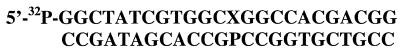Abstract
The oxidation of thymine in DNA can generate a base pair between 5-hydroxymethyluracil (HmU) and adenine, whereas the oxidation and deamination of 5-methylcytosine (5mC) in DNA can generate a base pair between HmU and guanine. Using synthetic oligonucleotides containing HmU at a defined site, HmU-DNA glycosylase activities in HeLa cell and human fibroblast cell extracts have been observed. An HmU-DNA glycosylase activity that removes HmU mispaired with guanine has been measured. Surprisingly, the HmU:G excision activity is 60 times greater than the corresponding HmU:A activity, even though the expected rate of formation of the HmU:A base pair exceeds that of the HmU:G base pair by a factor of 107. The HmU:G mispair would arise from the 5mC:G base pair, and, if unrepaired, would give rise to a transition mutation. The observation of an unexpectedly high HmU:G glycosylase activity suggests that human cells may encounter the HmU:G mispair much more frequently than expected. The conversion of 5mC to HmU must be considered as a potential pathway for the generation of 5mC to T transition mutations, which are often found in human tumors.
Oxidative DNA damage has been implicated in cancer and aging (1–3). The oxidation of the thymine methyl group of a T:A base pair can generate 5-hydroxymethyluracil (HmU; refs. 4 and 5). The biological implications of this DNA damage are currently unclear; however, the laboratories of Ames (6) and Teebor (7) have identified a specific glycosylase in higher eukaryotes that removes HmU from an HmU:A base pair in DNA. The need for DNA repair activities is generally discussed within the context of removing lesions that miscode or block the progression of a DNA or RNA polymerase. However, HmU is not miscoding (8), it does not perturb DNA structure (9, 10), and it does not impede polymerases (11, 12). Indeed, in some bacteriophage, HmU completely replaces T (13).
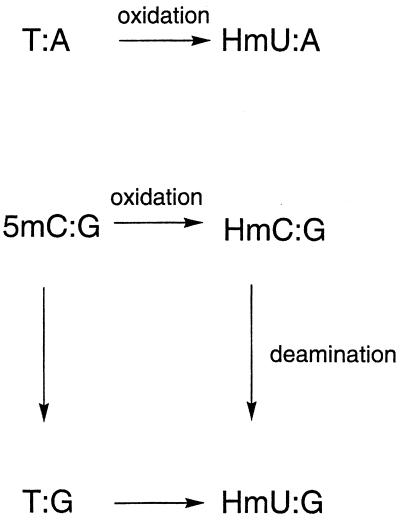
In the absence of an obvious need for the removal of HmU, the existence of the HmU-DNA glycosylase activity led to the proposal that the role of this activity was to remove HmU that could arise in DNA by a second pathway—the oxidation and deamination of 5-methylcytosine (5mC; refs. 7, 14, and 15). In support of this hypothesis, it was demonstrated that the HmU-DNA glycosylase is found only in higher eukaryotes that have 5mC in the genome (16). The methylated CpG dinucleotide is a reactive center for several carcinogens (17–19), and transition mutations at this site are frequently observed in human tumors (20–22). Recently, the apparent involvement of altered cytosine methylation patterns in the development of human cancer has renewed interest in understanding chemical mechanisms that perturb cytosine methylation patterns (23–27).
In the studies that initially identified HmU-DNA glycosylase activity, the substrate was the DNA of HmU-containing bacteriophage (6, 7). In these bacteriophage, the HmU replaces T and therefore pairs with A. Because HmU pairs with only A during polymerase-directed DNA synthesis, the preparation of substrates containing HmU paired with G required chemical synthesis. A viable synthetic scheme, which is now available (28), was used here to prepare substrates containing both HmU:A and HmU:G base pairs. Although the HmU:G rather than the HmU:A base pair had been proposed as the target of HmU-DNA glycosylase (7, 16), it had not been possible to demonstrate a glycosylase activity against HmU in an HmU:G base pair until synthetic substrates became available.
