Abstract
Traditionally, arthroscopic management of shoulder instability has been reserved for patients with isolated Bankart lesions without any capsular laxity or injury. To date, there are no animal studies evaluating the healing potential of capsular plication and/or capsulo-labral repair. The purpose of this in vivo animal study was to determine if the histological capsular healing of an open capsular plication simulating an arthroscopic plication is equivalent to the more traditional open capsular shift involving cutting and advancing the capsule. Twenty-six skeletally mature sheep were randomized to either an open capsular plication simulating arthroscopic plication (n=13), or an open traditional capsular shift (n=13). A sham operation (n=4) was also performed involving exposure to visualize the capsule. Normal non-operated control shoulders were also analyzed. A pathologist blinded to the treatment evaluated both hematoxylin and eosin (H&E) sections and polarized light microscopy. Qualitative scoring evaluated fibrosis, mucinous degeneration, fat necrosis, granuloma formation, vascularity, inflammatory infiltrate and hemosiderin (0 to 3 points). Both the capsular plication and open shift groups demonstrated healing by fibrosis at the site of surgical manipulation. There were no statistical differences in the capsular healing responses between the two groups with regard to fibrosis, granuloma formation and vascularity. The open shift group demonstrated significantly more mucinous degeneration (p=0.038). Fat necrosis was present in 4/13 specimens in the open shift group and none in the capsular plication specimens. Both groups demonstrated disorganized collagen formation under polarized light microscopy. There were no differences between non-operated control specimens and sham surgery specimens. Our findings support the hypothesis that histologic capsular healing is equivalent between the plication group and the open shift group. In addition, the open shift group demonstrated significantly more changes indicative of tissue injury. This basic science model confirms capsular healing after simulated arthroscopic plication, providing support for arthroscopic capsular plication in practice.
INTRODUCTION
Shoulder stabilization has historically been performed via open surgical procedures.2,8,24,26 Success rates following open stabilization have routinely been reported to be greater than 90%.2,17,18,22,24,26,28,36 Development of more advanced arthroscopic techniques over the last two decades has provided a less invasive and potentially more efficacious means of addressing glenohumeral instability.3,12,13,25,29,31 Although several early reports have shown that the management of shoulder instability through arthroscopic techniques has resulted in higher failure rates compared with open stabilization procedures,15,16,27 more recently, investigators have demonstrated comparable clinical results between these two procedures.8,10,12
Arthroscopic stabilization has several advantages over open procedures including: more thorough documentation of intraarticular pathology, the ability to address associated injuries and improve cosmesis, greater postoperative motion, faster recovery and increased cost effectiveness.1,6,15 It is becoming increasingly well accepted that shoulder instability primarily associated with labral pathology can successfully be addressed arthroscopically.8,9,11,19 However, instability associated with excessive capsular laxity is still considered by some to be a relative indication for shoulder arthrotomy.9 Nonetheless, recent advances in surgical technique and instrumentation have led to the performance of arthroscopic capsular plication in an increasing number of patients.12 Preliminary clinical results of this procedure indicate that arthroscopic plication is an effective method of eliminating or significantly reducing excessive capsular laxity without the use of an arthrotomy.3–5,12,37 To our knowledge, there are no animal studies nor is there basic science research evaluating the healing potential of the capsule after capsular plication performed in the manner that is required during the arthroscopic stabilization procedure. Histologic evaluation of capsular healing has not been well documented after any type of shoulder stabilization surgery, thus scientific evaluation and comparison of open capsular shift and arthroscopic plication has not been possible.
The purpose of this study was to evaluate the healing potential of side-to-side capsular placation via an open approach simulating arthroscopic capsular placation, compared to the healing potential of the more traditional open capsular shift involving cutting and advancing the capsule. Our primary hypothesis was that the capsular plication procedure would demonstrate similar histologic properties compared to the traditional open capsular shift. Thus, our first specific aim was to demonstrate that capsular plication is a viable surgical alternative for addressing redundant capsular tissue in the clinical arena. Our secondary hypothesis was that a sham operation group consisting of surgical exposure of the capsule without manipulation of the tissue, would demonstrate no significant differences in histology compared to non-operated limbs. Thus, our second specific aim was to demonstrate that the surgical approach and associated inflammatory mediators would, in and of themselves, have little effect on the capsular tissue. Validation of the potential to effectively address capsular laxity through arthroscopic capsular plication techniques using histological analysis is important to allow further advancement of these techniques.
