Abstract
Parallel tetramolecular quadruplexes may be formed with short oligodeoxynucleotides bearing a block of three or more guanines. We analyze the properties of sequence variants of parallel quadruplexes in which each guanine of the central block was systematically substituted with a different base. Twelve types of substitutions were assessed in more than 100 different sequences. We conducted a comparative kinetic analysis of all tetramers. Electrospray mass spectrometry was used to count the number of inner cations, which is an indicator of the number of effective tetrads. In general, the presence of a single substitution has a strong deleterious impact on quadruplex stability, resulting in reduced quadruplex lifetime/thermal stability and in decreased association rate constants. We demonstrate extremely large differences in the association rate constants of these quadruplexes depending on modification position and type. These results demonstrate that most guanine substitutions are deleterious to tetramolecular quadruplex structure. Despite the presence of well-defined non-guanine base quartets in a number of NMR and X-ray structures, our data suggest that most non-guanine quartets do not participate favorably in structural stability, and that these quartets are formed only by virtue of the docking platform provided by neighboring G-quartets. Two notable exceptions were found with 8-bromo-guanine (X) and 6-methyl-isoxanthopterin (P) substitutions, which accelerate quadruplex formation by a factor of 10 when present at the 5′ end. The thermodynamic and kinetic data compiled here are highly valuable for the design of DNA quadruplex assemblies with tunable association/dissociation properties.
INTRODUCTION
Guanine-rich regions abound in the human genome and they have the propensity to fold into higher order DNA structures such as quadruplexes (1,2) which result from the hydrophobic stacking of several guanine quartets (3) (Figure 1). A cation (typically Na+ or K+) located between two quartets participates in cation–dipole interactions with eight guanines, thereby reducing the repulsion of the central oxygen atoms, enhancing hydrogen bond strength and stabilizing quartet stacking. In the past decade, the level of interest in these peculiar structures has increased due to the putative roles of quadruplexes in key biological processes and to recent demonstrations of their existence in vivo (4–7). G-quadruplexes may have applications in areas ranging from supramolecular chemistry to medicinal chemistry and nanotechnology [reviewed in (8–11)]. Therefore, it is important to understand the rules that govern the formation of these complexes and to determine their stabilities and association kinetics.
Figure 1.
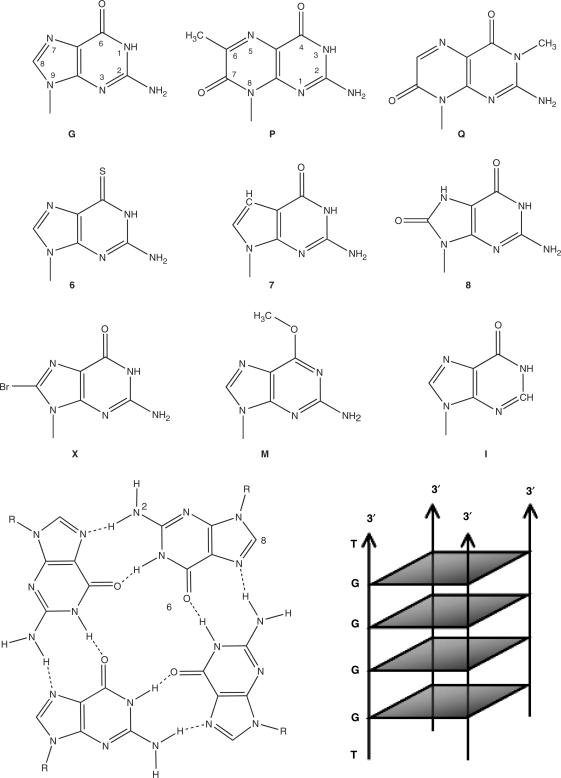
A G-quartet and bases tested here. Top: Chemical formulae of the bases tested here. I = Inosine; 6 = 6-thioguanine; 7 = 7-deazaguanine; 8 = 8-oxoguanine; P = 6MI = 6-methylisoxanthopterin; Q = 3MI = 3-methylisoxanthopterin; M = 6-methyl guanine; X = 8-bromo-guanine. Formula of the regular DNA and RNA bases (A, C, T, U) are not shown. Lower left: Cycling arrangement of four guanine into a G-quartet. Altering the NH2 group on position 2 will alter the external ring of H-bonds, whereas modifications of the 8-position should leave the H-bond pattern unaffected. Altering the carbonyl group at position 6 not only perturbs the central ring of H-bonds, but may also interfere with cation coordination. Lower right: Scheme of the general folding topology of the TG4T tetramolecular parallel quadruplex.
In the tetramolecular quadruplex configuration (G4-DNA, Figure 1), all strands are parallel, and all guanines are in the anti conformation. The conformations of guanines in G4-DNA are very well known due to a number of available high-resolution X-ray and NMR structures. This structural wealth might be explained in part by the extraordinary stiffness of the G4-DNA motif (12,13). On the other hand, less is known concerning the kinetics and thermodynamics of tetramolecular quadruplexes. Rules have been proposed to describe the properties of simple, short segments such as T2G4T2 (14). In previous studies, we analyzed the kinetics of quadruplex formation with short DNA sequences (15,16). The kinetic inertia of these quadruplexes allowed us to study association and dissociation processes independently. The association rate strongly depended on strand concentration, with an experimentally determined order close to four (14,15,17). The corresponding association rate constant kon decreased with increasing temperature (reflecting a negative activation energy Eon) and increased with ionic strength.
A number of recent reports demonstrate that tetramolecular quadruplexes may accommodate at least one unusual quartet (18,19). DNA quadruplex formation is therefore not restricted to G-repeat sequences. Rather, the quadruplex fold has a versatile and robust architecture that is accessible to a range of mixed sequences with the potential to form various tetrads or even hexads, heptads and octads. Many articles analyzed these ‘non-G quartets,’ often in the context of parallel tetramolecular quadruplexes. NMR studies have shown that the thymine in the center of the TG2TG2C four-stranded quadruplex forms a thymine quartet (20) and the cytosine in the TG3CGT quadruplex forms a cytosine quartet (21). Adenine quartets (22), uracil quartets (23) and bulges may also be accommodated in RNA quadruplexes (24), expanding the structural repertoire of quadruplexes. However, the contributions of these non-G quartets to the kinetics and energetics of the quadruplex are poorly understood, and structural methods provide only clues to the effects of these modifications. Little data is available for sequences in which the G-tract is interrupted by a ‘mismatch,’ i.e. any base (natural or synthetic) different from a guanine.
Using the canonical tetramolecular quadruplexes formed by TG4T and TG5T, we substituted each of the four or five guanines, respectively, with a variety of bases (the natural bases A, T, C and U, and the non-natural bases represented in Figure 1) and analyzed the impacts of these modifications on the kinetics of formation and thermal stabilities of the complexes. We demonstrate that, in most cases, the incorporation of a single modified quartet not only leads to decreased melting temperature but also to a decreased association rate. Non-guanine base quartets are, at best, tolerated in a parallel quadruplex and generally do not contribute to the stability of the structure, two exceptions being the 8-bromo-guanine (X) and 6-methyl-isoxanthopterin (P) substitutions.
MATERIALS AND METHODS
Nomenclature, synthesis and purification of oligonucleotide sequences
Oligonucleotides were synthesized by Eurogentec (Seraing, Belgium), except for P (= 6MI = 6-methylisoxanthopterin) and Q (= 3MI = 3-methylisoxanthopterin) (25,26), which were synthesized by Fidelity Systems, Inc. (Gaithersburg, MD, USA). Concentrations of all oligodeoxynucleotides were estimated using extinction coefficients provided by the manufacturer. A single letter/number code was chosen for all bases: I for inosine, 6 for 6-thioguanine, etc. (a complete list can be found in Figure 1, top). Sequences are given in the 5′ to 3′ direction; e.g. TG7GGGT is an oligonucleotide in which the second guanine has been replaced by 7-deazaguanine.
