Abstract
Yersiniabactin (Ybt) synthetase is a three-subunit, 17-domain [7 domains in high molecular weight protein (HMWP)2, 9 in HMWP1, and 1 in YbtE] enzyme producing the virulence-conferring siderophore yersiniabactin in Yersinia pestis. The 350-kDa HMWP1 subunit contains a polyketide synthase module (KS-AT-MT2-KR-ACP) and a nonribosomal peptide synthetase module (Cy3-MT3-PCP3-TE). The full-length HMWP1 was heterologously overexpressed in Escherichia coli and purified to near homogeneity. The purified HMWP1 showed thioesterase activity toward acyl-CoAs, such as acetyl-CoA, benzoyl-CoA, and malonyl-CoA, with saturation kinetics and relative catalytic efficiencies of 172:50:1. A chain-releasing thioesterase (TE) activity is ascribed to the C-terminal TE domain, and this was substantiated by the fact that acyl-N-acetylcysteamines were hydrolyzed by the didomain PCP3-TE fragment of HMWP1. However, PCP3-TE failed to hydrolyze any of the acyl-CoAs, suggesting the TE domain does not recognize CoA moiety, thus the acyl-CoA hydrolysis by HMWP1 must involve other domains. Ser-to-Ala mutants in each of the AT, ACP, PCP3, and TE domains reduced hydrolysis rates of the two fastest substrates, acetyl-CoA and benzoyl-CoA, by more than two orders of magnitude. Thus, the acyl-CoA hydrolysis activity requires 4 of the 9 domains of HMWP1, and it is consistent with autoacylation of the AT domain active site serine and subsequent passage of the itinerant acyl chain from AT to ACP to PCP3 to the TE domain, a cascade of four sequential acyl-enzyme intermediates, for hydrolytic turnover. This could represent an editing pathway for this polyketide synthase/nonribosomal peptide synthetase assembly line.
Yersiniabactin (Ybt) (Fig. 1), an iron-chelating siderophore, is biosynthesized and secreted by Yersinia pestis, a causative agent of bubonic plague (1–4). Ybt chelates ferric iron with a dissociation constant of 10−35 M (5), acting as a virulence factor in iron-deficient environments (1–4). The biosynthetic genes for Ybt include ybtE, encoding the one-domain 67-kDa YbtE, and irp1 and irp2, encoding two high molecular weight proteins, HMWP1 and HMWP2, respectively (Fig. 1A) (6). HMWP2 and HMWP1 form an assembly line of 16 predicted domains to convert salicylate, three cysteines, malonyl-CoA, and three adenosylmethionines to the mixed nonribosomal peptide/polyketide Ybt (Fig. 1A). YbtE activates salicylate and ligates it to the holo aryl carrier protein (ArCP) domain of HMWP2 to initiate Ybt biosynthesis (7). The seven-domain HMWP2, a dimodular nonribosomal peptide synthetase (NRPS), activates and incorporates two of the three cysteines to form the tandem five-membered heterocycles of Ybt (7–9). HMWP1 is predicted to be a hybrid of a five-domain polyketide synthase (PKS) (ketoacyl synthase, acyltransferase, methyltransferase 2, ketoacyl reductase, acyl carrier protein: KS-AT-MT2-KR-ACP) and a four-domain NRPS (cyclization domain 3, methyltransferase 3, peptidyl carrier protein 3, thioesterase: Cy3-MT3-PCP3-TE) (Fig. 1A) (6). The PKS module of HMWP1 probably synthesizes the t-butyl linker, whereas the NRPS module assembles the third five-membered heterocycle of Ybt (6). We have previously described the heterologous expression, purification, and biochemical characterization of HMWP2 to establish the biosynthetic mechanism of the tandem heterocycles of Ybt (7–9). In this work, we coexpressed the 350-kDa HMWP1 with Bacillus subtilis Sfp in Escherichia coli and isolated it as holoenzyme. We found that the full-length HMWP1, but not fragments thereof, selectively hydrolyzes acyl-CoAs in a catalytic process requiring four domains—AT, ACP, PCP3, and TE—that are thought to act in concert to generate a cascade of acyl-enzyme intermediates. This may be evidence of an editing route for the PKS/NRPS assembly line of Ybt.
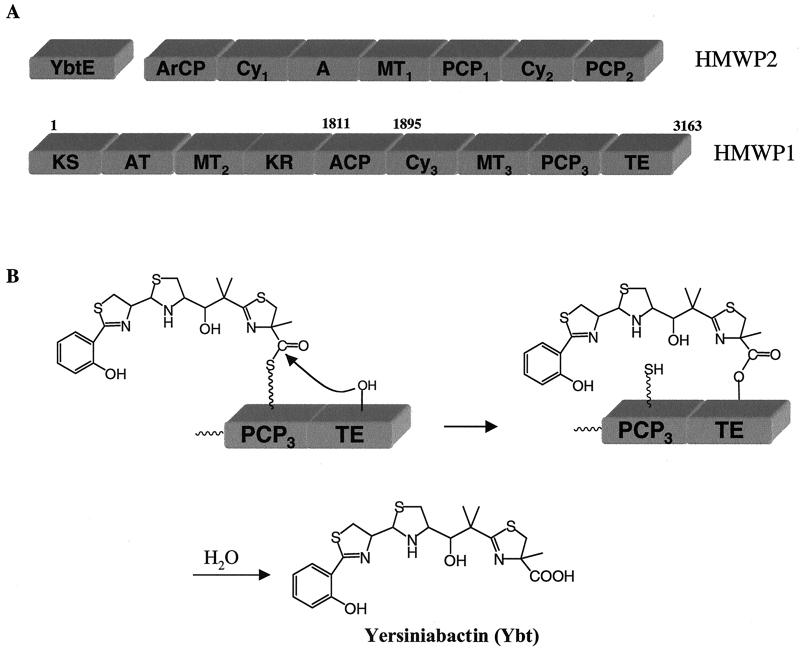
Figure 1.
