Abstract
Down syndrome (DS) is the most common genetic disorder associated with mental retardation (MR). It is believed that many of the phenotypic features of DS stem from enhanced expression of a set of genes located within the triplicated region on chromosome 21. Among those genes is DYRK1A encoding dual - specificity proline-directed serine/treonine kinase, which, as documented by animal studies, can potentially contribute to cognitive deficits in DS. Whether this contribution can be exerted through elevated levels of DYRK1A protein in the brain of DS subjects was the main goal of the present study. The levels of DYRK1A protein were measured by Western blotting in six brain structures that included cerebral and cerebellar cortices and white matter. The study involved large cohorts of DS subjects and age-matched controls representing infants and adults of different age, gender and ethnicity. Trisomic Ts65Dn mice, an animal model of DS, were also included in the study. Both in trisomic mice and in DS subjects, the brain levels of DYRK1A protein were increased approximately 1.5-fold, indicating that this protein is overexpressed in gene dosage-dependent manner. The exception was an infant group, in which there was no enhancement suggesting the existence of a developmentally regulated mechanism. We found DYRK1A to be present in every analyzed structure irrespective of age. This widespread occurrence and constitutive expression of DYRK1A in adult brain suggest an important, but diverse from developmental, role played by this kinase in adult central nervous system. It also implies that overexpression of DYRK1A in DS may be potentially relevant to MR status of these individuals during their entire life span.
Keywords: Minibrain DYRK1A kinase, Down syndrome (DS), Chromosome 21 trisomy, Down Syndrome Critical Region (DSCR)
Down syndrome (DS) is the most frequent genetic cause of mental retardation. It is assumed that many of the phenotypic features of DS stem from enhanced expression of a set of genes harbored within the triplicated region on chromosome 21, the so called Down syndrome critical region (DSCR) [14]. Among genes cloned from this region was a human homologue of the Drosophila minibrain/rat DYRK gene, which encodes a dual-specificity tyrosine phosphorylation-regulated kinase; DYRK1A [11, 22, 23]. This gene is highly expressed in the brain [12, 13, 19] and seems to play a role during brain development by regulating neurogenesis and neuronal differentiation [13, 23, 27]. That it also plays an important role in adult central nervous system was deduced from its expression patterns in the brain [9, 16] and the diverse learning and memory deficits observed in DYRK1A transgenic [1, 2, 4] and knock-out mice [10]. All the above observations implicate DYRK1A as a likely candidate for causing cognitive impairment in DS subjects. However to establish its full relevance to DS, proof is needed of enhanced brain levels of DYRK1A protein resulting from triplication of the gene. This proof is still missing, because previous studies on human subjects either were done at the level of RNA, as in the work of Guimera et al. [12], or were limited to fetuses [6] and adult tissue of control and AD cases [9, 25]. Here, we examined the brain levels of DYRK1A protein in large cohorts of DS subjects and age-matched controls representing infants and adults of different age and ethnicity. Ts65Dn trisomic mice, a current animal model of DS [7], were also included in the study. The results demonstrate for the first time overexpression of DYRK1A protein in DS brains in a gene dosage-dependent manner, thus strengthening the claim of altered levels of this kinase being at the root of mental retardation of DS patients.
Brain tissue was obtained from two sources: the Brain and Tissue Bank for Developmental Disorders of the NICHD, Baltimore, MD and the Institute for Basic Research Brain Bank (IBR Brain Bank). Both the control group (12 cases) and the DS group (17 cases) represented subjects of various age, gender, and ethnicity. The age range for the control group was 13 months to 71 years, while in the DS group the youngest subject was 12 months, and the oldest, 63 years old. The median postmortem interval time was 15 hours. The levels of DYRK1A were measured in six brain structures that included frontal (FC), temporal (TC), occipital (OC), and cerebellar (CX) cortices and cerebral (corpus callosum - CC) and cerebellar (CW) white matter. However, brain tissue from the NICHD Bank allowed dissection of only four structures i.e. frontal and cerebellar cortices and corresponding white matters.