Absorbance measurements
Isothermal and melting experiments were conducted as previously described (15). Starting from completely unfolded strands, absorbance was recorded at regular time intervals (120–300 s) at three to five different wavelengths in the presence of 110 mM KCl, NaCl or NH4Cl. Oligonucleotide strand concentration was fixed between 1 and 700 µM. For high concentrations, cuvettes of 0.5–1mm path length were used (Hellma France). Experimental points were fitted to a kinetic model, according to a previous study (15). To allow a comparison of the association rate constants, we arbitrarily defined the order of the reaction as four for all oligonucleotides. This value cannot be experimentally verified in all experimental conditions, and may somewhat differ [we previously reported values between 3.4 and 4.1 for unmodified G-rich oligonucleotides (15)]. To obtain an accurate value for kon, curves were fitted at all useable wavelengths (generally 240 and 295 nm, sometimes 260 and/or 375 nm for base P). Numerical values resulted from two to seven independent kon determinations. Most melting curves recorded by heating a preformed quadruplex do not correspond to equilibrium melting curves (hysteresis phenomenon), and the ‘T1/2’ deduced from these experiments depends on the heating rate (0.48°C/min here) (15). Apparent T1/2 above 90°C or below 20°C could not be accurately determined. Overall, >1000 kinetic or melting experiments were performed.
Gel electrophoresis
Purity of the provided oligonucleotides was initially tested by denaturing PAGE (data not shown). Samples in water and formamide were loaded on a 20% polyacrylamide gel containing Tris-Borate-EDTA (TBE) 1X and 7 M urea. Electrophoresis was performed at 14 W to reach a temperature close to 45°C. For kinetic experiments, association kinetic of G4-DNA was confirmed by non-denaturing PAGE. In that case, oligonucleotides were all incubated at a unique concentration (80–100 µM) during different times in lithium cacodylate 10 mM pH 7.2 buffer with 110 mM Na+ or 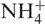 . Here, 10% sucrose was added just before loading. This method has a low throughput, but is useful for very long incubations and to confirm spectroscopic data. Oligothymidylate markers (dT6, dT12 or dT24) were also loaded on the gel. One should note that the migration of these markers (short 5′dTn oligonucleotides) does not necessarily correspond to single strands (27): these oligonucleotides were chosen here to provide an internal migration standard, not to identify single-stranded or higher order structures.
. Here, 10% sucrose was added just before loading. This method has a low throughput, but is useful for very long incubations and to confirm spectroscopic data. Oligothymidylate markers (dT6, dT12 or dT24) were also loaded on the gel. One should note that the migration of these markers (short 5′dTn oligonucleotides) does not necessarily correspond to single strands (27): these oligonucleotides were chosen here to provide an internal migration standard, not to identify single-stranded or higher order structures.
Mass spectrometry
ESI-MS experiments were performed as previously described (28,29). All experiments were performed on a Q-TOF Ultima Global (Micromass, now Waters, Manchester, UK) with the Z-spray ESI source. The capillary voltage was set to −2.2 kV and the cone voltage to 35 V. The RF lens 1 was set to 74 V for all the quadruplexes. The argon pressure inside the collision hexapole (3.0 × 10−5 mbar ± 5%) and the source pressure (2.70 mbar) were carefully kept constant. Quadruplexes were prepared in 150 mM ammonium acetate. Methanol (15%) was added to the samples just before injection to obtain a stable electrospray signal.
RESULTS
Formation of the canonical tetraplexes
All oligonucleotides studied here contain a single block of guanines and form tetramolecular species. Oligomers ending with a terminal 5′ or 3′ guanine, such as TG3–5 or G3–5T, are likely to form complex or higher order molecular species, as indicated by the CD studies of Lieberman and Hardin (30). For this reason, we chose two model sequences with terminal thymines, TG4T and TG5T. All studies were performed in K+, in Na+ and in 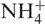 . Data concerning these canonical sequences may be found in Figure S1, which is published as supporting information. Interestingly, whereas K+ is the preferred cation for both association rate and thermal stability (highest apparent melting temperatures and highest association rate constants), Na+ and
. Data concerning these canonical sequences may be found in Figure S1, which is published as supporting information. Interestingly, whereas K+ is the preferred cation for both association rate and thermal stability (highest apparent melting temperatures and highest association rate constants), Na+ and 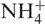 exhibit opposite trends: sodium leads to faster association than ammonium, but the quadruplexes have a higher melting temperature in the presence of
exhibit opposite trends: sodium leads to faster association than ammonium, but the quadruplexes have a higher melting temperature in the presence of 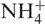 than in Na+. Similar conclusions were reached for other tetramolecular complexes (data not shown). These results illustrate that it is essential to evaluate the kinetics of dissociation and association to obtain a reliable estimate of the thermodynamic stability of these structures. The relative inefficiency of ammonium ions to promote quadruplex formation was relatively unexpected as this ion has an ionic radius close to potassium. One may propose that these ammonium ions could stabilize an undesired single-stranded conformation because of their greater propensity to interact with phosphate groups.
than in Na+. Similar conclusions were reached for other tetramolecular complexes (data not shown). These results illustrate that it is essential to evaluate the kinetics of dissociation and association to obtain a reliable estimate of the thermodynamic stability of these structures. The relative inefficiency of ammonium ions to promote quadruplex formation was relatively unexpected as this ion has an ionic radius close to potassium. One may propose that these ammonium ions could stabilize an undesired single-stranded conformation because of their greater propensity to interact with phosphate groups.
Quadruplex formation with the modified sequences
Variants of these sequences were designed. For most modifications, we systematically replaced one guanine at a time in the TG4T and TG5T oligonucleotides (i.e. nine different positions for single-substitutions). Examples are provided in Tables S1 and S2. We chose two different tetramolecular quadruplex motifs (TG4T and TG5T) for confirmatory purposes, but also because in thermal denaturation experiments, little or no dissociation was observed for the TG5T quadruplex and its variants, even at 90°C. The lower T1/2 of the TG4T quadruplex allowed us to observe and compare the unfolding process. On the other hand, the longer TG5T quadruplex, with an extra G-quartet and faster association kinetics, favors quadruplex formation even when highly destabilizing substitutions are incorporated, allowing us to quantitate the impact of these modifications on the association kinetics.
Determination of the 3D solution structure of all sequences studied here is beyond the scope of this article. Nevertheless, before comparing the kinetics and thermodynamics of these oligomers, we deemed it necessary to establish that these sequences have the same global architecture. Quadruplex formation was confirmed by four independent methods (Figures S2–S4). Oligonucleotides were analyzed by PAGE, and quadruplex formation was revealed by a slow-migrating band as compared to the migration pattern of the same ‘single-stranded’ oligomer. Complete or near complete conversion to a lower mobility band was obtained with most sequences. Furthermore, the isothermal difference and circular dichroism spectra of these structures were in agreement with the formation of quadruplexes (31–33). Finally, electrospray ionization mass spectrometry (ESI-MS) in the negative ion mode provided unambiguous data on strand stoichiometry (four identical strands are involved in a complex).
Association of the isolated strands at low temperature
Isothermal renaturation experiments were used to study the formation of the quadruplexes; representative examples are provided in Figures 2A and S5. Starting from the unfolded species, a time-dependent increase in absorbance at 295 nm was observed, while an opposite trend was seen at 240 nm, indicating a single-strands-to-quadruplex transition. Using various strand concentrations, one would expect the calculated kon to be concentration-independent if the order is correct. Association data for TG5T were fitted with n = 4, in agreement with previous observations (14,15,17). To allow a numerical comparison of the results, we defined n = 4 for all further studies. These fits were in nearly perfect agreement with the experimental points. Moreover, the kon values determined from the curves at different concentrations and at two different wavelengths (240 and 295 nm) were in excellent agreement, and a dual wavelength parametric test (34) failed to reveal the existence of more than two species (unfolded and associated; Figures 2B and S6).