(A) Domain organization of the three subunits of yersiniabactin synthetase, including the seven-domain high molecular weight protein (HMWP)2 and nine-domain HMWP1 subunits. ArCP, aryl carrier protein; Cy, cyclization domain; A, adenylation domain; MT, methyltransferase; PCP, peptidyl carrier protein; KS, ketoacyl synthase; AT, acyltransferase; KR, ketoacyl reductase; ACP, acyl carrier protein; TE, thioesterase. (B) Postulated mechanism for Ybt translocation and release by C-terminal TE domain in HMWP1.
Materials and Methods
Materials.
All of the chemicals were purchased from either Sigma or Aldrich except tris(2-carboxyethyl)phosphine hydrochloride (TCEP), which was from Molecular Probes. DNA primers were purchased from Integrated DNA Technologies (Coralville, IA). The vector DNA and E. coli BL21(DE3) were from Novagen. HMWP1 fragments 1–1895, 1896–3163, 1812–3163, and PCP3-TE were expressed and purified as will be described elsewhere (Z.S., C. Tseng, and C.T.W., unpublished work). Acyl thioesters of N-acetylcysteamine (SNACs) were prepared according to the procedure noted below.
Synthesis and Characterization of Acyl-SNACs.
Benzoyl-SNAC, salicyl-SNAC, 2-methyl-4-thiazolyl-SNAC (MT-SNAC), 4,5-dihydro-2-(2-hydroxyphenyl)-4-thiazolinyl-SNAC (HPT-SNAC), and 2′-(2-hydrophenyl)thiazolyl-2,4-thiazolinyl-SNAC (HPTT-SNAC) were prepared chemically. 1H NMR spectra were recorded on a 500-MHz Varian Unity NMR spectrometer. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was carried out with a Perspective Biosystems Voyager 4036 STR mass spectrometer. The procedure for preparing acyl-SNACs is described as follows: To the tetrahydrofuran (THF) solution of carboxylic acid (1 eq) and 1-hydroxybenzotriazole (1.2 eq) was added dicyclohexyl carbodiimide (1.2 eq) in THF, followed by N-acetylcysteamine (1.0 eq, except for salicylic acid, for which 5 eq was used). After stirring for 1 h at 24°C, potassium carbonate (1.0 eq.) was added and the reaction was stirred for 2 additional hours. The reaction mixture was then filtered and concentrated by rotary evaporation. The solid residue was dissolved in EtOAc and washed with 10% NaHCO3 and H2O. The organic layer was dried (MgSO4), concentrated, and purified by flash chromatography (3–8% MeOH in CHCl3) to give SNAC thioesters with 45–80% yield.
HPT-SNAC: 1H NMR (CDCl3) δ 7.45 (m, 2H), 7.08 (d, 2H), 6.92 (m, 1H), 5.99 (br s, 1H), 5.45 (dd, 1H), 3.67 (m, 2H), 3.46 (q, 2H), 3.08 (t, 2H), 1.99 (s, 3H); MALDI-TOF MS [M + H], 325.2 (325.1 calculated). HPTT-SNAC: 1H NMR (CDCl3) δ 8.07 (s, 1H), 7.63 (d, 1H), 7.37 (t, 1H), 7.09 (d, 1H), 6.95 (t, 1H), 5.85 (br s, 1H), 5.42 (dd, 1H), 3.75 (d, 2H), 3.46 (m, 2H), 3.08 (m, 2H), 1.97 (s, 3H); MALDI-TOF MS [M + H], 408.3 (408.1 calculated). Benzoyl-SNAC: 1H NMR (CDCl3) δ 7.96 (d, 2H), 7.59 (t, 1H), 7.46 (t, 2H), 6.07 (br s, 1H), 3.53 (dd, 2H), 3.23 (t, 2H), 1.97 (s, 3H); MALDI-TOF MS [M + H], 224.2 (224.1 calculated). Salicyl-SNAC: 1H NMR (CDCl3) δ 7.83 (d, 1H), 7.42 (dd, 1H), 6.98 (m, 2H), 6.15 (br s, 1H), 3.50 (dd, 2H), 3.21 (t, 2H), 1.96 (s, 3H); MALDI-TOF MS [M + H], 240.3 (240.1 calculated).
Cloning of Wild-Type and Mutants of HMWP1.