Three pairs, each consisting of one trisomic Ts65Dn mouse and its diploid littermate, were used in the study. Mice were maintained by mating females B6C3H-Ts65Dn to B6EiC3H F1 hybrid male mice. Genotyping by PCR on genomic DNA from the tail was performed according to the method described by Liu et al. [15], with some modifications. The brain tissue was removed at the age of 7 months (1 pair) and 8 months.
All animal handling and experimental procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and procedures involving human tissue, the Declaration of Helsinki. Our experimental protocols were approved by the Institutional Review Board of The NYS Institute for Basic Research in Developmental Disabilities.
Frozen brain tissue was mortar-ground in liquid nitrogen. 0.1g of powdered tissue was lysed in 1ml of 6M guanidine hydrochloride buffer (6M GuHCl, NaH2PO4, 10mM Tris, 20mM imidazole, pH 8.0) followed by short-pulse sonication. Protein concentration was determined by bicinchoninic acid assay (BCA, Pierce, IL,) and 4 mg of total protein of each brain lysate was taken for partial purification by immobilized metal affinity chromatography on Ni–charged Sepharose. The batch procedure was employed, in which 1 ml of cleared brain lysate in 1.5 ml tube was mixed with 50 μl of Ni-sepharose High Performance slurry (Amersham Biosciences, Sweden) and rotated for 2 hrs at 4° C. Ni-Sepharose beads were then washed 3 times with 1ml of 8M urea buffer (8M urea, 100mM NaH2PO4, 10mM Tris, 20mM imidazole, pH 5.9) for each wash. Samples were eluted by boiling for 2 min. in 50 μl of 1x tricine sample buffer containing 50mM EDTA.
10 μl of each eluate was separated electrophoretically on 8% tricine SDS-polyacrylamide gel and electro-transferred onto PVDF membrane. The membranes were blocked in 5% non-fat milk and incubated overnight with 1:5000 dilution of anti DYRK1A 7F3 monoclonal antibody. The antibody was raised against peptide containing the first 160 residues of rat DYRK1A [25]. The immunoreactive bands were visualized by using goat anti-mouse alkaline phosphatase-conjugated secondary antibody (Sigma, St. Louis, MO) and CDP-Star chemiluminescence reagent (New England Biolabs, Ipswich, MA). After chemiluminescence detection, some blots were developed by color reaction using BCIP/NBT substrate.
The densitometric quantification of immunoreactive bands on X-ray films was carried out with the 1D Scan EX 3.1 software program (Scanalytics Corp., Rockville, MD). Blots were compared by internal normalization with 20 ng of the recombinant GST– fusion protein containing the first 497 residues of rat DYRK1A (GST-497). The levels of DYRK1A protein were calculated as mean values of at least three independent sample evaluations. The data were evaluated for statistical significance with the two-tailed Student’s t test for independent samples using GraphPad Prism version 4.0 software program (GraphPad Software Inc., San Diego, CA) and the level of significance was considered at P < 0.05
Initial efforts to quantify DYRK1A levels directly in crude brain detergent extracts were hampered by inconsistency due to variable solubility of dissected brain tissue and poor separation during electrophoresis caused by the necessity of applying high protein load. Complete solubilization was achieved by a single-step lysis in 6M guanidine hydrochloride, while protein load was dramatically reduced as a result of partial purification by immobilized metal (Ni2+) ion affinity chromatography (IMAC). DYRK1A binds with high affinity to Ni-charged Sepharose through the histidine-rich domain in the C-terminal part of the molecule [12, 22]. Panel A of Fig. 1 shows immunoblots of IMAC-purified samples from the whole mouse brain (lane 2) and frontal cortex of a 32-year-old control subject (lane 3). Lane 1 was loaded with 20 ng of the GST-497 internal standard used for normalization. In both samples, 7F3 antibody detected three closely spaced bands: two major and one minor, with approximate molecular weights of 97, 94 and 87 kDa. This triplet of bands is not always clearly visible on blots developed by chemiluminescence, which produces a diffusible signal. Very similar immunoblotting profiles were also reported by others [4, 16, 19] for mouse and rat brain tissue.
Fig. 1. Quantification of DYRK1A brain levels in control and trisomic Ts65Dn mice.