Figure 2.
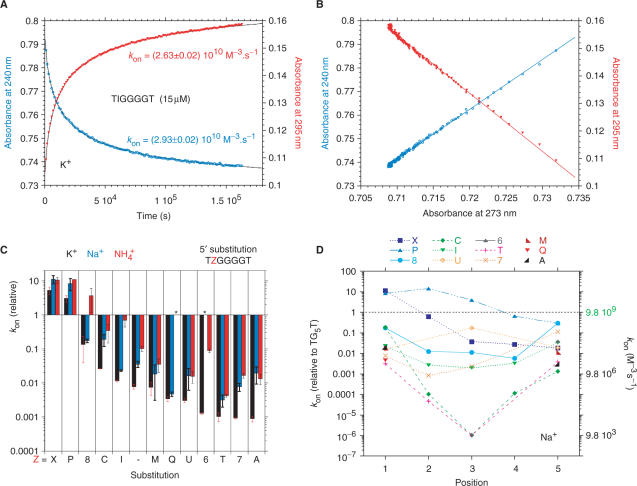
Analysis of the association curves. (A) Representative example of an isothermal renaturation experiment. Formation of a quadruplex with the inosine-containing oligonucleotide TIGGGGT (panel A) (15 µM strand concentration, at 4°C, 0.11 M K+). Raw absorbance was recorded simultaneously at two wavelengths (240 nm: blue circles and 295 nm: red inverted triangles). The fitted curves (full lines) are nearly indistinguishable from the experimental data. Fitted kon values are provided for each curve. (B) Example of a dual wavelength parametric test for TGGIGGT (identical conditions as in Panel A). In this example, absorbance at 240 nm (left Y-scale, blue circles) and absorbance at 295 nm (right Y-scale, red triangles) are plotted versus absorbance at 273 nm. Other examples are provided in Supplementary Data. (C) Relative association constant (kon) as compared to TG5T for oligomers in which the first guanine has been replaced by another base (code as in Figure 1). Data obtained in K+ (black), Na+ (blue) or 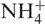 (red). ‘-’ corresponds to TG4T. Note that these relative values have been normalized for each cation compared to the unmodified oligonucleotide. kon values in K+ and Na+ are respectively 2000 and 10 times higher than in
(red). ‘-’ corresponds to TG4T. Note that these relative values have been normalized for each cation compared to the unmodified oligonucleotide. kon values in K+ and Na+ are respectively 2000 and 10 times higher than in 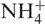 . (D) Association constants in Na+: Effect of a single guanine substitution on kon (corresponding curves in K+ or
. (D) Association constants in Na+: Effect of a single guanine substitution on kon (corresponding curves in K+ or 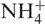 are provided as Supplementary Data). The position of the substitution is indicated on the X-axis: position 1 corresponds to the first guanine (5′ side), position 5 to the last guanine (3′ side). The relative kon values (±SD) for the formation of the TG5T variants are indicated on the left Y-axis (kon for the unmodified TG5T sequence under the same conditions = 1, corresponding to a horizontal dotted line). Absolute values are shown on the right Y-axis. Experiments were performed in 0.11 M Na+ at 3 ± 1°C. Note the semi-log scale: for many mutants, a single substitution may lead a tremendous decrease in kon. Only a few cases lead to a higher kon than TG5T, for example TXGGGGT (blue squares).
are provided as Supplementary Data). The position of the substitution is indicated on the X-axis: position 1 corresponds to the first guanine (5′ side), position 5 to the last guanine (3′ side). The relative kon values (±SD) for the formation of the TG5T variants are indicated on the left Y-axis (kon for the unmodified TG5T sequence under the same conditions = 1, corresponding to a horizontal dotted line). Absolute values are shown on the right Y-axis. Experiments were performed in 0.11 M Na+ at 3 ± 1°C. Note the semi-log scale: for many mutants, a single substitution may lead a tremendous decrease in kon. Only a few cases lead to a higher kon than TG5T, for example TXGGGGT (blue squares).
The association rate constants for the various oligonucleotides are provided in Tables S1–S3 and are compared in Figure 2C and D, and S7. All values are given in M−3 s−1, reflecting the order chosen to fit the data. Important differences may be found among the various sequences; values for association rate constants ranged from ∼1013 to 104 M−3 s−1 (i.e. 1 billion-fold difference). For this reason, all graphs are shown on a semi-log scale. One should note that, due to the order of four chosen for the fits, a 1 billion-fold decrease in kon corresponds to a ‘less impressive’, but still highly significant, 1000-fold higher strand concentration required to obtain a similar proportion of quadruplex species after the same incubation time. For nearly all sequences (modified or not), association was fastest in potassium and slowest in ammonium: 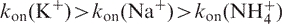 , as observed for the unmodified sequences.
, as observed for the unmodified sequences.
A vast majority of modified sequences associated at a much slower rate than the unmodified TG5T oligonucleotide. The most unfavorable modification found in this study was adenine in central positions. TGAG3T has a kon 108-times lower than TG5T in K+. Substitution effects were strongly position dependent. Contrasting with the >108-fold difference attained in central positions, the maximal destabilization for a terminal modification was 1000–5000-fold (meaning that 10–17-fold higher strand concentrations are required to obtain a similar proportion of quadruplex species as a function of time) (for e.g. Figure 2C). Overall, an unfavorable substitution had a lower detrimental effect when located at the extremities, leading to ‘V’- or ‘U’-shaped curves in Figures 2D and S7. This shows that the contributions of the quartets are not additive: a modified quartet also influences its neighboring G-quartets. Results obtained in the TG5T series were, in general, qualitatively confirmed in the TG4T series.
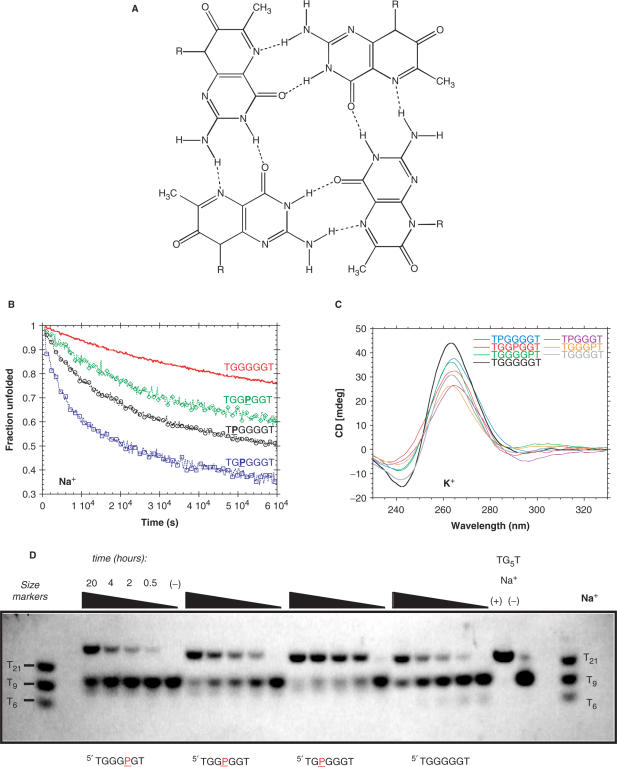
Unexpectedly, two types of substitutions resulted in faster association rates than for the canonical quadruplexes: 8-bromo-guanine (X) and 6-methyl isoxanthopterin (P) (Figure 3). These modifications do not show the ‘U’-shape dependence on position, but rather show a strong asymmetry, with 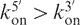 (see Figure S8B). These modifications accelerate quadruplex formation only when present at the 5′ end or 5′ half. These substitutions were further studied using non-denaturing gel electrophoresis (see Figure S9 for TXGGGGT). The case of 6-methyl-isoxanthopterin (P) is particularly interesting (Figure 3). This base was previously incorporated in a sequence compatible with quadruplex formation to act as a fluorescence reporter group, but its contribution to quadruplex stability was not investigated (35). This modified base can also form a quartet with eight hydrogen bonds (Figure 3A). An illustration of a renaturation experiment in Na+ is provided in Figure 3B. Faster quadruplex association was confirmed in K+ and
(see Figure S8B). These modifications accelerate quadruplex formation only when present at the 5′ end or 5′ half. These substitutions were further studied using non-denaturing gel electrophoresis (see Figure S9 for TXGGGGT). The case of 6-methyl-isoxanthopterin (P) is particularly interesting (Figure 3). This base was previously incorporated in a sequence compatible with quadruplex formation to act as a fluorescence reporter group, but its contribution to quadruplex stability was not investigated (35). This modified base can also form a quartet with eight hydrogen bonds (Figure 3A). An illustration of a renaturation experiment in Na+ is provided in Figure 3B. Faster quadruplex association was confirmed in K+ and 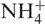 (Table S3). CD spectra of quadruplexes were very similar to TG4T and TG5T (Figure 3C). Confirmation of fast kinetics in Na+ was obtained by non-denaturing gel electrophoresis (Figure 3D).