Cloning of wild-type HMWP1.
Plasmid pET28b-HMWP1 was constructed from ligation of the NcoI/BsiWI fragment (containing fragment 1–5606 of the irp1 gene) from pQE60-PKS and the vector fragment (containing fragment 5607–9486 of irp1) from pET28b-1812–3163 (Z.S., C. Tseng, and C.T.W., unpublished work). Expression construct pET22b-HMWP1 was prepared by ligation of the XbaI/XhoI fragments of pET28b-HMWP1 into ET22b vector to give a C-terminal His6-tagged fusion protein when overexpressed in E. coli.
Site-directed mutagenesis.
All mutants were prepared by using the QuickChange site-directed mutagenesis kit (Stratagene), following the supplier's instructions. The mutation template was pET22b-HMWP1 and the mutation primers are listed below, with the mutating sites highlighted in boldface: TE-mut-forward (5′-GTG CTG GCG GGT TGG GCG TAT GGC GCG TTT CTT G-3′); TE-mut-reverse (5′-CAA GAA ACG CGC CAT ACG CCCAACCCGCCAGCAC-3′); AT-mut-forward (5′-GAC TTC GCC ATT GGG CAT GCC GTC GGT GAA TTT GCC GC-3′); AT-mut-reverse (5′-GCG GCA AAT TCA CCG ACG GCA TGC CCA ATG GCG AAG TC-3′; ACP-mut-forward (5′-GTT GCA ACT CGG CAT GGA CGC GCT GCT CTT CCT TGA ACT C-3′); ACP-mut-reverse (5′-GAG TTC AAG GAA GAG CAG CGC GTC CAT GCC GAG TTG CAA C-3′); PCP3-mut-forward (5′-CGA ACT GGG CGG CGA CGC CCT GAT GGC GAC AAG GAT GG-3′); and PCP3-mut-reverse (5′-CCA TCC TTG TCG CCA TCA GGG CGT CGC CGC CCA GTT CG-3′). The sequence of each mutant was confirmed by DNA sequencing.
Overexpression and Purification of Wild-Type HMWP1 and Various Mutants.
The expression vectors containing wild-type or mutant irp1 genes were transformed into BL21(DE3) competent cells that harbored pREP4-Sfp, which encodes a phosphopantetheinyltransferase from Bacillus subtilis (10). The cell culture was grown in LB medium supplemented with 100 μg/ml ampicillin and 40 μg/ml kanamycin. The cells were grown at 37°C to an OD600 of 0.6, and then were induced with 75 μl of isopropyl β-d-thiogalactopyranoside (IPTG) for 12 additional hours at 16–18°C.
Cells from six 1.5-liter cultures were harvested by centrifugation (10 min at 6,000 × g) and resuspended in 70 ml of buffer A (20 mM Tris-Cl/0.2 M NaCl/1.5 mM imidazole, pH 8.0). Resuspended cells were broken by French pressure cell [two passes at 15,000 psi (103 MPa)], and the cell debris was removed by centrifugation (30 min at 35,000 × g). The supernatant was incubated with 4 ml of Superflow nickel resin (Qiagen, Chatsworth, CA) overnight at 4°C with slow rotation. The resin was then packed into a column, washed with 20 bed volumes of wash buffer (buffer A with 20 mM imidazole), and the protein was eluted by a linear gradient of 20–300 mM imidazole. Fractions containing the protein (judged by SDS-PAGE) were pooled, dialyzed against buffer B [50 mM Tris-Cl, pH 8.0/10 mM MgCl2/0.1 mM EDTA/10% (vol/vol) glycerol], concentrated, flash frozen in liquid nitrogen, and stored at −80°C. Concentrations of the purified HMWP1 proteins were measured spectrophotometrically at 280 nm by using the calculated extinction coefficient of 0.49 μM−1⋅cm−1.
Time Courses of Hydrolysis of Acyl-SNACs and Acyl-CoAs by HMWP1 and PCP3-TE at 22°C.
A reaction mixture (500 μl) of 0.25 M KH2PO4 (pH 7.0), 0.25 μM wild-type or 1 μM mutant of HMWP1 or 1 μM PCP3-TE, 0.5 mM acyl-CoA or 0.3 mM acyl-SNAC, and 5 mM TCEP was incubated at 22°C. For the incubation of acyl-SNACs, 2.5% (vol/vol) dimethyl sulfoxide (DMSO) was included to solubilize the substrates. Samples (50 μl) were withdrawn at various times, quenched with 100 μl of 1.7% phosphoric acid (final pH ≈ 1.5), and flash frozen by liquid nitrogen. Each thawed sample was then injected into HPLC equipped with a C18 reverse-phase column (Vydac, 4.6 × 250 mm) with the detector monitoring at 254 nm. A linear gradient between buffer C (25 mM KH2PO4, pH 5.4; water plus 0.1% trifluoroacetic acid for methylmalonyl-CoA) and buffer D (100% acetonitrile, with addition of 0.1% trifluoroacetic acid for methylmalonyl-CoA) from 1% to 50% D over 14 min with a flow rate of 1 ml/min. A linear gradient from 4% to 80% D over 23 min was used to analyze the reactions of acyl-SNACs. The product and substrate peaks were identified by coinjection with authentic standards. The quantity of substrate consumption or product formation was deduced from the corresponding peak area by comparing with an calibrated standard HPLC curve of the pure compound.