In this paper, HmU-DNA glycosylase activities in human cell extracts have been measured against both HmU:A and HmU:G base pairs. An unexpectedly high activity against the HmU:G base pair has been observed. Compared with the expected rate of formation of the HmU:G base pair in eukaryotic DNA from established endogenous pathways, the magnitude of the observed activity is even more surprising. The abundant HmU:G glycosylase activity in human cell extracts suggests that as yet unknown mechanisms, potentially important in the development of human cancer, can modify the 5mC:G base pair.
Materials and Methods
Oligonucleotide Synthesis.
Oligonucleotides (24-mer; Fig. 1) were prepared by automated phosphoramidite synthesis, purified by RP-HPLC, and 5′-32P-end labeled by T4 polynucleotide kinase (New England Biolabs) with [γ-32P]ATP (Amersham Pharmacia). The 5-hydroxymethyl-2′-deoxyuridine was prepared by the method of Shiau et al. (29). The HmU phosphoramidite was synthesized by using a method developed by this laboratory (28). The 5-hydroxymethyl-2′-deoxycytidine and the 5-hydroxymethylcytosine (HmC) phosphoramidite were prepared by methods also developed by this laboratory (30). T:G, U:G, or HmU:G mispairs and T:A, U:A, HmU:A, C:G, 5mC:G, and HmC:G base pairs were prepared by annealing a 5-fold molar excess of unlabeled complementary strand to the appropriate labeled strand in 5 mM Hepes-KOH, pH 7.4, 1 mM NaCl, 0.1 mM EDTA, and 0.1 mM DTT. The oligonucleotides were heated together to 100°C and cooled slowly to room temperature.
Figure 1.
Sequence of the oligonucleotides used in this assay, where X = U, T, HmU, C, 5mC, or HmC and where P = A or G.
Assays with Cell Extracts and Cloned Enzymes.
Nuclear extracts of HeLa cells were obtained from Promega, stored as aliquots at −70°C, and used before a maximum of two freeze–thaw cycles. Normal human fibroblasts, HF-57, were obtained from Timothy O'Connor (Department of Biology, City of Hope National Medical Center, Duarte, CA). Whole fibroblast cell extracts were prepared from confluent cells according to a slightly modified procedure developed by Dignam et al. (31). Pelleted cells were suspended in 5 vol of 25 mM Tris-HCl (pH 7.5)/10 mM KCl/1.5 mM MgCl2/0.2 mM EDTA/0.1 mM EGTA/0.3 M sucrose/0.5 mM PMSF/2 mM β-mercaptoethanol/0.5% Nonidet P-40 and allowed to stand for 25 min on ice. The cells were then collected by centrifugation at 4°C for 10 min at 1,306 × g with an Eppendorf 5415C and suspended in 2.5 packed cell pellet vol (vol before the initial wash) with 25 mM Tris-HCl (pH 7.5)/1.5 mM MgCl2/0.2 mM EDTA/0.1 mM EGTA/5% glycerol/0.42 M NaCl/0.5 mM PMSF/2 mM β-mercaptoethanol. The cells were then homogenized vigorously for 1 min, left on ice for 10 min, and centrifuged at 4°C for 10 min at 11,268 × g. The supernatants from these steps were eluted through a NAP-10 column (Pharmacia Biotech) to exchange the extract into a buffer containing 20 mM Hepes (pH 7.5), 2 mM EDTA, 10 mM EGTA, 2 mM DTT, 0.5 mM PMSF, and 10 μg/ml leupeptin. Cloned uracil-DNA glycosylase (UDG) and reaction buffer were obtained from Amersham Pharmacia, and cloned thymine-DNA glycosylase (TDG) and reaction buffer were obtained from Trevigen (Gaithersburg, MD).
Base excision with human cell extracts was measured by using a slightly modified method developed by Wiebauer and Jiricny (32) and further modified by Jones and coworkers (33). End-labeled (5′-32P) duplexes or single-stranded oligonucleotides (5 pmol) were incubated with HeLa cell nuclear extract (5.5 μg protein/μl) or human fibroblast cell extract (6.0 μg protein/μl) in buffer containing 50 mM Pipes (pH 6.7), 1 mM DTT, 0.5 mM EDTA, and 10 μM ZnCl2 at 30°C in a total volume of 25 μl. In the cloned enzyme assays, 5 pmol end-labeled duplexes or single-stranded oligonucleotides were incubated with UDG (0.49 μg protein/μl) in reaction buffer or TDG (8.82 μg protein/μl) in reaction buffer at 37°C in a total volume of 10 μl. Protein concentrations were measured by using the Bio-Rad protein assay.
