Abstract
Using a process called quorum sensing (QS), bacteria communicate with extracellular signal molecules called autoinducers (AIs). Response to AIs allows bacteria to coordinate gene expression on a population-wide scale and thereby carry out particular behaviors in unison, much like multicellular organisms. In Vibrio cholerae El Tor, the etiological agent of the current cholera pandemic, AI information is transduced internally through a phosphorelay circuit that impinges on the transcription of multiple small regulatory RNAs (sRNAs). These RNAs base-pair with, and repress the translation of, the mRNA encoding the master transcriptional regulator HapR. In V. cholerae, HapR controls virulence factor expression and biofilm formation. Here we identify a sRNA-dependent, HapR-independent QS pathway in which the sRNAs base-pair with a new target mRNA and activate translation by preventing formation of a translation-inhibiting stem-loop structure. We show that the classical V. cholerae strain, which caused previous pandemics and is reportedly incapable of QS because of a nonfunctional HapR, nonetheless exhibits QS-controlled gene expression through this new HapR-independent pathway.
Keywords: autoinducer, sRNA, virulence, HapR
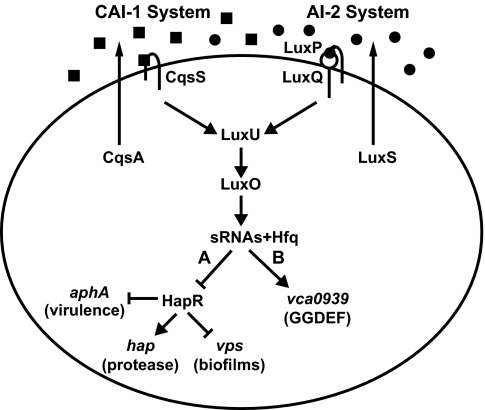
Quorum sensing (QS) is a process of cell–cell communication that enables bacterial populations to collectively control gene expression and thus coordinate group behaviors (1). QS is achieved by the synthesis, secretion, and detection of signal molecules called autoinducers (AIs) that accumulate in proportion to increasing cell density. The human pathogen Vibrio cholerae uses two AIs, CAI-1 and AI-2, to control QS target genes (Fig. 1). CqsA produces the intragenera signal CAI-1 (a compound of unknown structure), which is detected by the cognate sensor kinase protein CqsS (2, 3). LuxS synthesizes AI-2, a furanosyl borate diester, which fosters interspecies communication. AI-2 is detected by the LuxPQ receptor complex (4–7).
Fig. 1.
The V. cholerae QS circuit. HapR-dependent (A) and HapR-independent (B) pathways are controlled by Qrr sRNAs and Hfq. See Introduction for details. Squares, CAI-1; circles, AI-2.
At low cell density, in the absence of AIs, CqsS and LuxQ act as kinases, transferring phosphate to the response regulator LuxO via the phosphotransfer protein LuxU. LuxO∼P activates the transcription of genes encoding four small regulatory RNAs (sRNAs) called Qrr1–4 (quorum regulatory RNAs). The Qrr sRNAs, along with the sRNA chaperone, Hfq, base-pair with and destabilize the mRNA encoding hapR, and so no HapR is produced (8). Under this condition, HapR-repressed genes are expressed whereas HapR-activated genes are not (Fig. 1, pathway A). At high cell density, binding of AIs to their cognate sensors switches the sensors from kinases to phosphatases, reversing the phosphorylation cascade. Dephosphorylated LuxO is inactive, so transcription of the qrr genes terminates, HapR is produced, and the pattern of HapR-dependent gene regulation is inverted. Genes controlled by QS include those for virulence factor expression and biofilm formation (2, 9–13). Importantly, every V. cholerae QS target gene identified to date (>70 genes) requires HapR for regulation (Fig. 1).