Measurement of kcat and Km Values of Acyl-SNAC and Acyl-CoA Hydrolysis by HMWP1 and PCP3-TE at 22°C.
Reaction mixtures (100 μl) containing 0.23 M KH2PO4 (pH 7.0), 0.25 μM HMWP1 or PCP3-TE, acyl-SNAC or acyl-CoA at various concentrations, and 5 mM TCEP were incubated at 22°C for constant time (e.g., 17, 20, and 1.5 min for HPT-SNAC, benzoyl-CoA, and acetyl-CoA, respectively) before quenching by 20 μl of 8.5% phosphoric acid (final pH ≈ 1.5). The control reactions were performed in parallel. The reaction mixtures were analyzed by HPLC as described above.
Determination of kobs of Benzoyl-CoA and Acetyl-CoA Hydrolysis by Various Enzymes and the Complementation Experiments at 22°C.
Reaction mixtures (100 μl) contained 0.23 M KH2PO4 (pH 7.0), 0.5 μM wild-type HMWP1 or 1.0 μM mutants or fragments of HMWP1, 0.5 mM benzoyl- or 1 mM acetyl-CoA, and 5 mM TCEP. After 0- to 15-min incubation at 22οC, the reactions were terminated and subjected to HPLC analysis described above. For complementation experiments with mutant enzymes, three combinations were carried out in which the two mutants were included in the incubation at 1.0 μM (Table 3). The other conditions and HPLC analysis were exactly the same as the normal incubations.
Table 3.
Hydrolytic rates of 1 mM acetyl-CoA and 0.5 mM benzoyl-CoA by various enzymes
| Enzyme |
kobs,
min−1
|
|
|---|---|---|
| Acetyl-CoA | Benzoyl-CoA | |
| PCP3-TE | 0.29 | 0 |
| HMWP1 (1–1895) | ND | 0.19 |
| HMWP1 (1812–3163) | ND | 0.2 |
| HMWP1 (1896–3163) | ND | 0 |
| HMWP1 (wild-type) | 98.95 | 6.94 |
| HMWP1 (TE mutant) | 0.33 | 0.04 |
| HMWP1 (AT mutant) | 2.53 | 0.14 |
| HMWP1 (ACP mutant) | 7.52 | 0.17 |
| HMWP1 (PCP3 mutant) | 4.37 | 0.15 |
| AT mutant/TE mutant | 2.47 | ND |
| AT mutant/ACP mutant | 8.45 | ND |
| AT mutant/PCP3 mutant | 5.7 | ND |
Enzyme concentrations were 0.5 μM for acetyl-CoA and 1 μM for benzoyl CoA. ND, not determined.
Data Analysis.
For the HPLC time courses, data were fitted to a linear equation: [product] = kobsE0t + C, where E0 represents the enzyme concentration, kobs the observed steady-state rate, and C the product concentration at 0 min. For the measurement of kcat and Km of acyl-CoA or acyl-SNAC, the data were fitted to a Michaelis–Menten equation: [product] = [E0][S]kcat/(Km + [S]), where [E0] represents the total enzyme concentration, kcat the maximum steady-state turnover number, and [S] the substrate concentration.
Results
Overproduction and Purification of Wild-Type and Mutants of HMWP1 in Holo Form.
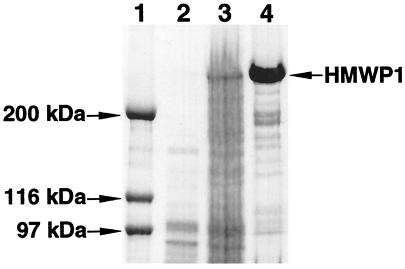
Various conditions were examined to optimize the yield of the soluble protein. It was found that low growth temperature (16–18°C), low inducer IPTG concentration (75 μM), and long induction (12 h) were essential to give a reasonable yield of soluble proteins (about 2 mg/liter). Overnight incubation of the clarified supernatant with nickel resin was required for sufficient binding of HMWP1 protein that carries a His6 tag on its C terminus. HMWP1 and mutant proteins were purified to near homogeneity as judged by SDS/PAGE (Fig. 2). Nearly all proteins purified were in holo form (data not shown) and their identity was confirmed by N-terminal peptide sequencing.
Figure 2.
SDS/5% PAGE of purified wild-type HMWP1 heterologously expressed in E. coli. Lane 1, molecular mass markers; lane 2, before IPTG induction; lane 3, after IPTG induction; lane 4, purified HMWP1.
Hydrolysis of Acyl-SNACs by HMWP1 and PCP3-TE.