The samples were removed at the indicated times. The reaction was stopped by adding 0.1 M NaOH or an equal volume of a formamide solution, which contained electrophoretic marker dyes, and heating at 90°C for 30 min. Labeled full-length oligonucleotide substrates and cleaved product oligonucleotides were subsequently separated by PAGE with 6 M urea and 18% polyacrylamide. A Molecular Dynamics PhosphorImager performed the visualization and quantitation.
Results
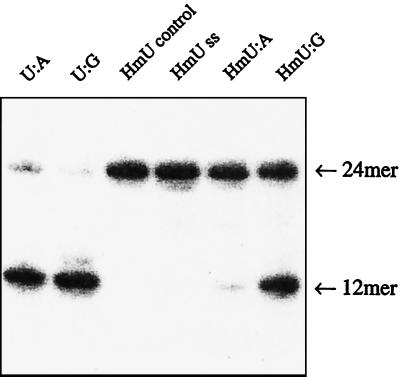
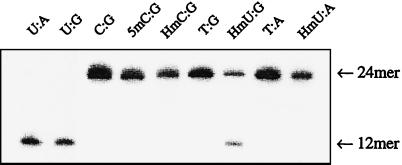
Fig. 1 shows the sequence of the oligonucleotides used in this study. The rates of excision of HmU from both HmU:G and HmU:A base pairs were measured by using normal human fibroblast cell extract and HeLa cell nuclear extract. Duplexes of the same sequence, but containing either an HmU:G or HmU:A base pair at the 13th position, were 5′-32P-end labeled on the strand containing HmU and incubated with the HeLa cell nuclear extract. The removal of HmU generated an abasic site that was subsequently cleaved by apurinic endonucleases present in the extract. The extent of HmU removal was assayed by electrophoresis on a denaturing gel that separated the cleaved oligonucleotides from the uncleaved oligonucleotides, followed by PhosphorImager analysis (Fig. 2). To compare the relative rates of base excision with different substrates by the HeLa cell nuclear extract, this assay was also performed with single-stranded HmU-containing oligonucleotides, and with U:G and U:A base pairs in the same sequence context (Fig. 2). Treatment of the U:G and U:A duplexes with the extract led to almost complete excision of U after incubation for 90 min. Treatment of the HmU:G and HmU:A duplexes under the same conditions resulted in ≈50% cleavage of the HmU:G duplex and substantially less cleavage of the HmU:A duplex. Single-stranded oligonucleotides with HmU were not effectively cleaved. Experiments were conducted to determine the initial rates for the removal of U and HmU from these single- and double-stranded oligonucleotides with the HeLa cell nuclear extract. Table 1 presents the measured activities.
Figure 2.
Polyacrylamide gel of oligonucleotides following incubation with HeLa cell nuclear extract. The sequence of the duplex is indicated in Fig. 1. The identity of the base pair at the cleavage site is indicated in the figure. Lanes marked HmU-control and HmU ss refer to the HmU-containing single strand not treated with extract, and the HmU-containing single strand treated with extract.
Table 1.
Excision activity in HeLa cell nuclear extract (μmol oligo/μg protein/min)
| Substrate | Activity |
|---|---|
| U ss | 2.3 × 10−8 |
| U:A | 6.3 × 10−8 |
| U:G | 8.0 × 10−8 |
| HmU ss | 3.2 × 10−11 |
| HmU:A | 4.7 × 10−11 |
| HmU:G | 2.7 × 10−9 |
Excision activities on oligonucleotide substrates containing U or HmU [single-stranded (ss), paired with A, or mispaired with G] were calculated from measurements of the initial rates.
