Abstract
Many treatments for this chronic skin disease are harmful to the developing fetus, so careful pre-conception planning and management adjustment are crucial for the pregnant patient
Psoriasis is a common skin condition that causes considerable morbidity and occupational disability.w1 It has an estimated lifetime prevalence of 1.5-2.2% in the adult population,1w2 with three quarters of patients presenting before the age of 40.w3 Incidence is similar for the two sexes, although women generally develop the disease earlier than men. The prevalence in pregnant women is unknown but probably reflects that of non-pregnant women of child bearing age. The scenario box on this page shows that balancing treatment of severe psoriasis with the decision to try to conceive requires forward planning, and there is always a chance that the disease will worsen. Our patient was fortunate to become pregnant so quickly; less fortunate women can have a prolonged and difficult course and must decide how long they will try to conceive before resuming systemic treatment.
Scenario
A 30 year old woman had had chronic plaque psoriasis since childhood. She had previously needed a prolonged course of PUVA (psoralen and ultraviolet A) and two admissions to achieve control of her psoriasis. She then began intermittent treatment with methotrexate, followed by continuous treatment. When she had been taking methotrexate for five years she decided she wanted to start a family. She therefore stopped methotrexate and took the oral contraceptive pill for three months. Within four weeks of stopping methotrexate her psoriasis flared up but was controlled with an eight week course of narrowband ultraviolet B. The patient became pregnant with twins four months after stopping methotrexate but has needed further ultraviolet B to control her psoriasis throughout pregnancy.
Methods
We searched national health information sources, the British Association of Dermatologists guidelines, the Cochrane database, and PubMed up to March 2007. Key search words included “psoriasis”, “maternal”, “pregnancy”, and “breastfeeding”. We sought advice from the National Teratology Information Service; the drug information department, Royal Victoria Infirmary, Newcastle upon Tyne; and colleagues
Does pregnancy affect psoriasis?
Chronic plaque psoriasis is thought to improve in 40-60% of patients during pregnancy, with most improvement during the late first and second trimesters.2w4-w6 This improvement has been associated with high concentrations of progesterone, which downregulates the T cell proliferative response.w5 Psoriasis is associated with an altered T cell response biased towards the T helper 2 profile. Pregnancy usually tips the balance towards a T helper 1 cytokine profile,w7 which may explain why psoriasis often changes in pregnancy. Psoriasis deteriorates in 10-20% of women during pregnancy and may require intensified treatment.w4-w6
Does psoriasis affect the outcome of pregnancy?
Psoriasis does not affect fertility or rates of miscarriage, birth defects, or premature birth.w8 Many treatments for psoriasis are associated with potential problems during pregnancy.
Psoriasis is associated with depression, but no studies have investigated whether pregnancy exacerbates depression more in patients with psoriasis than in the normal population. Having psoriasis should not affect the timing or mode of delivery. Psoriasis can localise into scars (Koebner's phenomenon), but this phenomenon has not been reported in perineal scars, although theoretically it can occur at any site of epidermal injury. The risk of infection and delayed healing of caesarean section wounds is theoretically higher, but no studies have assessed this risk.w9
Psoriasis has a multifactorial mode of inheritance. An individual's risk of developing psoriasis is estimated at 28% if one parent is affected and 65% if both parents have the disease.3
How are patients with psoriasis managed in pregnancy?
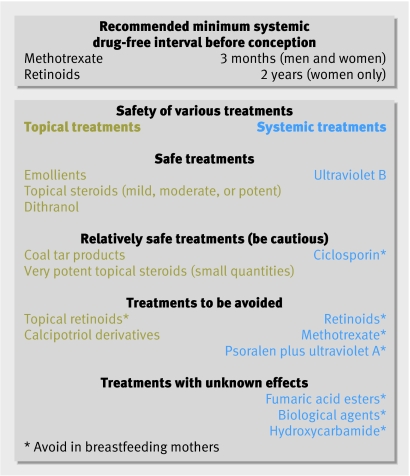
If possible, it makes sense to induce a period of remission or to optimise control of psoriasis before conception to help minimise flare-ups during pregnancy. Patients considering or taking systemic treatments should be warned in advance of the length of time that they will need to be off treatment before it can be considered safe for them to conceive (figure).