The C-terminal domain of many NRPS assembly lines, including HMWP1 (Fig. 1B), is a 30- to 35-kDa TE domain, thought to be responsible for release of the full-length peptidyl chain from its covalent tethering on the most downstream PCP domain. As an initial assay of catalytic activity of the purified HMWP1, we evaluated the ability of HMWP1 to hydrolyze various acyl thioesters as small molecule analogs of the late-stage acyl thioester covalent enzyme intermediate, yersiniabactinyl-S-PCP3 (Fig. 1B). The TE domain of HMWP1 was assayed in the context of the full-length HMWP1 or excised as PCP3-TE fragment. As shown in Table 1, the two-ring HPT-SNAC was a good substrate for the TE domain in either context (47 min−1 and 17 min−1), with a 3-fold higher catalytic efficiency (kcat/Km) in the PCP3-TE fragment. The three-ring HPTT-SNAC, which mimics the first half of Ybt, was hydrolyzed with a two orders of magnitude lower kcat (Table 1) and benzoyl-, salicyl-, and 2-methylthiazolyl-4-carboxyl-SNACs were not hydrolyzed at all, indicating the selective recognition of acyl group by the TE domain.
Table 1.
Kinetic parameters of acyl-SNAC hydrolysis at 22°C
| Enzyme | Substrate | kcat, min−1 | Km, μM | kcat/Km, mM−1⋅min−1 | Relative activity |
|---|---|---|---|---|---|
| PCP3-TE | HPT-SNAC | 47 ± 16 | 1,700 ± 689 | 27.6 | 31 |
| HMWP1 | HPT-SNAC | 17 ± 7 | 1,730 ± 905 | 9.8 | 11 |
| HMWP1 | HPTT-SNAC | 0.24 ± 0.03 | 265 ± 59 | 0.9 | 1 |
Hydrolysis of Acyl-CoAs by Wild-Type HMWP1.
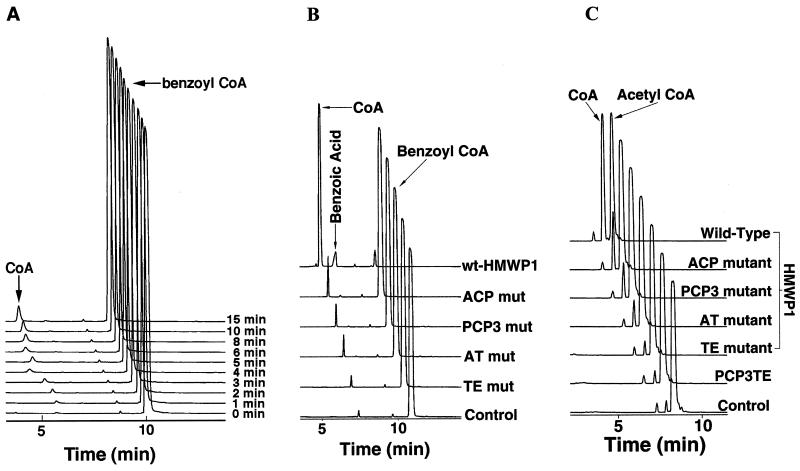
Hydrolysis of 0.5 mM benzoyl-CoA by 0.25 μM wild-type HMWP1 was evaluated over a 15-min time frame and gave a linear rate for CoA formation based on HPLC analysis (Fig. 3A). Hydrolysis was catalytic, as evidenced by typical saturation kinetics with a kcat of 8.1 min−1 and a Km of 85 μM (Table 2). The kinetic constants for the hydrolysis of several other acyl-CoAs were analyzed, and the comparison of catalytic efficiencies is presented in Table 2. Acetyl-CoA hydrolysis by HMWP1 was particularly robust, with a turnover number of 142 min−1, but was almost undetectable with the PCP3-TE fragment. Surprisingly, the PCP3-TE fragment was not able to sufficiently turn over any of the CoA substrates used by full-length HMWP1 (Table 3).
Figure 3.
(A) HPLC traces of a time course of 0.5 mM benzoyl-CoA hydrolysis by 0.25 μM wild-type HMWP1 at 22°C. The control reaction excluding HMWP1 was performed in parallel. (B) HPLC traces of the hydrolysis of 0.5 mM benzoyl-CoA by 1 μM wild-type HMWP1 and its various single-point Ser → Ala mutants for 120 min at 22°C. (C) HPLC traces of the hydrolysis of 1 mM acetyl-CoA by 0.5 μM wild-type and various single-point Ser → Ala mutants of HMWP1, and the PCP3-TE fragment for 20 min at 22°C. The HPLC traces were shifted progressively by 0.5 min for A and C and 0.4 min for B.
Table 2.
Substrate specificity of acyl-CoAs to wild-type HMWP1
| Substrate | kcat, min−1 | Km, μM | kcat/Km, mM−1⋅min−1 | Relative activity |
|---|---|---|---|---|
| Malonyl-CoA | 6.2 ± 0.1 | 3,290 ± 107 | 1.9 | 1 |
| Methylmalonyl-CoA | 4.3 ± 0.2 | 4,379 ± 436 | 0.98 | 0.5 |
| Acetyl-CoA | 142 ± 5 | 435 ± 47 | 326 | 171.6 |
| Acetoacetyl-CoA | 5 ± 1 | 2,442 ± 1,036 | 2.1 | 1.1 |
| Decanoyl-CoA | 2.1 ± 0.2 | 356 ± 123 | 5.9 | 3.1 |
| Benzoyl-CoA | 8.1 ± 0.1 | 85 ± 4 | 95.3 | 50.2 |
| Phenylacetyl-CoA | 4.6 ± 0.3 | 108 ± 28 | 42.6 | 22.4 |
Hydrolysis of Acyl-CoAs by Mutants and by Fragments of HMWP1.