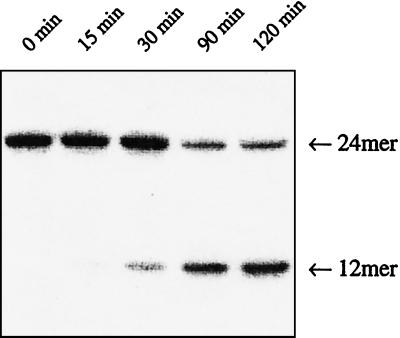
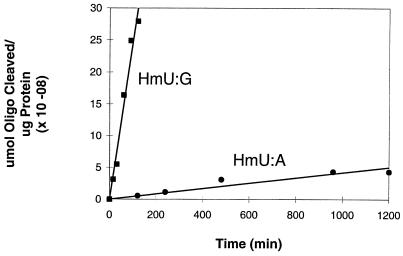
The degree of HmU excision from HmU:A and HmU:G base pairs by the HeLa cell nuclear extract was measured as a function of time (Figs. 3 and 4). Removal of HmU from both base pairs was also linear with increasing amounts of nuclear extract (data not shown). Surprisingly, measured activities were ≈4.7 × 10−11 μmol oligo/μg protein/min for the removal of HmU from HmU:A base pairs, but 2.7 × 10−9 μmol oligo/μg protein/min for the removal of HmU from HmU:G base pairs (Table 1, and Fig. 4).
Figure 3.
Cleavage of duplex containing HmU:G base pair at target site treated with HeLa cell nuclear extract for the periods of time indicated in the figure.
Figure 4.
Plot of the amount of duplex containing an HmU:G or HmU:A base pair cleaved per microgram of protein as a function of time.
Parallel studies were also conducted with duplexes containing a T:G base pair. However, the mispaired thymine glycosylase activity present in the extracts was observed only at trace levels in these assays and could not be quantified accurately. Other groups have similarly noted problems with observing thymine glycosylase activity in unfractionated cell extracts and in extracts containing high concentrations of salt (34, 35). In contrast to UDG and HmU-DNA glycosylase, purified TDG acts stoichiometrically rather than catalytically on T:G mispairs, potentially confounding the quantitation of activity (36).
To determine whether the UDG or TDG activities present in the cell extracts were contributing to the removal of HmU, oligonucleotide cleavage assays were conducted with cloned UDG (Escherichia coli) and TDG (Methanobacterium thermoautotrophicum). Table 2 summarizes excision data from the experiments using these cloned enzymes. UDG acted only on the uracil-containing substrates, whereas TDG acted on the U:G, T:G, and HmU:G base pairs. TDG was more active against T:G mispairs than HmU:G mispairs.
Table 2.
Excision activity of cloned UDG and TDG (μmol oligo/μg protein/min)
| Substrate | UDG | TDG |
|---|---|---|
| U ss | 4.3 × 10−6 | <1 × 10−12 |
| U:A | 7.8 × 10−6 | <1 × 10−12 |
| U:G | 1.0 × 10−5 | 2.3 × 10−6 |
| HmU ss | <1 × 10−12 | <1 × 10−12 |
| HmU:A | <1 × 10−12 | <1 × 10−12 |
| HmU:G | <1 × 10−12 | 9.0 × 10−8 |
| T:G | <1 × 10−12 | 1.5 × 10−7 |
Excision activities of cloned UDG and TDG on oligonucleotide substrates containing U, HmU, or T [single-stranded (ss), paired with A, or mispaired with G].
Furthermore, excision efficiencies of C:G, 5mC:G, HmC:G, T:G, HmU:G, T:A, and HmU:A base pairs in human fibroblast cell extract were measured to compare the relative rates of base excision of the different base pairs that might be involved in the generation of HmU:G and HmU:A base pairs (Scheme S1 and Fig. 5). U:A and U:G base pairs were included in the panel to verify that the enzymes in the extract were active. The human fibroblast cell extract did not induce observable cleavage of the labeled duplexes containing a C, 5mC, HmC, or T at the target site, incubated for 90 min. As with the HeLa cell nuclear extract, extracts from normal human fibroblasts had substantially more HmU:G than HmU:A activity.
Scheme 1.
Pathways by which HmU:G and HmU:A base pairs may be formed in DNA.
Figure 5.
Polyacrylamide gel of oligonucleotides following incubation with human fibroblast whole cell extract. The sequence of the duplex is indicated in Fig. 1. The identity of the base pair at the cleavage site is indicated in the figure.