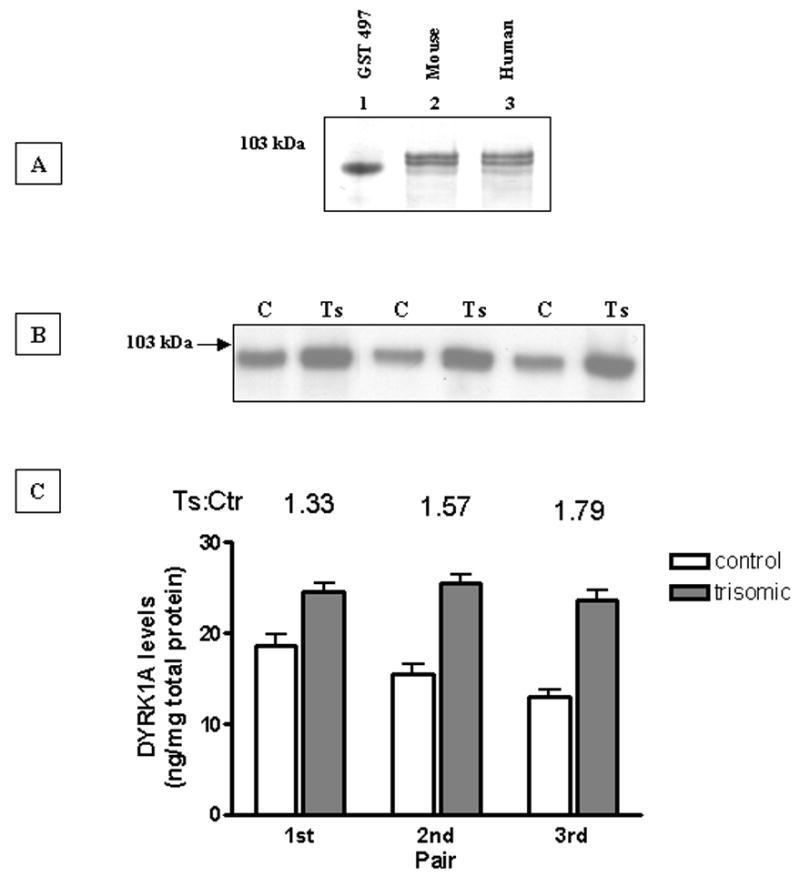
(A): Western blot analysis of DYRK1A isolated through Ni-affinity chromatography from mouse and human brain. Whole mouse brain (lane 2) and frontal cortex of 32-year old control subject (lane 3) were lysed in 6 M GuHCl, purified by IMAC, separated on 8% tricine SDS-polyacrylamide gel and immunobloted with 1:5000 dilution of 7F3 antibody. The blot was developed by color reaction using AP-conjugated secondary antibody and BCIP/NBP substrate. Lane 1 was loaded with 20 ng of GST-497 recombinant fusion DYRK1A protein standard.
(B): Representative Western-blot of three control (C)/trisomic(Ts) pairs of Ts65Dn mice. Equal loads of Ni-sepharose purified samples were resolved in 8% SDS-PAGE and immunoblotted with 1:5000 dilution of 7F3 antibody. Blots were developed by chemiluminescence using AP-conjugated secondary antibody.
(C): DYRK1A levels quantified by densitometric analysis of three independent Western blots as shown in (A). The density of immunoreactive bands was normalized with 20 ng of purified GST-497 recombinant DYRK1A protein and expressed as ng/mg of total protein of brain lysate. For all three pairs, the difference was statistically significant (P < 0.05) as established by paired Student’s t test.