Benzoyl-SNAC could not be processed by either the full-length HMWP1 or the PCP3-TE fragment (data not shown). However, benzoyl-CoA was rapidly hydrolyzed by the full-length HMWP1, but not the PCP3-TE fragment. These contrasting results indicated that the CoA moiety is clearly important for the benzoyl-CoA hydrolysis and the TE domain alone is not sufficient for this process. To evaluate this hypothesis, four single-point mutants were generated in which the active site Ser residue of the TE, AT, ACP, and PCP3 domains was changed to Ala. The active site Ser at the AT and TE domains is believed to be the linkage site for the covalent acyl enzyme intermediate, whereas the Ser at the ACP and PCP3 domains is posttranslationally modified with a prosthetic phosphopantetheinyl group, which serves as the thiol way station for the growing acyl chain during elongation. The HPLC traces in Fig. 3B demonstrate the dramatic reduction of benzoyl-CoA turnover by each of the mutants. The TE mutant was down 174-fold in rate, whereas AT, ACP, and PCP3 mutants were reduced by 40- 50-fold. Catalytic efficiencies were measured with 500-fold molar excess of substrate, and results are summarized in Table 3.
Similar results were obtained when acetyl-CoA was used as a substrate. In Fig. 3C, the HPLC traces for acetyl-CoA hydrolysis are displayed for wild-type and mutants of HMWP1, and the kobs values are summarized in Table 3. Mutation in any one of the four active site Ser residues significantly reduces the hydrolytic activity toward acetyl-CoA to near background activity for the TE mutant and PCP3-TE (≈0.3%). The other three domain mutants retained some residual hydrolytic activity (2.5–7.5%). Together, the kinetic data of the benzoyl-CoA and acetyl-CoA suggested that the AT, ACP, PCP3, and TE were all required for efficient catalytic hydrolysis of acyl-CoAs.
Hydrolysis of Malonyl-CoA by HMWP1.
It has been shown that the AT domain of HMWP1 uses [14C]malonyl-CoA for stoichiometric acylation of the ACP domain (Z.S., C. Tseng, and C.T.W., unpublished results). Malonyl-CoA was thus examined for hydrolysis catalyzed by HMWP1 under similar conditions. The relative catalytic efficiency for malonyl-CoA hydrolysis (kcat = 6.2 min−1 and Km = 3.3 mM) was 50- and 172-fold lower than that of benzoyl-CoA and acetyl-CoA, respectively (Table 2). Methylmalonyl-CoA, acetoacetyl-CoA, and decanoyl-CoA were likewise low catalytic efficiency substrates, whereas phenylacetyl-CoA was intermediate (Table 2).
Functional Complementation of the HMWP1 Fragments and Mutants.
Presently, the oligomerization state in a hybrid PKS/NRPS system is not known. While there was no evidence for oligomers of the purified HMWP1 subunit by gel filtration analysis (data not shown), we noted that the single HMWP1 mutants could be tested for functional complementation. As shown in Table 3, combinations of the AT/TE, AT/ACP, and AT/PCP3 mutants did not generate more than additive activity of both components. Attempts to reconstitute the benzoyl-CoA hydrolase activity by using the combination of the 1–1895 (PKS module) and 1896–3163 (NRPS module) fragments of HMWP1 failed to restore the hydrolytic activity (data not shown).
Discussion
PKS and NRPS are parallel enzymatic assembly lines that use the same principle of modular thiotemplates (11). It is not surprising that such assembly lines could intersect and domains/modules be mixed and matched to create a hybrid assembly line. Such hybrid assembly lines are responsible for the biosynthesis of therapeutically important molecules such as bleomycin (12), rapamycin (13), FK506 (14), and epothilone (15, 16). HMWP1 is a prototypical PKS/NRPS hybrid system with a five-domain PKS module fused to a four-domain NRPS module, and as such it may serve as a good example to deconvolute the molecular logic of a hybrid assembly line. The C-terminal TE domain of HMWP1 is believed to act as a chain-releasing catalyst to hydrolyze the mature Ybt from its acyl-S-enzyme intermediate at the most downstream thiol way station, the PCP3 domain (Fig. 1B). The results of Table 1 validate the thioesterase activity of this TE domain with a kcat of 47 min−1 for HPT-SNAC. The HMWP1 TE domain is selective for both the acyl and -SR sides of acyl thioester substrates. The fact that acyl-CoAs were hydrolyzed by HMWP1 rapidly with saturation kinetics but not by the two-domain PCP3-TE fragment prompted us to speculate that other domains of HMWP1 must work together with the C-terminal TE domain to carry out the observed hydrolysis.