Discussion
This paper describes an assay for the quantitative measurement of HmU-DNA glycosylase activity in human cell extracts. The oligonucleotide substrates, containing the HmU lesion at a defined site, were prepared by chemical synthesis using a method described previously (28). The composition of the oligonucleotides was verified by HPLC analysis of the liberated deoxynucleosides following enzymatic digestion and by GC/MS analysis following acid hydrolysis (37, 38). By using these synthetic substrates, the excision activity against HmU when mispaired with G has been measured, allowing direct comparison of HmU:A and HmU:G glycosylase activities.
Using this assay, an excision activity against HmU:G was demonstrated to be substantially greater than the observed, corresponding excision activity against HmU:A. In previous studies, the use of HmU-containing phage DNA limited the examination of HmU-DNA glycosylase activity to the HmU:A base pair (6, 7). If the results of the current study demonstrated similar activity against HmU:A and HmU:G, one could assume that the previously described HmU-DNA glycosylase activity was responsible, and that the target was the HmU base rather than the base pair, as is true for uracil-DNA glycosylase. However, because the HmU:G excision capacity is markedly greater than the corresponding HmU:A excision capacity, either a separate and previously undescribed activity exists in human cells to repair the HmU:G lesion, or the endogenous substrate of the previously described HmU-DNA glycosylase activity is the HmU:G mispair rather than the HmU:A base pair.
Previous structural studies suggest that the preference for the excision of the HmU:G lesion cannot be attributed to significant perturbation of the oligonucleotide duplex. The structures of duplexes containing both HmU:A and HmU:G base pairs have been studied by NMR spectroscopy (9). The replacement of a thymine residue by HmU in a T:A base pair does not alter overall DNA conformation or Watson–Crick base pairing, consistent with the replacement of T by HmU in some bacteriophage. When HmU is mispaired with G, both bases are observed to be intrahelical in a wobble geometry, and the 5-hydroxymethyl group of HmU forms an intrabase hydrogen bond with the O4-carbonyl group. The HmU:G base pair does not disturb overall DNA conformation, and the HmU:G mispair is essentially identical to T:G and U:G mispairs (39). Therefore, it is unlikely that the HmU:G excision activity is simply recognizing a substantial structural perturbation in DNA induced by the HmU:G mispair.
DNA repair activities in human cells are often redundant and overlapping. The possibility that the actual target of the apparent HmU-DNA glycosylase activity was some other lesion was considered. Human cells have high levels of UDG, and therefore the capacity of a cloned UDG to cleave the HmU-containing substrates was examined. Under conditions where the corresponding uracil-containing oligonucleotides were completely cleaved, no activity for this enzyme was observed against either the HmU:A or HmU:G base pairs (Table 2).
The deamination of 5mC to T generates a T:G mispair, structurally similar to the HmU:G mispair. The T:G mispair is repaired by a specific glycosylase activity (TDG). The activity of the cloned TDG was therefore examined with HmU-containing substrates. TDG does cleave HmU when mispaired with G, but not when paired with A. Nevertheless, the activity is in the order: U>>T>HmU. Prior studies, based on the relative reactivity of TDG, have argued that the actual substrate for TDG is not mispaired T, but mispaired U (36, 40). Because the activity of TDG against HmU is even less than that against T, TDG would not appear to explain the excision activity against HmU:G observed in the human cell extracts.
The magnitude of the observed HmU:G excision activity is much higher than expected, especially when taking into account the anticipated rate of formation of the HmU:G lesion generated in vivo. Spontaneous or endogenous reactions such as oxidation and deamination are relatively slow reactions under physiological conditions. Nevertheless, because of the size of the human genome, a substantial number of endogenous DNA damage events occur daily in every human cell (1–3). It has been estimated that the rate of formation of HmU from T is ≈620 per cell per day (2), corresponding to a rate constant of 2.1 × 10−7/day. The oxidation of T in DNA would generate an HmU:A base pair.