METHODS
After Animal Institute Care and Use Committee (AICUC) approval was obtained, thirty skeletally mature Columbian X Rambouillet ewes were allocated for use. The thirty sheep were randomly placed into three groups: 1) capsular plication group (n = 13); 2) open shift group (n = 13); 3) sham operation (n = 4).
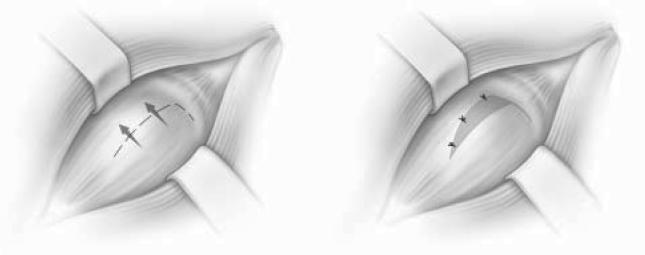
After standard prepping and draping, each of the shoulders was approached through a caudal (posterior) incision with the animal in lateral recumbency. The incision was made over the spine of the scapula and deepened to the level of the infraspinatus muscle tendon junction. The plane between the upper and lower portions of the infraspinatus was then developed to fully expose the caudal (posterior) capsule of the shoulder joint. For the capsular plication group, three interrupted horizontal mattress sutures were placed without disruption of the capsule, resulting in plication of the capsule (Figure 1). The sutures were placed midway between the proximal and distal portions of the caudal (posterior) capsule in line with the capsular fibers. The horizontal mattress sutures were placed so that 8 to 10 mm of capsular tissue was translated in a distal to proximal direction. For the open shift surgeries, a transverse incision was made in the caudal (posterior) capsule and the distal capsule was shifted proximally and sutured with three interrupted figure-of-eight sutures (Figure 2). The capsule was similarly shifted 8 to 10 mm with the open procedure. For the control animals, no further surgery was performed once the capsule was exposed. For all three groups, dissection down to the capsule was performed bluntly between muscle planes to minimize any soft tissue injury.
Figure 1.
Capsular plication was performed using three interrupted horizontal mattress sutures shifting the capsule from an inferior to superior direction without cutting the capsule.
Figure 2.
The open shift procedure was performed by cutting the capsule in a horizontal fashion, shifting it superiorly, and suturing it using three interrupted figure-of-eight sutures.
Postoperative activity was restricted for the first two weeks. The limbs were not immobilized. At six weeks, the animals were sacrificed and both the operated and non-operated limbs were harvested for analysis. Fresh tissue samples of the entire caudal (posterior) capsule were dissected from a cranial (anterior) approach to avoid tissue injury. Hematoxylin and eosin sections were prepared and scored by an attending pathologist blinded to the procedure performed. Polarized light microscopy was performed to further assess collagen orientation.
Statistical Methods
Qualitative scoring evaluated fibrosis, mucinous degeneration, fat necrosis, granuloma formation, vascularity, inflammatory infiltrate and hemosiderin (0=none; 1=slight; 2=moderate; 3=severe). Mean values and standard deviations were calculated for each of the categories within each test group (open shift group, plication group, sham group and non-operated controls). The Mann-Whitney test was used for pairwise comparisons between: The plication versus open shift group; sham group versus the open shift group; sham group versus plication group; and sham group versus non-operated controls. A significance level of 0.05 was used for all tests. Collagen organization was evaluated and graded with polarized light microscopy (0=organized; 1=disorganized). Fisher's exact test was used to identify significant differences between groups, again with the pvalue set at 0.05.