Several purified fragments of HMWP1, including 1–1895 (the PKS module), 1896–3163 (the NRPS module), and 1812–3163, were not capable of hydrolyzing benzoyl-CoA, implicating both the PKS and NRPS modules, connected in cis, were required for hydrolysis of acyl-CoAs. Mutation of the active site S2980 residue in the TE domain destroyed the activity both for HPT-SNAC and benzoyl-CoA, confirming that a functional TE domain is required for benzoyl-CoA hydrolysis. Together, these results implied that the hydrolysis involves an acyl enzyme intermediate (HPT-O-TE or benzoyl-O-TE). So how did the benzoyl-O-TE intermediate form, given that the direct processing of benzoyl-CoA by TE domain was ruled out?
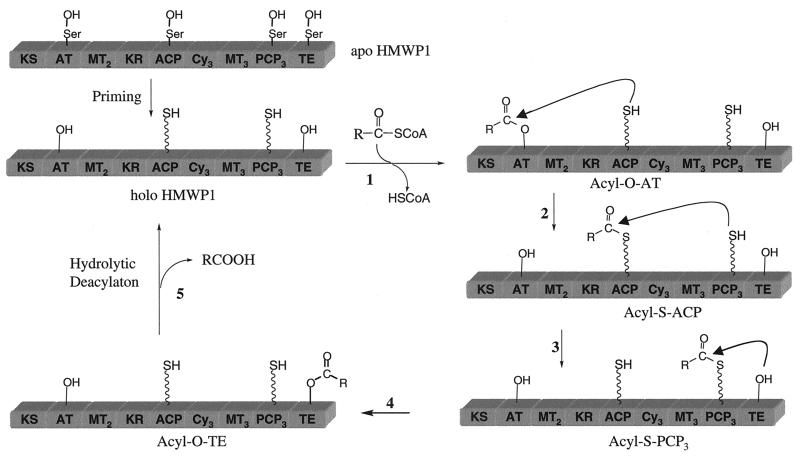
The CoA portion in benzoyl-CoA was important for the hydrolysis by HMWP1, since benzoyl-CoA was a good substrate whereas benzoyl-SNAC was not. Therefore, the hydrolytic process was hypothesized to be initiated by the AT domain in the PKS module. It has been demonstrated that the AT domain used [14C]malonyl-CoA and acylated the ACP domain fragment either in trans or in cis as part of full-length HMWP1 (data not shown). It is known that such malonyl-transferring AT domains in fatty acid synthases and polyketide synthases proceed via malonyl-O-seryl enzyme intermediates in the AT active sites (17). Mutation of the conserved Ser-641 to Ala at the AT domain of HMWP1 eliminated the malonyl transfer activity examined by autoradiography (data not shown) and reduced benzoyl-CoA and acetyl-CoA hydrolytic efficiency by 40- to 50-fold (Table 3). The function of the TE domain of this AT mutant was intact, as the mutant and wild-type enzymes showed similar activity toward HPT-SNAC (data not shown). Thus the great bulk of the hydrolytic flux requires a functional AT domain, and autoacylation of the AT domain is the likely route to recruit the acyl group onto the HMWP1 assembly line. The likely acceptor for the acyl group transfer by AT is the downstream ACP domain (Fig. 1 and Fig. 4) to yield the conventional acyl-S-ACP enzyme intermediate.
Figure 4.
Hydrolytic editing cycle involving four acyl enzyme intermediates progressively on HMWP1. Two acyl-O-Ser intermediates on the AT and TE domains (generated in steps 1 and 4 and two acyl-S-phosphopantetheinyl-carrier protein intermediates in the ACP and PCP3 domains (generated in steps 2 and 3) are shown. The catalytic hydrolysis by water occurs in the TE active site shown in step 5.
The explicit requirement for the holo-ACP form of HMWP1 for acyl-CoA hydrolysis was then evaluated with the ACP active site mutant (S1853A), incapable of receiving a translocating acyl group. Again, benzoyl- and acetyl-CoAs' hydrolytic efficiency was dramatically compromised, down to 2.5% and 7.6%, respectively (Table 3), suggesting acyl-S-ACP is an obligate acyl enzyme intermediate in hydrolysis. Now the question becomes whether the acyl-S-ACP could directly deliver the acyl group to the chain-releasing TE domain, or whether the acyl transfer via the PCP3 way station was necessary (Fig. 4). The HMWP1 PCP3 mutant (S2858A) was essentially inactive, retaining about 2% and 4.5% of activity for benzoyl-CoA and acetyl-CoA hydrolysis, respectively (Table 3). Therefore, the simple acyl-CoA hydrolysis by HMWP1 involves at least four of its domains, AT, ACP, PCP3, and TE, indicating a cascade of four acyl enzyme intermediates during catalysis as shown in Fig. 4.