The HmU:G base pair would be derived from the 5mC:G base pair. Scheme S1 shows that the reaction pathway could involve either deamination and then oxidation of 5mC or oxidation followed by deamination (7, 14, 15). The overall rates for each pathway can be estimated based on data reported in the literature. Jones and coworkers (41) have shown that 5mC in a 5mC:G base pair deaminates two to four times faster than C in a C:G base pair. The rate constant under physiological conditions is ≈5.0 × 10−8/day. The oxidation of T to HmU in a T:G base pair would be roughly similar to that of T to HmU in a T:A base pair, with a corresponding rate constant of 2.1 × 10−7/day as discussed above. Therefore, the overall rate constant for the deamination and subsequent oxidation of 5mC to HmU would be 1.1 × 10−14/day.
The rate of the second pathway, oxidation and then deamination, can be similarly estimated. In solution, 5mC is slightly more reactive than T toward free radical oxidation (42). The rate constant for the oxidation of 5mC in DNA would then be slightly higher than that of T, with a corresponding rate constant of approximately 2.3 × 10−7/day. Drake and Baltz (43) have measured the rate of deamination of HmC in DNA to be approximately 4.0 × 10−8/day, slightly lower than that of 5mC, but similar to the rate of C deamination in DNA. Thus, the overall rate constant for the oxidation and subsequent deamination of 5mC to HmU would be approximately 9.2 × 10−15/day.
Based on these two potential reaction pathways, the rate at which 5mC is converted to HmU, generating an HmU:G mispair, can be estimated to be approximately 2.0 × 10−14/day. In the human genome, there are approximately 7 × 107 5mC:G base pairs (44). Therefore, roughly one 5mC:G base pair per cell would be converted to an HmU:G base pair per 2,000 years. This number represents an upper limit, as this would be the expected frequency of formation of the HmU:G base pair if the intermediate T:G or HmC:G base pairs were not repaired. Because repair activities for both intermediate base pairs have been reported (14, 36), the rate of conversion of 5mC:G to HmU:G base pairs is expected to be vanishingly small under physiological conditions.
Enzymatic pathways by which cytosine methylation patterns in eukaryotes may be altered have been proposed. Activities that remove (45, 46), deaminate (47), and directly demethylate (48, 49) 5mC residues in DNA have been discussed. In each case, subsequent studies either have not supported these mechanisms or the activities have proven difficult to characterize (50–53). Data reported here obtained with both HeLa cells and normal human fibroblasts (Figs. 2 and 5) clearly demonstrate an HmU:G glycosylase activity. In fact, of the possible base pairs that might arise from the 5mC:G base pair by established reaction pathways (Scheme S1 and Fig. 5), the HmU:G base pair is acted on most readily. The astonishingly low expected frequency of formation of the HmU:G base pair, coupled with the unexpectedly abundant excision activity, indicates that additional, and perhaps enzyme-mediated mechanisms may exist for modification of 5mC:G base pairs.
The transition mutation from 5mC:G to T:A is the most frequent substitution mutation found in human cancer (21). In the p53 gene, nearly half of the observed point mutations are C:G to T:A changes at methylated CpG dinucleotides (20, 22). The mechanism for this conversion is generally believed to be hydrolytic deamination of 5mC to T with the generation of the poorly repaired T:G mispair. The coding properties of T and HmU are the same during polymerase-directed DNA replication (8). Therefore, the modification of 5mC to T or HmU would result in the same mutation following DNA replication. The observation of a high HmU:G glycosylase activity in human cells suggests that the HmU:G mispair is encountered more frequently than anticipated. Therefore, the possibility that transition mutations at methylated CpG dinucleotides might proceed via the HmU:G mispair must be considered. The HmU:G glycosylase activity described here may thus be important in preventing transforming mutations in human cells.
Acknowledgments
This work was supported in part by National Institutes of Health Grants GM 50351 and CA 84487.