V. cholerae El Tor (V. choleraeEl) is responsible for the current cholera pandemic, whereas classical V. cholerae (V. choleraeCl) was responsible for previous pandemics (14). V. choleraeCl and some other V. cholerae strains carry frameshift mutations in hapR. Canonical HapR-controlled reporters are not properly regulated in these strains, and, because of this, these strains have been deemed incapable of QS (15).
Results
Identification of a HapR-Independent QS Target Gene.
We designed a genetic screen to identify V. cholerae AI-regulated target genes. A library of random V. choleraeEl genomic fragments fused to a promoterless luxCDABE cassette was introduced into ΔcqsA, ΔluxS (CAI-1−, AI-2−) V. choleraeEl. After parallel coculture with either a V. choleraeEl AI-producing (CAI-1+, AI-2+) or AI-nonproducing (CAI-1−, AI-2−) strain (referred to below as AI+ donor and AI− control, respectively), the library was screened for altered lux expression. Twenty-five unique promoters displayed differential regulation; five fusions were repressed, and 20 were activated by AIs.
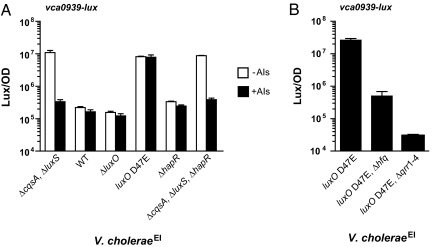
Here we focus on one AI-repressed gene (vca0939) that exhibited a particularly interesting pattern of QS regulation. vca0939 is predicted to encode a GGDEF domain-containing protein. Bacterial GGDEF proteins synthesize the intracellular small-molecule second messenger cyclic di-GMP, which regulates numerous processes including biofilm formation (16). We found that, in the V. choleraeEl ΔcqsA, ΔluxS strain, vca0939-lux is expressed, and addition of exogenous AIs reduces the expression ≈30-fold (Fig. 2A). Consistent with repression by AIs, at high cell density, in the wild-type strain (i.e., CAI-1+, AI-2+) and in the ΔluxO mutant that is “locked” in high cell density mode, there is only low-level expression of vca0939-lux irrespective of the presence of additional AIs. Again, as expected, in the locked low cell density luxO D47E mutant, which carries a constitutive LuxO∼P mimic, vca0939-lux expression is activated ≈30-fold relative to the wild type, irrespective of the presence of exogenous AIs. However, vca0939-lux expression is not activated in the ΔhapR mutant. This result was unanticipated because both the ΔhapR and luxO D47E mutants simulate the low cell density state. Thus, the ΔhapR result suggests that AIs repress expression of vca0939-lux in the absence of HapR, and, as mentioned, all V. cholerae AI-responsive genes identified to date require HapR for their regulation (2, 9–13). To explore this peculiar expression pattern, vca0939-lux expression was measured in a ΔcqsA, ΔluxS, ΔhapR, triple mutant (CAI-1−, AI-2−, HapR−) cocultured with the AI+ donor or AI− control. vca0939-lux expression in the ΔcqsA, ΔluxS, ΔhapR triple mutant with or without AIs is identical to that in the ΔcqsA, ΔluxS double mutant (Fig. 2A), showing that indeed the AI-mediated repression of vca0939 expression occurs in the absence of HapR.
Fig. 2.
HapR-independent expression of vca0939. Shown is vca0939-lux expression in V. choleraeEl strains incubated in a 1:1 coculture with either a V. choleraeEl CAI-1−, AI-2− strain (−AIs, open bars) or a V. choleraeEl CAI-1+, AI-2+ strain (+AIs, filled bars) (A) and V. choleraeEl monocultures (B). Each panel shows the mean bioluminescence/OD (Lux/OD) and the standard deviations of triplicate cultures.
vca0939 Expression Is Controlled by Qrr sRNAs and Hfq.