RESULTS
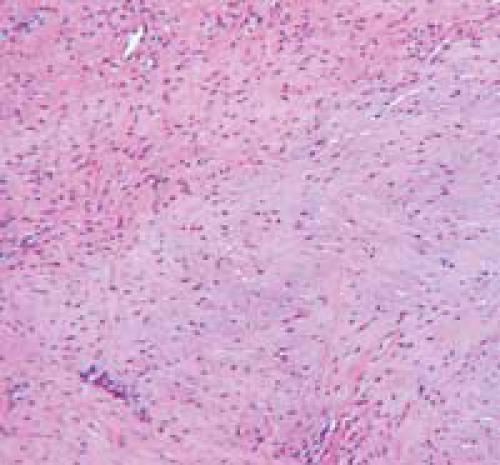
There were no gross failures at the capsule from either surgical procedure. There was one infection in the plication group that was noted at the time of dissection and confirmed with histological evaluation. This animal was eliminated from any statistical analyses. Both the capsular plication group (Figure 3), and the open shift group (Figure 4) demonstrated healing by fibrosis at the site of surgical manipulation. The size of the fibrotic scar was measured by the number of high-power fields. There were no statistically significant differences in the capsular healing responses between these two groups using the histological criteria of: fibrosis (p=0.13); granuloma formation (p=1); and vascularity (p=0.1). Both groups demonstrated disorganized collagen formation under polarized light microscopy. The open shift group (Figure 5), however, demonstrated significantly more mucinous degeneration compared to the plication group (Figure 6) (p=0.038). Furthermore, fat necrosis was present in 4/13 specimens in the open shift group (Figure 7), while no fat necrosis was present after any of the plication or sham surgeries.
Figure 3.
H&E-stained photomicrograph (2.5x) of the posterior capsule after the plication procedure was performed. This section demonstrates healing by fibrosis at the site of surgical manipulation.
Figure 4.
H&E-stained photomicrograph (2.5x) of the posterior capsule after the open shift procedure was performed. Histologically, there were no differences between groups in terms of the size of the fibrotic scar.
Figure 5.
H&E-stained photomicrograph (10x) showing mucinous degeneration the posterior capsule after the open shift procedure.
Figure 6.
H&E-stained photomicrograph (10x) showing no mucinous degeneration in the posterior capsule after the capsular plication procedure. There was significantly less mucinous degeneration after capsular plication compared to open shift.
Figure 7.
H&E-stained photomicrograph (10x) showing the presence of fat necrosis after the open shift procedure. No cases demonstrated fat necrosis after the plication procedure while four out of 13 of the open shift specimens demonstrated areas of fat necrosis.
The sham group, compared to the open shift group, had significantly less of the following histological findings: fibrosis (p=0.03); mucinous degeneration (p=0.03); granuloma formation (p=0.03); vascularity (p=0.03); and collagen organization (p=0.015).
Compared to the plication group, the sham group scored significantly lower in the histological criteria of: fibrosis (p=0.03); granuloma formation (p=0.03); vascularity (p=0.03); and collagen organization (p=0.015). There was no significant difference in the presence of mucinous degeneration in the sham group compared to the plication group (p=0.2) (minimal in both groups). There were no significant differences in the presence of inflammatory infiltrates between any of the three groups (minimal in all groups).
There were no significant differences in the histological appearance of the capsule after sham surgeries compared to non-operated controls in any of the qualitative scoring categories (Figures 8 and 9). Furthermore, under polarized light microscopy, both the sham surgery group and the non-operated controls demonstrated equally organized and well-aligned collagen.
Figure 8.
H&E-stained photomicrograph (2.5x) of the capsule after the sham surgery
Figure 9.
H&E-stained photomicrograph (2.5x) of the capsule in non-operated control animals. There were no differences in the histological appearance of the capsule in any of the qualitative scoring categories between the sham surgeries and non-operated control specimens.
DISCUSSION
Arthroscopic management of shoulder instability has become increasingly popular with several reports demonstrating comparable results to the more traditional open technique.8,12,20,21,30 Previous reports have suggested that the success of the arthroscopic procedure is related to careful patient selection, with the best results occurring in patients with instability due to a discrete Bankart lesion without significant capsular laxity or injury.8,32–35
With the continued refinement of arthroscopic techniques, surgeons have begun to expand the indications of arthroscopy to patients in whom significant capsular laxity is a component of the instability pattern. There have been numerous reports demonstrating the clinical efficacy of arthroscopic posterior labral repair and capsular plication for recurrent posterior subluxation of the shoulder.3–5,21,23,37 Recently, Kim et al.20 reported a 4% recurrence rate in 167 patients treated with arthroscopic capsulolabral repairs for recurrent traumatic anterior instability. These authors emphasized the importance of adequate tensioning of the redundant anterior aspect of the capsule in addition to repair of the Bankart lesion, as plastic deformation of the capsular ligament may precede the failure of the anterior-inferior labral attachment.7,20
Successful re-tensioning of both the anterior and posterior capsule can be achieved arthroscopically by plication and proximal shift of the inferior aspect of the capsule. However, this is individualized depending upon the condition of the capsular tissue and the location of the capsular laxity. Cadaveric biomechanical studies have demonstrated the importance of tensioning different portions of the capsule in different arm positions.14 A greater appreciation of the importance of both the placement of plication sutures and the position of the arm during tensioning will likely improve the results of selective capsulorrhaphy procedures. Unfortunately, there continues to be a lack of basic science evaluating the healing potential of capsule-to-capsule plication in the manner that is required for arthroscopic shoulder stabilization procedures.