Abbreviations
- HmU
5-hydroxymethyluracil
- 5mC
5-methylcytosine
- HmC
5-hydroxymethylcytosine
- UDG
uracil-DNA glycosylase
- TDG
thymine-DNA glycosylase
References
- 1.Loeb L A. Cancer Res. 1989;49:5489–5496. [PubMed] [Google Scholar]
- 2.Mullaart E, Lohman P H M, Berends F, Vijg J. Mutat Res. 1990;237:189–210. doi: 10.1016/0921-8734(90)90001-8. [DOI] [PubMed] [Google Scholar]
- 3.Ames B N, Gold L S, Willett W C. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teebor G W, Frenkel K, Goldstein M S. Proc Natl Acad Sci USA. 1984;75:318–321. doi: 10.1073/pnas.81.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenkel K, Cummings A, Solomon J, Cadet J, Steinberg J, Teebor G W. Biochemistry. 1985;24:4527–4533. doi: 10.1021/bi00338a007. [DOI] [PubMed] [Google Scholar]
- 6.Hollstein M C, Brooks P, Linn S, Ames B N. Proc Natl Acad Sci USA. 1984;81:4003–4007. doi: 10.1073/pnas.81.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon-Carlson S V, Gokhale H, Teebor G W. J Biol Chem. 1989;264:13306–13312. [PubMed] [Google Scholar]
- 8.Levy D D, Teebor G W. Nucleic Acids Res. 1991;19:3337–3343. doi: 10.1093/nar/19.12.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellac S, Fazakerley G V, Sowers L C. Biochemistry. 1993;32:7779–7786. doi: 10.1021/bi00081a025. [DOI] [PubMed] [Google Scholar]
- 10.Pasternack L B, Bramham J, Mayol L, Galeone A, Jia X, Kearns D R. Nucleic Acids Res. 1996;24:2740–2745. doi: 10.1093/nar/24.14.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrala A M, Vilpo J A. Biochemistry. 1989;28:8274–8277. doi: 10.1021/bi00447a003. [DOI] [PubMed] [Google Scholar]
- 12.Vilpo J A, Vilpo L M. Mutat Res. 1995;316:123–131. doi: 10.1016/0921-8734(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Kallen R G, Simon M, Marmur J. J Mol Biol. 1962;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- 14.Cannon S V, Cummings A, Teebor G W. Biochem Biophys Res Commun. 1988;151:1173–1179. doi: 10.1016/s0006-291x(88)80489-3. [DOI] [PubMed] [Google Scholar]
- 15.Boorstein R J, Teebor G W. Cancer Res. 1988;48:5466–5470. [PubMed] [Google Scholar]
- 16.Boorstein R J, Chiu L-N, Teebor G W. Nucleic Acids Res. 1989;17:7653–7661. doi: 10.1093/nar/17.19.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wojciechowski M F, Meehan T. J Biol Chem. 1984;259:9711–9716. [PubMed] [Google Scholar]
- 18.Jones P A, Buckley J D. Adv Cancer Res. 1990;54:1–23. doi: 10.1016/s0065-230x(08)60806-4. [DOI] [PubMed] [Google Scholar]
- 19.Weitzman S A, Turk P W, Milkowski D H, Kozlowski K. Proc Natl Acad Sci USA. 1994;91:1261–1264. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones P A, Rideout W M, III, Shen J-C, Spruck C H, Tsai Y C. BioEssays. 1992;14:33–36. doi: 10.1002/bies.950140107. [DOI] [PubMed] [Google Scholar]
- 21.Spruck C H, III, Rideout W M, III, Jones P A. In: DNA Methylation: Molecular Biology and Biological Significance. Jost J P, Saluz H P, editors. Basel: Birkhauser; 1993. [Google Scholar]
- 22.Laird P W, Jaenisch R. Hum Mol Genet. 1994;3:1487–1495. doi: 10.1093/hmg/3.suppl_1.1487. [DOI] [PubMed] [Google Scholar]
- 23.Smith S S, Hardy T A, Baker D J. Nucleic Acids Res. 1987;15:6899–6916. doi: 10.1093/nar/15.17.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hepburn P A, Margison G P, Tisdale M J. J Biol Chem. 1991;266:7985–7987. [PubMed] [Google Scholar]
- 25.Smith S S, Kaplan B E, Sowers L C, Newman E M. Proc Natl Acad Sci USA. 1992;89:4744–4748. doi: 10.1073/pnas.89.10.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turk P W, Laayoun A, Smith S S, Weitzman S A. Carcinogenesis. 1995;16:1253–1255. doi: 10.1093/carcin/16.5.1253. [DOI] [PubMed] [Google Scholar]