(Table S3). CD spectra of quadruplexes were very similar to TG4T and TG5T (Figure 3C). Confirmation of fast kinetics in Na+ was obtained by non-denaturing gel electrophoresis (Figure 3D).
Figure 3.
6MI (‘P’) leads to faster association (A) Putative quartet formed by ‘P’ (6MI, or 6-methyl isoxanthopterin). (B) Isothermal quadruplex formation experiments for TGGGGGT (red) TPGGGGT (black), TGPGGGT (blue) and TGGPGGT (green) in 0.11 M Na+ at 2.9°C deduced from absorbance measurements at 295 nm. All strand concentrations were identical (10 µM). The fraction of unfolded oligonucleotide is plotted versus time. Note that the fluorescence emission of 6MI is quenched by adjacent purines, preventing us from following kinetics by fluorescence spectroscopy (25). (C) Examples of CD spectra for the quadruplexes containing the base P at 4°C in 0.11 M K+. Experimental conditions provided in Supplementary Data. (D) Gel experiments showing that quadruplex formation is faster for TGPGGGT rather than for TGGGGGT (right) in 0.11 M Na+ at 4°C. All strand concentrations were identical (80 µM). Size markers (single-stranded oligothymidylate T21, T9 and T6) are provided. Time-dependent formation of the tetramolecular quadruplex leads to the apparition of a retarded band. Its mobility is close to the mobility of the reference TG5T quadruplex.
Dissociation of the preformed quadruplexes
Starting from preformed quadruplexes (several days at 0–5°C and high strand concentration, 100–1000 µM), the denaturation was followed by recording the absorbance at 240 or 295 nm (15,36) (examples shown in Figures S1C, and 4A and B). This led to a ‘cooperative’ curve that does not reflect an equilibrium denaturation process: upon subsequent cooling, little renaturation of the DNA quadruplex was obtained, in agreement with the low kon values. Furthermore, the apparent melting temperature did not depend on oligonucleotide concentration but strongly depended on the rate of heating (data not shown) (33), again indicating that this profile does not correspond to an equilibrium curve but solely reflects the dissociation of the quadruplex. T1/2 values are provided for most oligonucleotides in Figures 4 and S10 and Tables S1–S3. In general, we found 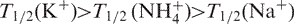 . Differences in T1/2 reflect differences in thermal lability (15) and dissociation rate constant (koff) values can be extracted from the UV-melting curves (16). For most TG5T variants, no dissociation of the quadruplex could be observed in potassium (T1/2 > 90°C). Hence, thermal denaturation data could be collected only for a subset of sequences. In general, the apparent melting temperature was highest in K+ and lowest in Na+, as observed for the unmodified sequences.
. Differences in T1/2 reflect differences in thermal lability (15) and dissociation rate constant (koff) values can be extracted from the UV-melting curves (16). For most TG5T variants, no dissociation of the quadruplex could be observed in potassium (T1/2 > 90°C). Hence, thermal denaturation data could be collected only for a subset of sequences. In general, the apparent melting temperature was highest in K+ and lowest in Na+, as observed for the unmodified sequences.
Figure 4.
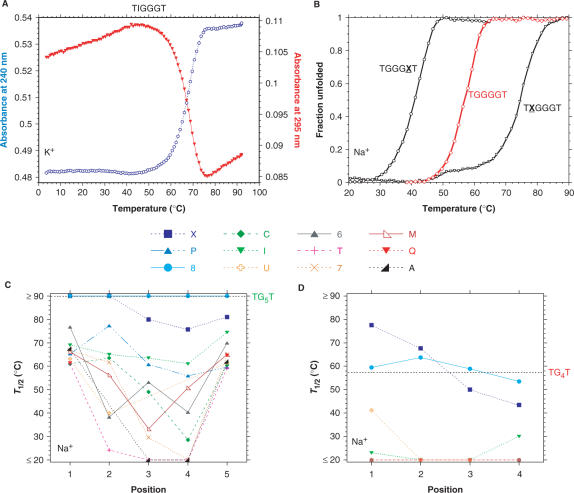
Thermal melting experiments. (A) Representative example of a thermal denaturation experiment. Melting of the quadruplex formed with the TIGGGT oligonucleotide may be followed at 240 nm (blue circles; left Y-scale) and 295 nm (red triangles; right Y-scale). The quadruplex was preformed at 4°C at high concentration (300 µM). T1/2 values determined from the absorbance at 240 and 295 nm are identical (67°C). (B) Comparison of the thermal stability of three different quadruplexes: TGGGXT (open circles), TGGGGT (red diamonds) and TXGGGT (inverted triangles) in 0.11 M Na+. Raw absorbance data has been converted to fraction unfolded, using linear baseline assumptions for single strands and quadruplexes. Most melting curves are monophasic; a few samples give a pretransition illustrated here for TXGGGT (black triangles). Temperature gradient: 0.48°C/min. (C and D) Effect of a single guanine substitution on the apparent melting temperature of TG5T (panel C) and TG4T (panel D) variants. The position of the substitution is indicated on the X-axis: position 1 corresponds to the first guanine (5′ side), position 5 (or 4 in the case of TG4T variants) to the last guanine (3′ side). Here, 12 different replacements were tested, each corresponding to a different symbol. The T1/2 values are provided with a ±1°C accuracy. Experiments were performed in 0.11 M Na+ using a temperature gradient of 0.48°C/min. In some cases, no melting of the quadruplex was observed: T1/2 was arbitrarily fixed at ≥90°C.
Most modified quadruplexes had lower thermal stabilities than the unmodified oligonucleotide. Differences in T1/2 could be extreme; e.g. the T1/2 for TGTGGGT in Na+ was more than 60°C lower than the T1/2 for TG5T under identical conditions (Figure 4C). From the T1/2 values, the various modifications could be ranked from mildly stabilizing to very destabilizing (note that the stabilizing modifications could only be studied for TG4T variants in Na+ and 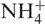 , the T1/2 being >90°C in other cases). For substitution of the first guanine of the G5 stretch, X ≈ 8 > G >> all others. The ranking of the other modifications depended on the position and the cation, with T, A, 7 and C often being very destabilizing (higher dissociation rate). The ranking was almost independent on the nature of the monocation (Figures 4 and S10). Interestingly, this dissociation ranking is different from the one found for association rates. For example, P, which was found to accelerate quadruplex formation, nevertheless led to a significant decrease in T1/2.