The primary purpose of this project was to perform a histological evaluation of the healing potential of an open side-to-side capsular plication simulating arthroscopic capsular plication, and compare this to the healing potential of the more traditional open capsular shift involving cutting and advancing the capsule. Our findings confirmed the hypothesis that capsular healing was equivalent between the plication group and the open shift group. There were no differences in the size of the fibrotic scar, the amount of granuloma formation, or the degree of vascularity seen in the surgically manipulated tissue. In addition, the open shift group demonstrated significantly more changes indicative of tissue damage demonstrated by increased mucinous degeneration in the shifted capsular tissue, and the presence of fatty degeneration in nearly one-third of the open shift specimens. Evaluation of collagen organization by polarized light microscopy demonstrated disorganized collagen formation in both groups. However, these results represent early post-surgical healing (six weeks), and longer-term studies will be required to further evaluate the potential for maturation of fibrotic scar into organized collagen.
The most important limitations of this study were that an open approach was used to simulate an arthroscopic technique and the procedure was evaluated in an animal model. Unfortunately, there is no ideal animal model to truly assess arthroscopic techniques. For both the open shift procedure as well as the plication procedure, an intermuscular caudal (posterior) approach through the two heads of the infraspinatus (the ovine infraspinatus is divided into a discreet upper and lower portion) was performed to access the posterior capsule. Preliminary dissections of the ovine shoulder revealed that the posterior capsule could be easily approached without detaching any musculotendinous structures. Unlike the human shoulder, the caudal (posterior) capsule was robust, and in most specimens, thicker than the cranial (anterior) capsule; thus it was felt to be an appropriate model to assess capsular tissue healing.
In order to confirm that the surgical approach itself and any potential inflammatory mediators would have little effect on the capsular tissue, a sham operation group consisting of surgical exposure of the capsule without manipulation of the tissue was performed and histological specimens were compared to non-operated limbs. We found that the capsular tissue from animals that underwent the sham operation was indistinguishable from non-operated control animals. Thus, we feel that the lack of tissue response seen in our control sham operation group suggests that the histological capsular healing in the plication group was truly related to the surgical intervention of capsular plication with suture placement.
The management of the capsule in the plication group did accurately simulate what is performed clinically during arthroscopic stabilization procedures. In fact, we believe that this animal model represents the worst-case scenario for capsule-to-capsule healing, because the intraarticular portions of the capsule were folded side-to- side without any prior abrasion or manipulation of the tissue, which is typically performed clinically in order to stimulate an inflammatory response. A potential explanation for the ability of this tissue to form fibrotic scar similar to that seen after an open shift, is that the actual penetration of the capsule with the suture needle results in sufficient tissue bleeding and inflammation to mount a healing response. However, we still recommend light abrasion of the capsule prior to plication in the clinical setting.
The previously reported inferior clinical results seen after arthroscopic plication relative to open shift for shoulder instability may be improved with current arthroscopic techniques. Most surgeons feel that arthroscopic stabilization procedures should be reserved for patients with isolated labral pathology without significant capsular injury or laxity. The patient criteria, however, is expanding and validation of the potential to effectively address capsular laxity through arthroscopic capsular plication techniques using histological analysis will provide further evidence that these procedures can be successfully performed. To date, we are aware of no scientific investigations that have looked at capsular healing after any type of shoulder stabilization procedure (open or arthroscopic). This basic science evaluation confirms the capsular healing potential after arthroscopic plication and supports its use in clinical practice. Future research in this area will address functional and biomechanical considerations to further delineate the role of arthroscopic management of excessive capsular laxity.