Our results show that vca0939 expression requires LuxO but not HapR. Hfq and the Qrr sRNAs lie between LuxO and HapR in the QS circuit (Fig. 1) (8). To examine their roles in vca0939 regulation, we deleted either hfq or the four qrr genes and measured vca0939-lux expression. Deletion of hfq causes a ≈100-fold reduction in luxO D47E-dependent expression of vca0939-lux, and elimination of the Qrr sRNAs causes an even more severe reduction (Fig. 2B), showing that both Hfq and the Qrr sRNAs are required for QS regulation of vca0939. We note that higher residual vca0939-lux expression occurs in the Δhfq mutant than in the Δqrr1–4, mutant suggesting that the sRNAs may possess a low level ability to activate vca0939-lux in the absence of Hfq.
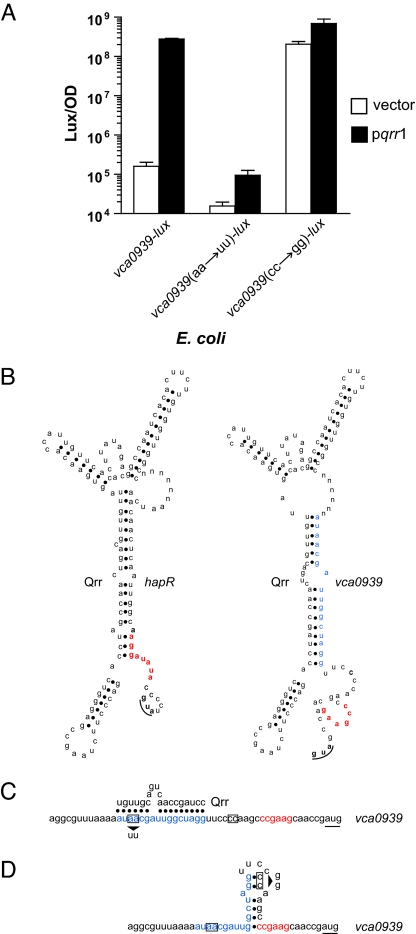
The only previously predicted function for the Qrr sRNAs is to destabilize a single target mRNA, that encoding hapR (8). To determine whether the Qrr sRNAs function directly or indirectly to control vca0939 expression, we measured vca0939-lux expression in Escherichia coli in the absence and the presence of constitutively produced Qrr1. E. coli encodes an Hfq homolog but lacks the other V. cholerae QS components (17). Expression of vca0939-lux is activated >1,000-fold by Qrr1 in E. coli (left bars in Fig. 3A). This transcription pattern reflects VCA0939 protein production, because C-terminally FLAG-tagged VCA0939 is detected in the presence of Qrr1 but cannot be detected when Qrr1 is absent (data not shown). Thus, in addition to directly repressing hapR expression, Qrr1 and, we infer, the other Qrr sRNAs also directly activate vca0939 expression.
Fig. 3.
Base-pairing between Qrr sRNAs and hapR and vca0939 mRNAs. (A) E. coli carrying either a vector control (open bars) or a qrr1-expressing vector (filled bars), along with a plasmid containing vca0939-lux or the mutagenized reporters vca0939(aa→uu)-lux or vca0939(cc→gg)-lux. (B) MFOLD predictions (18) for mRNA hybrids containing the Qrr sRNA sequence fused by a six-base linker (denoted n) to hapR (Qrr/hapR) or vca0939 (Qrr/vca0939) mRNA. (C) Predicted Qrr/vca0939 duplex showing the location of the vca0939(aa→uu)-lux mutation (arrow under the boxed nucleotides). (D) The predicted inhibitory stem-loop structure in the mRNA of vca0939 showing the location of the vca0939(cc→gg)-lux mutation (arrow adjacent to the boxed nucleotides) (19). In B–D the RBSs are shown in red, the initiation codons are underlined, and the predicted sRNA binding site in the vca0939 mRNA is in blue.
The V. cholerae Qrr sRNAs Base-Pair with Multiple Target mRNAs.