, the T1/2 being >90°C in other cases). For substitution of the first guanine of the G5 stretch, X ≈ 8 > G >> all others. The ranking of the other modifications depended on the position and the cation, with T, A, 7 and C often being very destabilizing (higher dissociation rate). The ranking was almost independent on the nature of the monocation (Figures 4 and S10). Interestingly, this dissociation ranking is different from the one found for association rates. For example, P, which was found to accelerate quadruplex formation, nevertheless led to a significant decrease in T1/2.
Substitution effects were strongly position-dependent. Overall, an unfavorable substitution had a less detrimental effect when located at the extremities. However, asymmetrical effects were also observed, e.g. for the 8 and X modifications. A similar observation was reached in another study: TXGGT and TGXGT formed a more stable quadruplex than the unmodified sequence, whereas TGGXT was much less stable than the natural counterpart (18). Within ‘central’ positions (2, 3 or 4 in the TG5T variants), no general rule emerged. Position 3 was not necessarily more destabilizing than position 2 or 4. Results obtained in the TG5T series were qualitatively confirmed in the TG4T series (compare Figure 4C and D). However, a number of modified sequences failed to melt in the TG5T series, as mentioned previously.
Whereas the canonical TG5T quadruplex resisted boiling in Na+ for a few minutes, variant quadruplexes incorporating a single central A, T or 7 base could collapse below physiological temperature (Figure 4). Only a few modifications (X and 8) led to an equal or higher thermal stability than a guanine, and this effect was generally restricted to the terminal positions (1 and 5, or 1 and 4). This property could not be evidenced for TG5T variants, as the canonical quadruplex already exhibits a T1/2 ≥ 90°C under all conditions. In contrast, the denaturation of the TG4T quadruplex in Na+ (Figure 4D) and 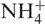 (Figure S10D) could be observed.
(Figure S10D) could be observed.
Addressing the relative equilibrium stability of the quadruplexes
As explained above, the thermal denaturation experiments do not give access to equilibrium data. Dissociation rate constant (koff) values could be extracted from the UV-melting curves (16). Most modified quadruplexes had a higher dissociation rate constant than the canonical quadruplex. In an Arrhenius representation, data points could be fitted with a straight line, in agreement with a simple melting process, allowing us to determine a positive activation energy of dissociation (Eoff) (Figure S11). To illustrate the differences in the dissociation process, one can also calculate the lifetimes of the different quadruplexes (t1/2 = ln(2)/koff) at a given temperature. For example, at 44°C [d(TGGGXT)]4 has a 20-fold shorter lifetime than the corresponding unmodified [d(TG4T)]4. A notable exception to this rule is the [d(TXGGGT)]4 quadruplex, which is 50-fold longer lived than [d(TG4T)]4. Thus, most substitutions, but not all, had very debilitating effects on quadruplex thermal stability and lifetime.
The determination of the equilibrium association constant can, in principle, be done by calculating the kon/koff ratio at a given temperature. Unfortunately, kon and koff values are experimentally accessible in a different temperature range: the higher the T1/2, the less reliable the koff extrapolation at 3°C, not talking of the sequences with T1/2 > 90°C. Nevertheless, it is clear that, at low temperature and at the chosen concentrations, the equilibrium is highly displaced towards the tetramer in all cases (as confirmed by mass spectrometry), so the estimation of relative equilibrium stabilities by traditional methods is hardly conceivable. We therefore used a mass spectrometry-based approach consisting in counting the number of ammonium cations present in the tetramers. In contrary to Na+ and K+ cations, non-tightly bound 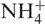 cations escape from the complex before it reaches the detector, but
cations escape from the complex before it reaches the detector, but 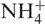 cations coordinated between stable adjacent tetrads remain in the complex (28). For the unmodified sequences, when the proper soft experimental conditions are used, (n − 1) ammonium ions are found in the [d(TGnT)]4 quadruplexes, as shown in Figure S4. In the case of [d(T8GGGGT)]4, four ammonium ions were detected, suggesting that this modified tetrad forms a sufficiently stable architecture to keep the coordinated ammonium ion sandwiched between adjacent G4-tetrads. However, for all other modifications, an average of less than four ammonium ions is detected. The number of ammonium ions embedded in the structure is plotted for each substitution in Figure 5. This number can be interpreted as indicative of the number of effective tetrads in the quadruplex. There is usually a good agreement between the mass spectrometry results and the association/dissociation data: for example TGAGGGT, which has a very low kon, also displays the lowest average number of ammoniums (1.7).
cations coordinated between stable adjacent tetrads remain in the complex (28). For the unmodified sequences, when the proper soft experimental conditions are used, (n − 1) ammonium ions are found in the [d(TGnT)]4 quadruplexes, as shown in Figure S4. In the case of [d(T8GGGGT)]4, four ammonium ions were detected, suggesting that this modified tetrad forms a sufficiently stable architecture to keep the coordinated ammonium ion sandwiched between adjacent G4-tetrads. However, for all other modifications, an average of less than four ammonium ions is detected. The number of ammonium ions embedded in the structure is plotted for each substitution in Figure 5. This number can be interpreted as indicative of the number of effective tetrads in the quadruplex. There is usually a good agreement between the mass spectrometry results and the association/dissociation data: for example TGAGGGT, which has a very low kon, also displays the lowest average number of ammoniums (1.7).
Figure 5.
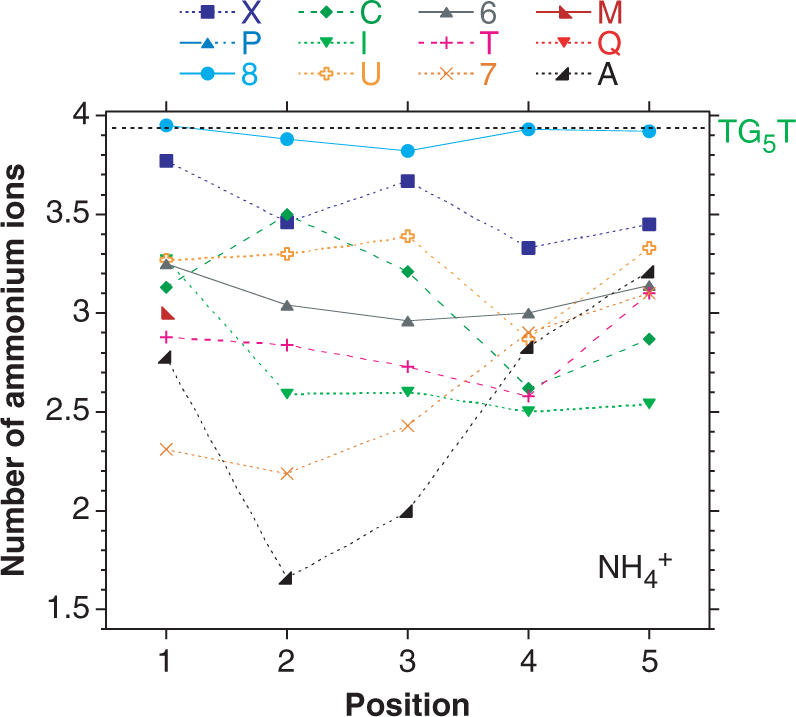
Effect of guanine substitution on the mean number of 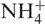 ions present in the quadruplexes: MS analysis. ESI-MS spectra obtained in gentle condition help in understanding the formation of these tetramolecular structures, not only by providing the strand stoichiometry but also an unambiguous determination of the number of contributing structural cations. The position of each substitution is indicated on the X-axis. The mean number of ammonium ions (NNH4) present in the complexes is obtained from equation: NNH4 = [4 × I(G4with 4NH4) + 3 × I(G4with 3NH4) + 2 × I(G4with 2NH4) + 1 × I(G4with 1NH4)]/Sum[I(G4all)] where I(G4n) are the relative intensities of the quadruplex with different number of ammonium ions. Note that the P and Q modifications were not shown here.
ions present in the quadruplexes: MS analysis. ESI-MS spectra obtained in gentle condition help in understanding the formation of these tetramolecular structures, not only by providing the strand stoichiometry but also an unambiguous determination of the number of contributing structural cations. The position of each substitution is indicated on the X-axis. The mean number of ammonium ions (NNH4) present in the complexes is obtained from equation: NNH4 = [4 × I(G4with 4NH4) + 3 × I(G4with 3NH4) + 2 × I(G4with 2NH4) + 1 × I(G4with 1NH4)]/Sum[I(G4all)] where I(G4n) are the relative intensities of the quadruplex with different number of ammonium ions. Note that the P and Q modifications were not shown here.