References
- 1.Allen AA, Drakos MC. Arthroscopic instability repairs: are they as good as open? Current Opinion in Orthopedics. 2001;12(4):315–318. [Google Scholar]
- 2.Altchek DW, Warren RF, Skyhar MJ, Ortiz G. T-plasty modification of the Bankart procedure for multidirectional instability of the anterior and inferior types. J Bone Joint Surg. (Am) 1991;73(1):105–112. [PubMed] [Google Scholar]
- 3.Antoniou J, Duckworth DT, Harryman DT., II Capsulolabral augmentation for the management of posteroinferior instability of the shoulder. J Bone Joint Surg. (Am) 2000;82(9):1220–1230. doi: 10.2106/00004623-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Harryman DT., II Arthroscopic posterior capsular repair. Clin Sports Med. 2000;19(1):101–114. vi–vii. doi: 10.1016/s0278-5919(05)70298-2. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou J, Harryman DT., II Posterior instability. Orthop Clin North Am. 2001;32(3):463–473. ix. doi: 10.1016/s0030-5898(05)70215-7. [DOI] [PubMed] [Google Scholar]
- 6.Barber FA, Click SD, Weideman CA. Arthroscopic or open bankart procedures: what are the costs. Arthroscopy. 1998;14:671–674. doi: 10.1016/s0749-8063(98)70092-1. [DOI] [PubMed] [Google Scholar]
- 7.Bigliani LU, Pollock RG, Soslowsky LJ, Flatow EL, Pawluk RJ, Mow VC. Tensile properties of the inferior glenohumeral ligament. J Orthop Res. 1992;10(2):187–197. doi: 10.1002/jor.1100100205. [DOI] [PubMed] [Google Scholar]
- 8.Cole BJ, L'Insalata J, Irrgang J, Warner JJ. Comparison of arthroscopic and open anterior shoulder stabilization. A two to six-year follow- up study. J Bone Joint Surg. (Am) 2000;82-A(8):1108–1114. doi: 10.2106/00004623-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19–48. doi: 10.1016/s0278-5919(05)70294-5. [DOI] [PubMed] [Google Scholar]
- 10.Cole BJ, Warner JJ. Prospectively determined arthroscopic versus open shoulder stabilization: 2-6 year follow-up. J Shoulder Elbow Surg. 1998;7:313. [Google Scholar]
- 11.Fealy S, Drakos M, Allen AA, Warren RF. Arthroscopic Bankart Repair: Experience With an Absorbable, Transfixing Implant. Clinical Orthopaedics & Related Research. 2001;1(390):31–41. [PubMed] [Google Scholar]
- 12.Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior- inferior glenohumeral instability. Two to five-year follow-up. J Bone Joint Surg. (Am) 2000;82-A(7):991–1003. doi: 10.2106/00004623-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Gerber A, Warner JJ. Thermal capsulorrhaphy to treat shoulder instability. Clin Orthop. 2002. pp. 105–116. [DOI] [PubMed]
- 14.Gerber C, Werner CM, Macy JC, Jacob HA, Nyffeler RW. Effect of selective capsulorrhaphy on the passive range of motion of the glenohumeral joint. J Bone Joint Surg. (Am) 2003;85-A(1):48–55. doi: 10.2106/00004623-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Green MR, Christensen KP. Arthroscopic versus open bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371–374. doi: 10.1016/s0749-8063(05)80308-1. [DOI] [PubMed] [Google Scholar]
- 16.Guanche CA, Quick DC, Sodergren KM, Buss DD. Arthroscopic versus open reconstruction of the shoulder in patients with isolated Bankart lesions. Am J Sports Med. 1996;24(2):144–148. doi: 10.1177/036354659602400204. [DOI] [PubMed] [Google Scholar]
- 17.Hovelius L. Anterior dislocation of the shoulder in teen-agers and young adults. Five-year prognosis. J Bone Joint Surg. (Am) 1987;69(3):393–399. [PubMed] [Google Scholar]
- 18.Jobe FW, Giangarra CE, Kvitne RS, Glousman RE. Anterior capsulolabral reconstruction of the shoulder in athletes in overhand sports. Am J Sports Med. 1991;19(5):428–434. doi: 10.1177/036354659101900502. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Ha KI. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755–763. doi: 10.1053/jars.2002.31701. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Ha KI, Cho YB, Ryu BD, Oh I. Arthroscopic anterior stabilization of the shoulder: two to six-year follow-up. J Bone Joint Surg. (Am) 2003;85-A(8):1511–1518. [PubMed] [Google Scholar]