Because Qrr1 directly regulates vca0939 expression (Fig. 3A), we speculated that the Qrr sRNAs base-pair with the mRNA encoding vca0939. To test this hypothesis, an MFOLD algorithm was used to compare the predicted secondary structures of Qrr/hapR and Qrr/vca0939 mRNA hybrids (Fig. 3B) (18). In the case of Qrr/hapR, the algorithm predicts the exact base-pairing identified previously (8), giving us confidence in this approach. In the case of Qrr/vca0939, identical interactions between vc0939 mRNA and each of the four Qrr sRNAs are predicted, and, importantly, the critical base-pairing region in each sRNA is identical to that required for the Qrr/hapR interactions. However, a distinct region of the vca0939 mRNA appears to participate in base-pairing to the sRNAs. Specifically, the ribosome binding site of hapR is occluded in the Qrr/hapR duplex but not in the predicted interactions between the Qrr sRNAs and vca0939 (Fig. 3B). Rather, analysis of the 5′ UTR of the vca0939 mRNA suggests that, in the absence of the Qrr sRNAs, a single inhibitory stem-loop structure exists in a region overlapping both the RBS and the putative sRNA binding site (19). Qrr sRNA interaction with the vca0939 mRNA apparently prevents formation of this inhibitory structure, allows access to the ribosome, and promotes translation. These predictions suggest a molecular explanation underlying the different outcomes (i.e., activation versus inhibition of translation) of Qrr/mRNA target base-pairing interactions.
The V. cholerae Qrr sRNAs Disrupt an Inhibitory Stem Loop Structure in the vca0939 mRNA.
To test the validity of the above predictions, we altered sites in the vca0939 5′ UTR predicted to be crucial for the interaction with the Qrr sRNAs or for the formation of the inhibitory stem-loop structure. We subsequently measured their effects on vca0939-lux expression. We disrupted the putative sRNA binding site in vca0939 by changing an AA doublet to a UU (see arrow in Fig. 3C) while leaving the nucleotides required for the formation of the putative inhibitory stem-loop structure intact. The AA to UU alteration reduced expression of vca0939-lux in E. coli carrying Qrr1 to background levels [Fig. 3A, center bars, denoted vca0939(aa→uu)-lux], suggesting that Qrr1 no longer binds to vca0939 mRNA and promotes its translation. In the reciprocal experiment, we mutated the site predicted to be required for forming the inhibitory stem-loop by changing a CC pair to GG (see arrow in Fig. 3D). This disruption was engineered into vca0939 in a region outside of the site proposed to be required for sRNA binding. This mutation caused maximal expression of vca0939-lux in the presence and absence of the Qrr sRNA [Fig. 3A, right bars, denoted vca0939(cc→gg)-lux]. Thus, disruption of the stem-loop structure alleviates any requirement for a Qrr sRNA in activation of vca0939 expression. Together, our results demonstrate that indeed the Qrr sRNAs directly base-pair with vca0939 mRNA, and, furthermore, they function to activate its expression by disrupting an inhibitory stem-loop structure at the RBS.
QS Occurs in V. cholerae Strains Carrying hapR Null Mutations.