We initially hoped that further refinements of the relative ammonium stabilities could be obtained by tandem mass spectrometry experiments (selecting a complex with a given number of ammoniums and fragmenting it at variable collision energies), and these details are provided as supporting information for the interested reader (Figure S12 and S13). Unfortunately, we found a weak correlation between the stability in the gas phase deduced from MS/MS experiments and the stability in solution, at least for this system. Nevertheless, these MS/MS experiments may still be useful to obtain further insight into the possible dissociation pathway of the structural cations, and they might be of interest for those interested in the modeling/calculation of cation–quadruplex interactions.
DISCUSSION
In the present study, we analyzed the effects of 12 different base substitutions on the kinetics and thermodynamics of parallel tetramolecular quadruplexes. The data were compared with the parallel-stranded tetramolecular quadruplexes formed by TG4T and TG5T. Most isothermal and melting experiments could be analyzed in the framework of an all-or-none process, in agreement with Petraccone et al., who demonstrated that the quadruplex-to-single strand transition of TG4T involved only two significant spectral species, suggesting a simple dissociation pathway (17). To our knowledge, the present work is the first experimental attempt to quantify and compare a variety of modified quadruplex sequences.
Although many oligomers adopt relatively similar conformations, the kinetics of these complexes may vary greatly. We showed that the consideration of Tm (or T1/2) as the sole indicator of quadruplex thermodynamics may lead to a profound underestimation of the energetic penalty imposed by a single guanine replacement. It is essential to evaluate the kinetics of both dissociation and association to obtain a reliable estimate of the thermodynamic penalty imposed by the sequence modification. It is striking that for quadruplexes, a ‘mismatch’ has a deleterious impact on both the association and dissociation processes, whereas for duplexes and triplexes, a mismatched base-pair or base-triplet affects the dissociation process (37,38). A possible explanation for this behavior comes from the differences in length among these motifs. Only four to five base quartets are formed in quadruplexes, and a mismatch is more likely to affect the nucleation event for initial quadruplex association.
The 5′/3′ asymmetry observed in the influence of stabilizing modifications also gives interesting insights into the nucleation process. One may therefore be tempted to propose that the rate-limiting step involves the 5′ side of the strands. All three favorable modifications (8, X and P) accelerated formation or decelerated dissociation of quadruplexes only when located on the 5′ side. In X and P modifications, the respective bromo- and methyl substituents may favor the initial hydrophobic collapse that brings strands together. However, as this asymmetry is not observed for all substitutions, this putative directional nucleation-zipping mechanism for quadruplex formation is probably less pronounced than for triplexes (39). The extremely deleterious impact of a central guanine substitution on association also indicates that the central guanines participate in the rate-limiting step. It is also worth noticing that with these three modifications (P, X and 8), the syn conformation is (or is likely to be) favored as compared to a regular guanine (40) suggesting the implication of the syn G at the 5′ end in the nucleation process. In the publications reporting quadruplex structures based on the (3 + 1) or mixed parallel–antiparallel scaffold (41–46) the Gs on the 5′ part of the quadruplex are mostly syn. These 5′ syn bases might also participate in the stability of the quadruplex (for X and 8).
The structure of this kinetic intermediate remains elusive but some observations help to eliminate some possibilities: (i) a Hoogsteen duplex or triplex is an extremely unstable, and therefore unlikely, intermediate (13), (ii) transient strand dimers and trimers have been evidenced by mass spectrometry (47), (iii) monocations participate in the stabilization of this kinetic intermediate (15,16), (iv) association is faster at low temperature (15), (v) the experimental order of the reaction is close to four (14–17) while (vi) a four-body collision is an impossible event. Starting from the double-dimer to tetramer pathway proposed by Wyatt et al. (14) and the ‘cross-like’ two-stranded assemblies proposed by Stefl et al. (13), one may envision that the rate-limiting step is the formation of ‘nucleation’ quartets, with four guanines unlikely to originate from four different strands. Two of these guanines must then originate from the same strand (for example, one ‘central’ and the other towards the 5′ end, thereby explaining a certain asymmetry) and some of these bases transiently adopt a syn conformation. This transient geometry could be facilitated by the presence of some modifications, (X or P for example), for which the syn conformation is preferred. A long guanine tract facilitates the formation of two (and perhaps three) stacked quartet, which captures one to two monocations and defines the nucleation event. This could explain the puzzling observation that the longer the guanine tract, the faster the association and this is in agreement with the negative activation energy of association (Eon = −29 kcal/mol for TG4T) found for tetramolecular quadruplexes (15). These initial quartet(s) are embedded in a two-stranded dimer, rather than a Hoogsteen duplex, and will then undergo a series of rearrangements involving the association of additional strands, possible formation of a trimer, syn to anti conversion (again, the presence of syn bases in the final structure may be proposed for some of the analogs; in that case anti to syn conversion of a few residues could be imagined), formation of extra quartets and progressive slippage of strands in order that all guanines in a quartet correspond to the same base in the four strands.
The wealth of data compiled here can serve as a basis for future structural interpretation. Interestingly, Stefl et al. already performed molecular dynamics simulations of DNA quadruplex molecules containing modified bases (48). The incorporation of 6-thioguanine (6) or 6-methylguanine (M) sharply destabilized four-stranded G-DNA structures, whereas inosine (I) had a limited effect. The first two modifications prevented proper cation coordination and created a steric clash in the central part of the quartet, whereas inosine could still form a quartet, even though the external ring of H-bonds is lost. All these predictions are verified in our experiments. Also, the higher destabilization observed with central modifications, together with the mass spectrometry measurements of the number of coordinated cations, suggest that the stability should be interpreted in terms of nearest neighbors (two neighboring quartets and the associated cations) instead of quartets only.
One of the major findings of our study is that most substitutions are extremely detrimental to quadruplex stability, as shown by substantial decreases in both the association rate and the thermal stability of the complex. In particular, all natural bases (A, C, T and U) fall in this category. Non-G quartets in genomic DNA are therefore clearly not favorable to the energetics of the quadruplexes: they are tolerated at best. This is independent of the nature of the monocation: with a few exceptions, an unfavorable substitution in K+ remains unfavorable in Na+ and 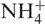 . Despite the presence of well-defined non-guanine base quartets in a number of NMR and X-ray structures, our data suggest that these quartets do not participate favorably in structural stability and are formed only by virtue of the docking platform provided by neighboring G-quartets.
. Despite the presence of well-defined non-guanine base quartets in a number of NMR and X-ray structures, our data suggest that these quartets do not participate favorably in structural stability and are formed only by virtue of the docking platform provided by neighboring G-quartets.