- 21.Kim SH, Ha KI, Park JH, Kim YM, Lee YS, Lee JY, Yoo JC. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg. (Am) 2003;85-A(8):1479–1487. doi: 10.2106/00004623-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery WH, III, Jobe FW. Functional outcomes in athletes after modified anterior capsulolabral reconstruction. Am J Sports Med. 1994;22(3):352–358. doi: 10.1177/036354659402200310. [DOI] [PubMed] [Google Scholar]
- 23.Murrell GA, Warren RF. The surgical treatment of posterior shoulder instability. Clin Sports Med. 1995;14(4):903–915. [PubMed] [Google Scholar]
- 24.Neer CS, II, Foster CR. Inferior capsular shift for involuntary inferior and multidirectional instability of the shoulder. A preliminary report. J Bone Joint Surg. (Am) 1980;62(6):897–908. [PubMed] [Google Scholar]
- 25.Nelson BJ, Arciero RA. Arthroscopic management of glenohumeral instability. Am J Sports Med. 2000;28(4):602–614. doi: 10.1177/03635465000280042801. [DOI] [PubMed] [Google Scholar]
- 26.Pagnani MJ, Dome DC. Surgical treatment of traumatic anterior shoulder instability in american football players. J Bone Joint Surg. (Am) 2002;84-A(5):711–715. doi: 10.2106/00004623-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Pagnani MJ, Warren RF, Altchek DW, Wickiewicz TL, Anderson AF. Arthroscopic shoulder stabilization using transglenoid sutures. A four- year minimum followup. Am J Sports Med. 1996;24(4):459–467. doi: 10.1177/036354659602400409. [DOI] [PubMed] [Google Scholar]
- 28.Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg. (Am) 1978;60(1):1–16. [PubMed] [Google Scholar]
- 29.Savoie FH, III, Field LD. Thermal versus suture treatment of symptomatic capsular laxity. Clin Sports Med. 2000;19(1):63–75. vi. doi: 10.1016/s0278-5919(05)70296-9. [DOI] [PubMed] [Google Scholar]
- 30.Savoie FH, III, Miller CD, Field LD. Arthroscopic reconstruction of traumatic anterior instability of the shoulder: the Caspari technique. Arthroscopy. 1997;13(2):201–209. doi: 10.1016/s0749-8063(97)90155-9. [DOI] [PubMed] [Google Scholar]
- 31.Sekiya JK, Ong BC, Bradley JP. Thermal capsulorrhaphy for shoulder instability. Instr Course Lect. 2003;52:65–80. [PubMed] [Google Scholar]
- 32.Speer KP, Warren RF. Arthroscopic shoulder stabilization. A role for biodegradable materials. Clin Orthop. 1993. pp. 67–74. [PubMed]
- 33.Speer KP, Warren RF, Pagnani M, Warner JJ. An arthroscopic technique for anterior stabilization of the shoulder with a bioabsorbable tack. J Bone Joint Surg. (Am) 1996;78(12):1801–1807. doi: 10.2106/00004623-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Warner JJ, Miller MD, Marks P. Arthroscopic Bankart repair with the Suretac device. Part II: Experimental observations. Arthroscopy. 1995;11(1):14–20. doi: 10.1016/0749-8063(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 35.Warner JJ, Miller MD, Marks P, Fu FH. Arthroscopic Bankart repair with the Suretac device. Part I: Clinical observations. Arthroscopy. 1995;11(1):2–13. doi: 10.1016/0749-8063(95)90082-9. [DOI] [PubMed] [Google Scholar]
- 36.Wirth MA, Blatter G, Rockwood CA., Jr The capsular imbrication procedure for recurrent anterior instability of the shoulder. J Bone Joint Surg. (Am) 1996;78(2):246–259. doi: 10.2106/00004623-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Wolf EM, Eakin CL. Arthroscopic capsular plication for posterior shoulder instability. Arthroscopy. 1998;14(2):153–163. doi: 10.1016/s0749-8063(98)70034-9. [DOI] [PubMed] [Google Scholar]