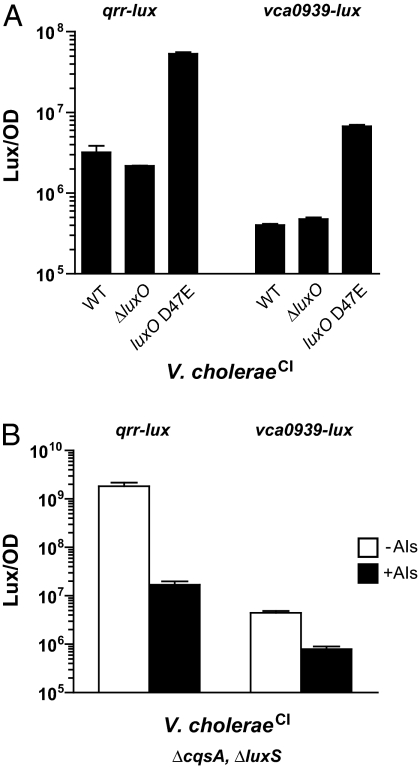
Many previously characterized clinical isolates of V. cholerae have been deemed incapable of QS based on DNA sequence data documenting the absence of a functional HapR master regulator. For example, V. cholerae classical strain O395 (V. choleraeCl) possesses a frameshift mutation in hapR generating a truncated polypeptide (79 aa, compared with the 204-aa protein of V. choleraeEl) (15). Our inspection of the V. choleraeCl genome shows that all of the other components of its QS circuit are intact and that a homolog of vca0939 is present. Based on the above results, we predict that V. choleraeCl is capable of QS (i.e., responding to AIs) through the HapR-independent pathway identified here. To verify that the overall V. choleraeCl QS circuit functions, we introduced qrr-lux and vca0939-lux fusions into wild-type, ΔluxO, and luxO D47E V. choleraeCl strains. Fig. 4A shows that the expression patterns of both reporters exactly mimic those in the corresponding V. cholerae El Tor strains (see Fig. 2A) (8). Specifically, both the qrr sRNA and vca0939 genes are maximally expressed in the low cell density state (luxO D47E mutant) and minimally expressed in the high cell density state (WT and ΔluxO mutant).
Fig. 4.
QS occurs in V. choleraeCl strain O395 despite a nonfunctional HapR. (A) qrr-lux (left bars) and vca0939-lux (right bars) expression in V. choleraeCl monocultures. (B) V. choleraeCl CAI-1−, AI-2− mutant carrying either qrr-lux (left bars) or vca0939-lux (right bars) in a 1:1 coculture with either a V. choleraeCl CAI-1−, AI-2− strain (−AIs, open bars), or a V. choleraeCl CAI-1+, AI-2+ strain (+AIs, filled bars). Mean bioluminescence/OD (Lux/OD) and the standard deviations of triplicate cultures are shown.
To establish that the V. choleraeCl biotype is capable of a QS response, we assayed whether a V. choleraeCl ΔcqsA, ΔluxS (i.e., CAI-1−, AI-2−) mutant could regulate qrr-lux and vca0939-lux expression in response to the exogenous addition of AIs. To do this, as in Fig. 2, we used coculture, this time with either a V. choleraeCl AI+ donor or V. choleraeCl AI− control strain. Expression of qrr-lux and vca0939-lux is repressed 100- and 6-fold respectively, by the presence of AIs (Fig. 4B). Thus, V. cholerae isolates, including classical strains, like O395, that lack functional HapR proteins, nonetheless use QS to regulate expression of vca0939 and very likely other target genes.
Discussion
HapR has been designated the master regulator of QS in V. cholerae because all QS target genes identified to date require it for their control (2, 9–13). Previous studies supported a model in which all QS AI information was channeled exclusively to HapR via the four Qrr sRNAs (8). Here we show that another target, vca0939, encoding a putative GGDEF protein, is also regulated by AIs and by the Qrr sRNAs. Critically, however, unlike the hapR mRNA that is negatively controlled by the Qrr sRNAs, the vca0939 mRNA is positively controlled by the Qrr sRNAs and in a HapR-independent manner. Together, our results show that the Qrr sRNAs, in association with the mRNA chaperone Hfq, act as both positive and negative regulators of mRNA translation, and, furthermore, both HapR-dependent and HapR-independent QS pathways exist in V. cholerae.
Only two other Hfq-dependent sRNAs (DsrA and RyhB, both in E. coli) are known to have dual functions as posttranscriptional activators and repressors. Base-pairing of the DsrA sRNA to the rpoS mRNA, encoding the stationary phase σ factor RpoS, activates translation by preventing formation of an inhibitory secondary structure (20). DsrA also prevents translation by binding to the mRNA encoding the nucleoid protein H-NS (21). Likewise, the RyhB sRNA controls iron scavenging by base-pairing to and activating translation of the shiA mRNA, which encodes a permease. RyhB also functions in the E. coli stress response by binding to, and triggering the degradation of, the sodB mRNA encoding superoxide dismutase (22, 23).