Our study also provides useful guidelines for the future conception of synthetic DNA assemblies based on quadruplex formation. Comparing the association constants found for a variety of substitutions led us to propose the following conclusions: (i) the central part of the quartet (the central ring of H-bonds and O6 carbonyl groups) is vital to its stability: altering this part not only leads to the loss of one H-bond, but may also hamper coordination of the central cation. (ii) Removal of the external ring of H-bonds leads to a moderate decrease in the association rate (ex: inosine). However, if one not only remove these H-bonds but perturbs the geometry/planarity of the quartet as a result of a steric clash, as for 7-deazaguanine, the penalty is more severe. (iii) One is left with a limited freedom to play with the 8-position and, in a few cases (8-bromo-guanine), substitutions may even become favorable. Modifications that do not affect the cyclic hydrogen bond pattern nor the central carbonyl groups are well tolerated and may effectively replace guanines, although syn/anti sugar configuration preferences play a role. (iv) Finally, the purine geometry is not an absolute requirement to form a stable quartet: isoxanthopterine is fully compatible with quadruplex formation, and other planar bicyclic groups may also form a quartet. In that case, we believe that the presence of a central carbonyl group is required (i.e. at a position equivalent to the O6 group of guanine) and should be H-bonded to a H-bond donor group (likely an amino group) from another base. (v) The conclusions reached here apply to base quartets in which, by virtue of the tetramolecular system, all four bases are substituted. It should be interesting to compare this system with intramolecular quadruplexes, in which a single base may be replaced in each quartet [for example: (49)].
The two ‘non-canonical’ modifications X and P even lead to faster quadruplex formations than the all-guanine reference sequences. The only substitution that leads to a stability improvement in both association and dissociation parameters (as compared to guanine) is 8-bromo-guanine (X), when inserted at the 5′ end (position 1). However, the case of P substitution is also highly interesting on the application point of view, because this modification in the 5′ side leads to an increase of both the association and dissociation rates. Reversible devices based on P-modified quadruplexes could therefore have a higher turnover than the classical G-quadruplexes. The thermodynamic and kinetic data compiled here is highly valuable for the design of DNA quadruplex assemblies with tunable association/dissociation properties. So far, guanines are still a quartet′s best friends!
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Rougée, M.E. Hawkins and both referees for helpful discussions and comments. We are indebted to Marilyn Monroe whose song inspired, among other things, the title of this article. This work was supported by ARC (# 3365 to J.L.M), E.U. FP6 ‘MolCancerMed’ (LSHC-CT-2004-502943), and FRFC (2.4623.05) grants. S.A. is the recipient of a ‘Fondation Jérôme Lejeune’ fellowship. V.G. is a Research Associate of the FNRS (Fonds National de la Recherche Scientifique, Belgium) and F.R. is currently a FNRS post-doctoral fellow. Funding to pay the Open Access publication charge was provided by INSERM.
Conflict of interest statement. None declared.
REFERENCES
- 1.Neidle S., Parkinson G.N. The structure of telomeric DNA. Curr. Opin. Struct. Biol. 2003;13:275–283. doi: 10.1016/s0959-440x(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 2.Burge S., Parkinson G.N., Hazel P., Todd A.K., Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gellert M., Lipsett M.N., Davies D.R. Helix formation by guanylic acid. Proc. Natl. Acad. Sci. USA. 1962;48:2013–2018. doi: 10.1073/pnas.48.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grand C.L., Powell T.J., Nagle R.B., Bearss D.J., Tye D., Gleason-Guzman M., Hurley L.H. Mutations in the G-quadruplex silencer element and their relationship to c-MYC overexpression, NM23 repression, and therapeutic rescue. Proc. Natl. Acad. Sci. USA. 2004;101:6140–6145. doi: 10.1073/pnas.0400460101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Schaffitzel C., Berger I., Postberg J., Hanes J., Lipps H.J., Plückthun A. In vitro generated antibodies specific for telomeric guanine quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl. Acad. Sci. USA. 2001;98:8572–8577. doi: 10.1073/pnas.141229498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duquette M.L., Handa P., Vincent J.A., Taylor A.F., Maizels N. Intracellular transcription of G-rich DNAs induces formation of G-loops, novel structures containing G4 DNA. Genes Dev. 2004;18:1618–1629. doi: 10.1101/gad.1200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paeschke K., Simonsson T., Postberg J., Rhodes D., Lipps H. Telomere end-binding proteins control the formation of G-quadruplex DNA structures in vivo. Nat. Struct. Mol. Biol. 2005;12:847–854. doi: 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- 8.Davis J.T. G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 9.Alberti P., Bourdoncle A., Saccà B., Lacroix L., Mergny J.L. DNA nanomachines and nanostructures involving quadruplexes. Org. Biomol. Chem. 2006;4:3383–3391. doi: 10.1039/b605739j. [DOI] [PubMed] [Google Scholar]
- 10.Maizels N. Dynamic roles for G4 DNA in the biology of eukaryotic cells. Nature Struct. Mol. Biol. 2006;13:1055–1059. doi: 10.1038/nsmb1171. [DOI] [PubMed] [Google Scholar]
- 11.Oganesian L., Bryan T.M. Physiological relevance of telomeric G-quadruplex formation: a potential drug target. Bioessays. 2007;29:155–165. doi: 10.1002/bies.20523. [DOI] [PubMed] [Google Scholar]
- 12.Spackova N., Berger I., Sponer J. Nanoseconds molecular dynamics simulations of parallel and antiparallel guanine quadruplex DNA molecules. J. Am. Chem. Soc. 1999;121:5519–5534. [Google Scholar]
- 13.Stefl R., Cheatham T.E., Spackova N., Fadrna E., Berger I., Koca J., Sponer J. Formation pathways of a guanine-quadruplex DNA revealed by molecular dynamics and thermodynamic analysis of the substates. Biophys. J. 2003;85:1787–1804. doi: 10.1016/S0006-3495(03)74608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyatt J.R., Davis P.W., Freier S.M. Kinetics of G-quartet-mediated tetramer formation. Biochemistry. 1996;35:8002–8008. doi: 10.1021/bi960124h. [DOI] [PubMed] [Google Scholar]
- 15.Mergny J.L., De Cian A., Ghelab A., Saccà B., Lacroix L. Kinetics of tetramolecular quadruplexes. Nucleic Acids Res. 2005;33:81–94. doi: 10.1093/nar/gki148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mergny J.L., De Cian A., Amrane S., Webba da Silva M. Kinetics of double-chain reversals bridging contiguous quartets in tetramolecular quadruplexes. Nucleic Acids Res. 2006;34:2386–2397. doi: 10.1093/nar/gkl098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petraccone L., Pagano B., Esposito V., Randazzo A., Piccialli G., Barone G., Mattia C.A., Giancola C. Thermodynamics and kinetics of PNA-DNA quadruplex-forming chimeras. J. Am. Chem. Soc. 2005;127:16215–16223. doi: 10.1021/ja0545923. [DOI] [PubMed] [Google Scholar]
- 18.Esposito V., Randazzo A., Piccialli G., Petraccone L., Giancola C., Mayol L. Effects of an 8-bromodeoxyguanosine incorporation on the parallel quadruplex structure [d(TGGGT)](4) Org. Biomol. Chem. 2004;2:313–318. doi: 10.1039/b314672c. [DOI] [PubMed] [Google Scholar]
- 19.Petraccone L., Erra E., Esposito V., Randazzo A., Galeone A., Barone G., Giancola C. Biophysical properties of quadruple helices of modified human telomeric DNA. Biopolymers. 2005;77:75–85. doi: 10.1002/bip.20189. [DOI] [PubMed] [Google Scholar]