Treatment advice to be given before conception in patients with psoriasis
Topical treatments are first line treatments for psoriasis, and emollients, topical steroids, and dithranol are considered safe in pregnancy. Manufacturers of vitamin D analogues such as calcipotriol advise avoidance, although significant systemic absorption is unlikely to occur when they are used for localised disease.2 The safety of coal tar products is unclear as animal studies have suggested teratogenicity, although this has not been reported in humans. Such products are probably safe for use in the second and third trimesters.
If these agents fail to control disease, referral for specialist care should be considered. Ultraviolet B is the safest second line agent, followed by ciclosporin.2 The efficacy of ultraviolet B has not been assessed specifically in pregnant patients, but randomised controlled trials in the general population with psoriasis have shown it to be effective in around 65% of patients.4 Similar efficacy was found in a controlled trial with an average dosing regimen of ciclosporin in psoriasis.5 PUVA is mutagenic and is contraindicated,w10 w11 although no adverse fetal outcomes have been reported for women who become pregnant while being treated with PUVA. Some studies have suggested that localised topical PUVA may be relatively safe,6 but insufficient evidence exists, and in view of the mutagenic potential of PUVA it cannot be recommended.
All retinoids—both topical and oral—are contraindicated in pregnancy due to teratogenicity, especially in the first trimester.7 A period of at least two years between stopping oral retinoids and pregnancy is recommended to negate the teratogenic effects.w12 Women considering pregnancy or at risk of becoming pregnant should be aware of the risks before starting treatment.
Methotrexate is contraindicated in pregnancy as it is associated with spontaneous miscarriage, cleft palate, and skeletal abnormalities.2w13 w14 Most evidence for teratogenicity comes from the use of high dose methotrexate for chemotherapy or abortion. Although malformations can be seen after maternal exposure to low doses of methotrexate, there are many reports of healthy infants born after exposure in early pregnancy.8w15 w16 Methotrexate can also affect spermatogenesis, causing chromosomal aberrations. Both men and women should therefore avoid conception for at least three months after taking methotrexate, although the risk to the fetus is largely theoretical for men.w17
Hydroxycarbamide (hydroxyurea) is a neoplastic agent occasionally used as a second line systemic treatment for psoriasis. Although teratogenic in animals, no problems have been reported in the limited number of women exposed to this agent during pregnancy.9w18 w19 In view of the limited data available, manufacturers advise avoidance in pregnancy
Fumaric acid esters are widely used as systemic treatment for psoriasis in Europe (particularly Germany)w20 w21 but are not licensed in the United Kingdom; they are available on a named patient basis at some UK centres. There are no studies in pregnancy so they should be used cautiously on a case by case basis.
Manufacturers of etanercept and infliximab, which are licensed for use in severe psoriasis, advise avoidance during pregnancy. No toxicity or teratogenicity has been reported in animal studies of etanercept, and limited human data suggest that neither agent causes harm to the fetus.10 However, these drugs are often used in combination with methotrexate. Manufacturers of etanercept have set up a registry to follow patients who have been exposed to etanercept in pregnancy. More information about its safety profile will therefore be available in the future.
How is psoriasis managed postpartum?
More than half of patients have a flare-up within six weeks of delivery, although this is usually not worse than their prepregnancy state.w4-w6 Making women aware of this can allow therapeutic options to be planned in advance, leading to prompt treatment and minimising disease as far as possible.
