of transfusion and response to Epoetin alfa in orthopedic surgery patients. Am J Orthop. 1996;25(8):533–42. [PubMed] [Google Scholar]
- 12.Faris PM, Spence RK, Larholt KM, Sampson AR, Frei D. The predictive power of baseline hemoglobin for transfusion risk in surgery patients. Orthopedics. 1999;22(1 Suppl):s135–s140. doi: 10.3928/0147-7447-19990102-06. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg MA, McCutchen JW, Jove M, Di Cesare P, Friedman RJ, Poss R, Guilfoyle M, Frei D, Young D. A safety and efficacy comparison study of two dosing regimens of epoetin alfa in patients undergoing major orthopedic surgery. Am J Orthop. 1996;25(8):544–552. [PubMed] [Google Scholar]
- 14.Schlaeppi B, Gunter P, Nydegger UE. Enhancing the efficacy of preoperative autologous blood donation by erythropoietin. Transfus Sci. 1994;15(2):171–177. doi: 10.1016/0955-3886(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 15.Tryba M. Epoetin alfa plus autologous blood donation and normovolemic hemodilution in patients scheduled for orthopedic or vascular surgery. Semin Hematol. 1996;33(2 Suppl 2):34–36. discussion 37-8. [PubMed] [Google Scholar]
- 16.Mears DC, Durbhakula SM, Miller B. Developments in blood management: the potential therapeutic role for epoetin alfa in orthopedic trauma. Orthopedics. 1999;22(1 Suppl):s151–s154. doi: 10.3928/0147-7447-19990102-10. [DOI] [PubMed] [Google Scholar]
- 17.van Papendrecht Hoynck, Jeekel H, Busch OR, Marquet RL. Efficacy of recombinant erythropoietin for stimulating erythropoiesis after blood loss and surgery. An experimental study in rats. Eur J Surg. 1992;158(2):83–87. [PubMed] [Google Scholar]