The hydrolysis of the cognate substrate, e.g., malonyl-CoA, by wild-type HMWP1 proceeded with much lower catalytic efficiency than that of the noncognate substrate acetyl-CoA (Table 2). The slow hydrolysis of the cognate malonyl chain and the accelerated hydrolysis of noncognate acyl chains were consistent with a hydrolytic editing process. While the correctly loaded malonyl chain persists in place, waiting on the ACP domain to accept the upcoming chain transferring from the HMWP2 subunit, an incorrect acyl intermediate misloaded by the AT domain has to be removed promptly to reactivate HMWP1 during Ybt biosynthesis. This hydrolytic editing goes at 142 events per min for acetyl group and at 8 per min for benzoyl group dislodged from the PKS module and swept down the assembly line to be finally cleaved away by the TE domain. The five-step hydrolytic editing path in Fig. 4 is a variant of the normal chain elongation and termination process. It remains to be seen whether such a composite hydrolytic editing function is found in other PKS and NRPS systems.
The failure of HMWP1 fragments and their combinations to carry out acyl-CoA hydrolytic editing suggested that the orientation of the domains in the intact HMWP1 subunit is important for the directional covalent passage of acyl chains from AT to ACP to PCP3 to TE way stations. In addition to the Ser-to-Ala point mutants demonstrating each domain's essentiality for the acyl-CoA catalytic hydrolysis, the lack of any functional complementation by mixing two HMWP1 mutants argues against acyl chain transfer across HMWP1 subunits. This finding is in contrast to interchain transfer that has reported for fatty acid synthase dimers (11, 17, 18).
Acknowledgments
We thank Dr. Gary W. Ashley of Kosan Biosciences for providing 2-methylthiazolyl-4-carboxyl SNAC. This work was supported by National Institutes of Health Grant AI 42736 (C.T.W.). Z.S. is a Postdoctoral Fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
Abbreviations
- ACP
acyl carrier protein
- AT
acyltransferase
- DMSO
dimethyl sulfoxide
- HPT-COOH
4,5-dihydro-2-(2-hydroxyphenyl)-4-thiazolylcarboxylic acid
- HPTT-COOH
2′-(2-hydroxyphenyl)thiazolyl-2,4-thiazolinyl-4-caboxylic acid
- HMWP
high molecular weight protein
- MALDI-TOF
matrix-assisted laser desorption ionization time-of-flight
- IPTG
isopropyl β-d-thiogalactopyranoside
- SNAC
N-acetylcysteamine thioester
- PCP
peptidyl carrier protein
- TCEP
tris(2-carboxyethyl)phosphine hydrochloride
- TE
thioesterase
- Ybt
yersiniabactin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260495697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260495697
References
- 1.Fetherston J D, Lillard J W, Jr, Perry R D. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson S, Burrows T W. J Exp Pathol. 1956;37:577–583. [PMC free article] [PubMed] [Google Scholar]
- 3.Perry R D, Fetherston J D. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden S W, Fetherston J D, Perry R D. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry R D, Balbo P B, Jones H A, Fetherston J D, DeMoll E. Microbiology. 1999;145:1181–1190. doi: 10.1099/13500872-145-5-1181. [DOI] [PubMed] [Google Scholar]
- 6.Gehring A M, DeMoll E, Fetherston J D, Mori I, Mayhew G F, Blattner F R, Walsh C T, Perry R D. Chem Biol. 1998;5:573–586. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 7.Gehring A M, Mori I, Perry R D, Walsh C T. Biochemistry. 1998;37:17104–17115. doi: 10.1021/bi9850524. [DOI] [PubMed] [Google Scholar]
- 8.Suo Z, Walsh C T, Miller D A. Biochemistry. 1999;37:14023–14035. doi: 10.1021/bi991574n. [DOI] [PubMed] [Google Scholar]
- 9.Keating T A, Miller D A, Walsh C T. Biochemistry. 2000;39:4729–4739. doi: 10.1021/bi992923g. [DOI] [PubMed] [Google Scholar]
- 10.Lambalot R H, Gehring A M, Flugel R S, Zuber P, LaCelle M, Marahiel M A, Reid R, Khosla C, Walsh C T. Chem Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 11.Cane D, Walsh C T. Chem Biol. 1999;6:R319–R325. doi: 10.1016/s1074-5521(00)80001-0. [DOI] [PubMed] [Google Scholar]
- 12.Du L, Sanchez C, Chen M, Edwards D J, Shen B. Chem Biol. 2000;7:623–642. doi: 10.1016/s1074-5521(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 13.Kleinkauf H, von Dohren H. J Antibiot. 1995;48:563–567. doi: 10.7164/antibiotics.48.563. [DOI] [PubMed] [Google Scholar]
- 14.Motamedi H, Cai S J, Shafiee A, Elliston K O. Eur J Biochem. 1998;244:74–80. doi: 10.1111/j.1432-1033.1997.00074.x. [DOI] [PubMed] [Google Scholar]
- 15.Molnar I, Schupp T, Ono M, Zirkle R, Milnamow M, Nowak-Thompson B, Engel N, Toupet C, Stratmann A, Cyr D D, et al. Chem Biol. 2000;7:97–109. doi: 10.1016/s1074-5521(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B. Science. 2000;287:640–642. doi: 10.1126/science.287.5453.640. [DOI] [PubMed] [Google Scholar]
- 17.Smith S. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 18.Witkowski A, Joshi A, Smith S. Biochemistry. 1996;35:10569–10575. doi: 10.1021/bi960910m. [DOI] [PubMed] [Google Scholar]