- 20.Patel P.K., Hosur R.V. NMR observation of T-tetrads in a parallel stranded DNA quadruplex formed by Saccharomyces cerevisiae telomere repeats. Nucleic Acid Res. 1999;27:2457–2464. doi: 10.1093/nar/27.12.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel P.K., Bhavesh N.S., Hosur R.V. NMR observation of a novel C-tetrad in the structure of the SV40 repeat sequence GGGCGG. Biochem. Biophys. Res. Commun. 2000;270:967–971. doi: 10.1006/bbrc.2000.2479. [DOI] [PubMed] [Google Scholar]
- 22.Pan B.C., Xiong Y., Shi K., Deng J.P., Sundaralingam M. Crystal structure of an RNA purine-rich tetraplex containing adenine tetrads: implications for specific binding in RNA tetraplexes. Structure. 2003;11:815–823. doi: 10.1016/s0969-2126(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 23.Cheong C.J., Moore P.B. Solution structure of an unusually stable RNA tetraplex containing G-quartet and U-quartet structures. Biochemistry. 1992;31:8406–8414. doi: 10.1021/bi00151a003. [DOI] [PubMed] [Google Scholar]
- 24.Pan B.C., Xiong Y., Shi K., Sundaralingam M. Crystal structure of a bulged RNA tetraplex at 1.1 angstrom resolution: implications for a novel binding site in RNA tetraplex. Structure. 2003;11:1423–1430. doi: 10.1016/j.str.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Driscoll S.L., Hawkins M.E., Balis F.M., Pfleiderer W., Laws W.R. Fluorescence properties of a new guanosine analog incorporated into small oligonucleotides. Biophys. J. 1997;73:3277–3286. doi: 10.1016/S0006-3495(97)78352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins M.E., Pfleiderer W., Balis F.M., Porter D., Knutson J.R. Fluorescence properties of pteridine nucleoside analogs as monomers and incorporated into oligonucleotides. Anal. Biochem. 1997;244:86–95. doi: 10.1006/abio.1996.9879. [DOI] [PubMed] [Google Scholar]
- 27.Kejnovska I., Kypr J., Vorlickova M. Oligo(dT) is not a correct native PAGE marker for single-stranded DNA. Biochem. Biophys. Res. Commun. 2007;353:776–779. doi: 10.1016/j.bbrc.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 28.Rosu F., Gabelica V., Houssier C., Colson P., De Pauw E. Triplex and quadruplex DNA structures studied by electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2002;16:1729–1736. doi: 10.1002/rcm.778. [DOI] [PubMed] [Google Scholar]
- 29.Rosu F., Gabelica V., Shin-ya K., DePauw E. Telomestatin induced stabilization of the human telomeric DNA quadruplex monitored by electrospray mass spectrometry. Chem. Commun. 2003;34:2702–2703. doi: 10.1039/b309394h. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman D.V., Hardin C.C. Extraction of information on the buildup and consumption of reactive intermediates from quadruplex DNA assembly time courses. Biochim. Biophysica. Acta. 2004;1679:59–64. doi: 10.1016/j.bbaexp.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Mergny J.L., Phan A.T., Lacroix L. Following G-quartet formation by UV-spectroscopy. FEBS Lett. 1998;435:74–78. doi: 10.1016/s0014-5793(98)01043-6. [DOI] [PubMed] [Google Scholar]
- 32.Mergny J.L., Li J., Lacroix L., Amrane S., Chaires J.B. Thermal difference spectra: a specific signature for nucleic acid structures. Nucleic Acids Res. 2005;33:e138. doi: 10.1093/nar/gni134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petraccone L., Erra E., Randazzo A., Giancola C. Energetic aspects of locked nucleic acids quadruplex association and dissociation. Biopolymers. 2006;83:584–594. doi: 10.1002/bip.20591. [DOI] [PubMed] [Google Scholar]
- 34.Wallimann P., Kennedy R.J., Miller J.S., Shalango W., Kenp D.S. Dual wavelength parametric test of two-state models for circular dichroism spectra of helical polypeptides: anomalous dichroic properties of alanine-rich peptides. J. Am. Chem. Soc. 2003;125:1203–1220. doi: 10.1021/ja0275360. [DOI] [PubMed] [Google Scholar]
- 35.Myers J.C., Moore S.A., Shamoo Y. Structure-based incorporation of 6-methyl-8-(2-deoxy-beta-ribofuranosyl)isoxanthopteridine into the human telomeric repeat DNA as a probe for UP1 binding and destabilization of G-tetrad structures. J. Biol. Chem. 2003;278:42300–42306. doi: 10.1074/jbc.M306147200. [DOI] [PubMed] [Google Scholar]
- 36.Saccà B., Lacroix L., Mergny J.L. The effect of chemical modifications on the thermal stability of different G-quadruplexes-forming oligonucleotides. Nucleic Acids Res. 2005;33:1182–1192. doi: 10.1093/nar/gki257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic – oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J. Mol. Biol. 1971;62:361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- 38.Rougée M., Faucon B., Mergny J.L., Barcelo F., Giovannangeli C., Garestier T., Hélène C. Kinetics and thermodynamics of triple-helix formation: effects of ionic strength and mismatches. Biochemistry. 1992;31:9269–9278. doi: 10.1021/bi00153a021. [DOI] [PubMed] [Google Scholar]
- 39.Alberti P., Arimondo P.B., Mergny J.L., Garestier T., Hélène C., Sun J.S. A directional nucleation-zipping mechanism for triple-helix formation. Nucleic Acids Res. 2002;30:5407–5415. doi: 10.1093/nar/gkf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dias E., Battiste J.L., Williamson J.R. Chemical probe for glycosidic conformation in telomeric DNAs. J. Am. Chem. Soc. 1994;116:4479–4480. [Google Scholar]
- 41.Wang Y., Patel D.J. Solution structure of the Tetrahymena telomeric repeat d(T(2)G(4))(4) G-tetraplex. Structure. 1994;2:1141–1156. doi: 10.1016/s0969-2126(94)00117-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang N., Phan A.T., Patel D.J. (3 + 1) Assembly of three human telomeric repeats into an asymmetric dimeric G-quadruplex. J. Am. Chem. Soc. 2005;127:17277–17285. doi: 10.1021/ja0543090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai J.X., Dexheimer T.S., Chen D., Carver M., Ambrus A., Jones R.A., Yang D.Z. An intramolecular G-quadruplex structure with mixed parallel/antiparallel G-strands formed in the human BCL-2 promoter region in solution. J. Am. Chem. Soc. 2006;128:1096–1098. doi: 10.1021/ja055636a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambrus A., Chen D., Dai J., Bialis T., Jones R.A., Yang D. Human telomeric sequence forms a hybrid-type intramolecular G-quadruplex structure with mixed parallel/antiparallel strands in potassium solution. Nucleic Acids Res. 2006;34:2723–2735. doi: 10.1093/nar/gkl348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luu K.N., Phan A.T., Kuryavyi V., Lacroix L., Patel D.J. Structure of the human telomere in K+ Solution: an intramolecular (3 + 1) G-quadruplex scaffold. J. Am. Chem. Soc. 2006;128:9963–9970. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan A.T., Luu K.N., Patel D.J. Different loop arrangements of intramolecular human telomeric (3 + 1) G-quadruplexes in K+ solution. Nucleic Acids Res. 2006;34:5715–5719. doi: 10.1093/nar/gkl726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mergny J.L., Gros J., De Cian A., Bourdoncle A., Rosu F., Saccà B., Guittat L., Amrane S., Mills M., et al. In: Quadruplex Nucleic Acids, Cambridge, UK, RSC, Neidle S., Balasubramanian S., editors. 2006. pp. 31–80. [Google Scholar]
- 48.Stefl R., Spackova N., Berger I., Koca J., Sponer J. Molecular dynamics of DNA quadruplex molecules containing inosine, 6-thioguanine and 6-thiopurine. Biophys. J. 2001;80:455–468. doi: 10.1016/S0006-3495(01)76028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez de la Osa J., Gonzalez C., Gargallo R., Rueda M., Cubero E., Orozco M., Avino A., Eritja R. Destabilization of quadruplex DNA by 8-aminoguanine. Chembiochem. 2006;7:46–48. doi: 10.1002/cbic.200500281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.