The target of Qrr regulation defined here, vca0939, encodes a protein containing a GGDEF motif. GGDEF proteins are guanylate cyclases that synthesize the intracellular second messenger c-di-GMP. Another set of proteins, containing EAL or HD-GYP motifs, are phosphodiesterases responsible for breaking down c-di-GMP. Increased c-di-GMP levels, among other things, induce expression of exopolysaccharide biosynthetic genes required for biofilm formation in V. cholerae (14). Deletion of vca0939 in V. cholerae El Tor did not alter expression of exopolysaccharide biosynthesis genes or biofilm formation (data not shown). This result was not unexpected because V. cholerae is predicted to possess 41 GGDEF proteins and 31 EAL and HD-GYP proteins (24). Significant redundancy exists in the roles these proteins play in controlling c-di-GMP levels in V. cholerae, and, frequently, deletion of the gene encoding one GGDEF or EAL protein, as in the case of vca0939, does not result in an observable phenotype.
The genomes of all sequenced Vibrio species show that they possess QS circuits extremely similar to that of V. cholerae El Tor strain C6706 (1). V. cholerae classical strain O395 and V. cholerae El Tor strain N16961 harbor nonsense mutations in their hapR genes (15), but, by contrast, these strains have only conservative mutations in genes encoding the other components of their QS circuitry. Strikingly, all four qrr DNA sequences in these V. cholerae strains are 100% identical to those in V. cholerae El Tor strain C6706, suggesting that, irrespective of the presence or absence of HapR, maintenance of functional Qrr sRNAs is critical. We suggest that this is because the Qrr sRNAs play an essential regulatory role in HapR-independent QS pathways that make HapR− V. cholerae strains capable of cell–cell communication.
We propose that, at low cell density (low AI levels), LuxO∼P activates expression of the genes encoding the Qrr sRNAs, which, along with Hfq, bind to target mRNAs and alter their fates (Fig. 1). In the case of the hapR mRNA, the Qrr sRNAs base-pair over the RBS and destabilize the message. This interaction alters HapR-dependent gene expression (Fig. 1, pathway A). In the case of vca0939, base-pairing of the Qrr sRNAs antagonizes an inhibitory stem-loop and allows translation of VCA0939 (Fig. 1, pathway B). In V. cholerae strains containing a functional HapR (e.g., pandemic El Tor C6706), both pathways are used (Fig. 1, pathways A and B). In strains lacking HapR (e.g., pandemic classical O395 and El Tor N16961), only the latter pathway is relevant (Fig. 1, pathway B). Our screen was performed in a HapR+ strain. Fortuitously, it allowed us to identify one HapR-independent target gene. We predict that additional target mRNAs like vca0939 exist and are regulated directly by the Qrr sRNAs in V. cholerae strains. We expect this to be the case in other Vibrio species because, as mentioned, they also contain V. cholerae-like QS systems. We are currently performing genetic screens (in HapR− Vibrio strains) and in silico analyses to identify additional targets of this HapR-independent, AI-responsive QS pathway.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
Bioluminescence was measured in the following V. choleraeEl C6706str strains carrying the vca0939-lux reporter: WT (BH2028); ΔcqsA, ΔluxS (BH1842); ΔluxO (BH2029); luxO D47E (BH2031); ΔhapR (BH2030); ΔcqsA, ΔluxS, ΔhapR (BH2111); luxO D47E, Δhfq (BH2183); and luxO D47E, Δqrr1–4 (BH2133). Bioluminescence was measured in the following V. choleraeCl O395str strains carrying a vca0939-lux reporter: WT (BH2341); ΔluxO (BH2332); luxO D47E (BH2323), and ΔcqsA, ΔluxS (BH2386). Bioluminescence was measured in the following V. choleraeCl O395str strains carrying a qrr-lux reporter: WT (BH2302); ΔluxO (BH2330); luxO D47E (BH2325); and ΔcqsA, ΔluxS (BH2385). E. coli DH10B strains used in Fig. 3A carry either pKKqrr1 (BH2130, BH2470, and BH2472) or a control vector pKK177 (BH2128, BH2469, and BH2471), as described in ref. 8, along with the vca0939-lux (BH2130, BH2128), vca0939(aa→uu)-lux (BH2470, BH2469), or vca0939(cc→gg)-lux (BH2472, BH2471) reporter plasmids, respectively. The QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce the aa→uu and cc→gg mutations into the vca0939-lux reporter plasmid. V. cholerae strains were constructed as described (25). V. choleraeEl C6706str WT (BH1718) and V. choleraeCl O395str WT (BH2388) strains and the corresponding ΔcqsA, ΔluxS (BH1720) and ΔcqsA, ΔluxS (BH2387) strains carrying the vector pBBRlux (described in ref. 8) served as AI+ donors and AI− controls, respectively. Cultures were grown in LB medium at 30°C with aeration. Chloramphenicol (10 mg·liter−1); and ampicillin (100 mg·liter−1) were used to maintain plasmids.
Bioluminescence Assays.
Overnight cultures were diluted 1:100 in LB. In coculture experiments, donor strains were mixed at a 1:1 ratio with recipients. V. choleraeEl cocultures were incubated for 8 h, and V. choleraeCl cocultures were incubated for 6 h; monocultures were incubated overnight. Absorbance and bioluminescence were quantified thereafter. Lux/OD is defined as counts per min−1·ml−1/OD600.
Acknowledgments
We thank the members of the B.L.B. laboratory for insightful discussions. We are grateful to G. Chimalakonda for help with the screen and J. Irgon for bioinformatics. This research was supported by the Howard Hughes Medical Institute (B.L.B.) and by National Institutes of Health grants (to B.L.B. and B.K.H.).
Abbreviations
- QS
quorum sensing
- AI
autoinducer
- sRNA
small regulatory RNA.
Footnotes
The authors declare no conflict of interest.
References
- 1.Waters CM, Bassler BL. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 2.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 3.Henke JM, Bassler BL. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 5.Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Mol Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Neiditch MB, Federle MJ, Pompeani AJ, Kelly RC, Swem DL, Jeffrey PD, Bassler BL, Hughson FM. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Hammer BK, Bassler BL. Mol Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. Mol Microbiol. 2004;53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Proc Natl Acad Sci USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacikova G, Skorupski K. Mol Microbiol. 2002;46:1135–1147. doi: 10.1046/j.1365-2958.2002.03229.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Mekalanos JJ. Dev Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 14.Faruque SM, Albert MJ, Mekalanos JJ. Microbiol Mo Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joelsson A, Liu Z, Zhu J. Infect Immun. 2006;74:1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camilli A, Bassler BL. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storz G, Altuvia S, Wassarman KM. Annu Rev Biochem. 2005;74:199–217. doi: 10.1146/annurev.biochem.74.082803.133136. [DOI] [PubMed] [Google Scholar]
- 18.Mandin P, Repoila F, Vergassola M, Geissmann T, Cossart P. Nucleic Acids Res. 2007;35:962–974. doi: 10.1093/nar/gkl1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abreu-Goodger C, Merino E. Nucleic Acids Res. 2005;33:W690–W692. doi: 10.1093/nar/gki445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. Proc Natl Acad Sci USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lease RA, Cusick ME, Belfort M. Proc Natl Acad Sci USA. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prevost K, Salvail H, Desnoyers G, Jacques J, Phaneuf E, Masse E. Mol Microbiol. 2007;64:1260–1273. doi: 10.1111/j.1365-2958.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 23.Masse E, Escorcia FE, Gottesman S. Genes Dev. 2003;17:2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galperin MY, Nikolskaya AN, Koonin EV. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- 25.Skorupski K, Taylor RK. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]