Additional educational resources
Oumeish OY, Al-Fouzan AW. Miscellaneous diseases affected by pregnancy. Clin Dermatol 2006;24:113-7
Tauscher AE, Fleischer AB Jr, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg 2002;6:561-70
Zip C. A practical guide to dermatological drug use in pregnancy. Skin Thera Lett 2006;11:1-4
Janssen NM, Genta MS. The effects of immunosuppressive and anti-inflammatory medications on fertility, pregnancy, and lactation. Arch Intern Med 2000;160:610-9
National Teratology Information Service (www.nyrdtc.nhs.uk/Services/teratology/teratology.html)—UK based service providing information about drug use in pregnancy and lactation. The service is available on +44 (0)191 232 1525; 8.30 am to 5 pm Monday to Friday
First line treatment options for breastfeeding women are primarily limited to emollients, moderate to low potency topical steroids, and dithranol. Topical treatment should be applied after breastfeeding, and washed off thoroughly before the next feed.
Acetretin, methotrexate, ciclosporin, hydroxycarbamide, biological treatments, and PUVA are all contraindicated in breastfeeding women; the safest second-line agent is ultraviolet B. Breastfeeding may need to be curtailed if further treatment options are required.
Supplementary Material
Thanks to Peter Farr for critically reviewing the manuscript. The National Teratology Information Service and the Drug Information Department, Royal Victoria Infirmary, Newcastle upon Tyne provided advice.
Contributors: SW and NJR searched the literature and drafted the initial manuscript. All three authors helped write the article and approved the final version. NJR is guarantor.
Funding: SW receives a Wellcome clinical research training fellowship.
Competing interests: The department has received financial income and support from Stiefel Laboratories, Leo Pharmaceuticals, Schering Plough, and Merck Serono.
Provenance and peer review: Commissioned; externally peer reviewed.
This is one of a series of occasional articles about how to manage a pre-existing medical condition during pregnancy. If you would like to suggest a topic for this series please email Kirsten Patrick (kpatrick@bmj.com)
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol 2005;141:1537-41. [DOI] [PubMed] [Google Scholar]
- 2.Tauscher AE, Fleischer AB Jr, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg 2002;6:561-70. [DOI] [PubMed] [Google Scholar]
- 3.Swanbeck G, Inerot A, Martinsson T, Enerback C, Enlund F, Samuelsson L, et al. Genetic counselling in psoriasis: empirical data on psoriasis among first-degree relatives of 3095 psoriatic probands. Br J Dermatol 1997;137:939-42. [PubMed] [Google Scholar]
- 4.Gordon PM, Diffey BL, Matthews JN, Farr PM. A randomized comparison of narrow-band TL-01 phototherapy and PUVA photochemotherapy for psoriasis. J Am Acad Dermatol 1999;41:728-32. [DOI] [PubMed] [Google Scholar]
- 5.Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med 1991;324:277-84. [DOI] [PubMed] [Google Scholar]
- 6.Pham CT, Koo JY. Plasma levels of 8-methoxypsoralen after topical paint PUVA. J Am Acad Dermatol 1993;28:460-6. [DOI] [PubMed] [Google Scholar]
- 7.Rosa FW, Wilk AL, Kelsey FO. Teratogen update: vitamin A congeners. Teratology 1986;33:355-64. [DOI] [PubMed] [Google Scholar]
- 8.Lewden B, Vial T, Elefant E, Nelva A, Carlier P, Descrotes J, et al. Low dose methotrexate in the first trimester of pregnancy: results of a French collaborative study. J Rheumatol 2004;31:2360-5. [PubMed] [Google Scholar]
- 9.Pata O, Tok CE, Yazici G, Pata C, Oz AU, Aban M, et al. Polycythemia vera and pregnancy: a case report with the use of hydroxyurea in the first trimester. Am J Perinatol 2004;21:135-7. [DOI] [PubMed] [Google Scholar]
- 10.Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn's disease and rheumatoid arthritis. Am J Gastroenterol 2004;99:2385-92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.