- 27.Graff J R, Herman J G, Myohanen S, Baylin S B, Vertino P M. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- 28.Sowers L C, Beardsley G P. J Org Chem. 1993;58:1664–1665. doi: 10.1021/jo00059a011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiau G T, Schinazi R F, Chen M S, Prusoff W H. J Med Chem. 1980;23:127–133. doi: 10.1021/jm00176a005. [DOI] [PubMed] [Google Scholar]
- 30.Tardy-Planechaud S, Fujimoto J, Lin S S, Sowers L C. Nucleic Acids Res. 1997;25:553–558. doi: 10.1093/nar/25.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dignam J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiebauer K, Jiricny J. Nature (London) 1989;339:234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- 33.Schmutte C, Yang A S, Beart R W, Jones P A. Cancer Res. 1995;55:3742–3746. [PubMed] [Google Scholar]
- 34.Neddermann P, Jiricny J. J Biol Chem. 1993;268:21218–21224. [PubMed] [Google Scholar]
- 35.Griffin S, Karran P. Biochemistry. 1993;32:13032–13039. doi: 10.1021/bi00211a012. [DOI] [PubMed] [Google Scholar]
- 36.Waters T R, Swann P F. J Biol Chem. 1998;273:20007–20014. doi: 10.1074/jbc.273.32.20007. [DOI] [PubMed] [Google Scholar]
- 37.Kasai H, Crain P F, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H. Carcinogenesis. 1986;7:1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- 38.LaFrancois C J, Yu K, Sowers L C. Chem Res Toxicol. 1998;11:786–793. doi: 10.1021/tx970233c. [DOI] [PubMed] [Google Scholar]
- 39.Carbonnaux C, Fazakerley G V, Sowers L C. Nucleic Acids Res. 1990;18:4075–4081. doi: 10.1093/nar/18.14.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neddermann P, Jiricny J. Proc Natl Acad Sci USA. 1994;91:1642–1646. doi: 10.1073/pnas.91.5.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen J-C, Rideout W M, III, Jones P A. Nucleic Acids Res. 1994;22:972–976. doi: 10.1093/nar/22.6.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda T, Shinohara H, Kondo M. J Radiat Res. 1975;16:153–161. doi: 10.1269/jrr.16.153. [DOI] [PubMed] [Google Scholar]
- 43.Baltz R H, Bingham P M, Drake J W. Proc Natl Acad Sci USA. 1976;73:1269–1273. doi: 10.1073/pnas.73.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown T C, Jiricny J. Cell. 1987;50:945–950. doi: 10.1016/0092-8674(87)90521-6. [DOI] [PubMed] [Google Scholar]
- 45.Vairapandi M, Duker N J. Nucleic Acids Res. 1993;21:5323–5327. doi: 10.1093/nar/21.23.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vairapandi M, Duker N J. Oncogene. 1996;13:933–938. [PubMed] [Google Scholar]
- 47.Zingg J M, Shen J C, Yang A S, Rapoport H, Jones P A. Nucleic Acids Res. 1996;24:3267–3275. doi: 10.1093/nar/24.16.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya S K, Ramchandani S, Cervoni N, Szyf M. Nature (London) 1999;397:579–583. doi: 10.1038/17533. [DOI] [PubMed] [Google Scholar]
- 49.Cedar H, Verdine G L. Nature (London) 1999;397:568–569. doi: 10.1038/17492. [DOI] [PubMed] [Google Scholar]
- 50.Steinberg R A. Nucleic Acids Res. 1995;23:1621–1624. doi: 10.1093/nar/23.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmutte C, Jones P A. Biol Chem. 1998;379:377–388. doi: 10.1515/bchm.1998.379.4-5.377. [DOI] [PubMed] [Google Scholar]
- 52.Wolffe A P, Jones P L, Wade P A. Proc Natl Acad Sci USA. 1999;96:5894–5896. doi: 10.1073/pnas.96.11.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu B, Zheng Y, Hess D, Angliker H, Schwarz S, Siegmann M, Thiry S, Jost J P. Proc Natl Acad Sci USA. 2000;97:5135–5139. doi: 10.1073/pnas.100107597. . (First Published April 25, 2000; 10.1073/pnas.100107597) [DOI] [PMC free article] [PubMed] [Google Scholar]