The experimental protocol was first tested in the animal model of DS consisting of Ts65Dn trisomic mice. Panel B of Fig. 1 shows a representative Western blot of three control/trisomic pairs together with plotted mean values calculated for three independent repeats. In all three pairs the levels of DYRK1A in trisomic mouse were significantly higher (P = 0.0278) than in their diploid littermate. The values of trisomic to control ratio ranged from 1.3 to 1.8. The DYRK1A levels expressed in ng/mg of total protein in brain lysate were calculated on the basis of intensity of 20 ng of GST-497 standard band, which was run on each gel. The mean value for control was 17, and for trisomic, 25 ng/mg, giving an aneuploid to euploid ratio of 1.5
Initially, we measured the levels of DYRK1A protein in two cohorts (6 control and 12 DS cases) received from the IBR Brain Bank. The levels of DYRK1A in each individual case were quantitated as described above, and the mean values in ng/mg of total protein in six structures are presented in panel A of Fig. 2. In all six structures, there was statistically significant (P = 0.0313) enhancement of DYRK1A levels in the DS group. The DS to control ratio varied from 1.3 to 1.5 for cortices, while for both white matters (1.8 - CC; 1.7 - CW), ratios were slightly above the expected theoretical value of 1.5.
Fig. 2. Quantification of DYRK1A protein levels in selected structures of human brain: Summary of results obtained in 6 control and 12 DS subjects.
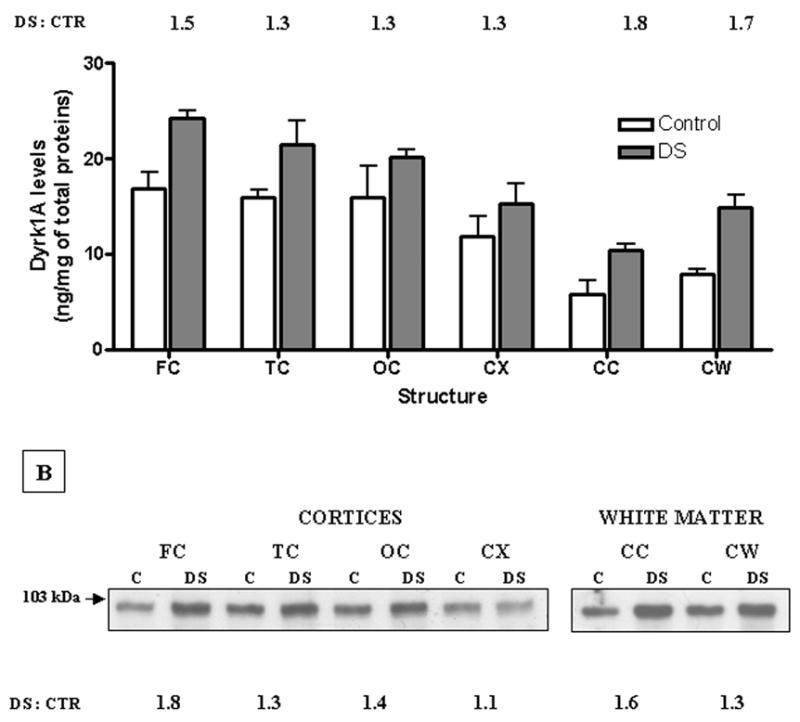
(A) Samples of frontal (FC), temporal (TC), occipital (OC), and cerebellar (CX) cortices and two white matters dissected from corpus callosum (CC) and cerebellum (CW) were prepared and quantified by Western blotting according to the standard procedure as described earlier. The results expressed as ng/mg of total protein in brain lysate represent mean ± SD of all individual cases within each group, where each case was independently evaluated at least three times. In all six structures, the difference between the control and the DS group was statistically significant (P < 0.05), as determined by the two-tailed Student’s t test for independent samples.
(B) Representative Western blot of pooled tissue from cases analyzed individually in (A). Mixing equal amounts of powdered brain tissue from each case made pools, which were then processed for Western blotting and densitometric quantification exactly in the same way as individual cases. Values of the DS : CTR ratio for each structural pool were calculated on the basis of scans of three independent Western blots.
Next, we measured DYRK1A levels in the pools of brain tissue from all previously analyzed cases. This measure was aimed at minimizing the influence of variation caused by unavoidable experimental errors associated with multiple testing of individual cases. The representative Western blot and the mean values of DS to control ratio for each pooled structure are shown in panel B of Fig. 2. Consistent with the results obtained for testing cases individually, the levels of DYRK1A in all six DS pools were higher, although in cerebellar cortex, the difference was less pronounced. Results for frontal cortex and cerebellar white matter were slightly discrepant in exact values (FC: 1.8 vs. 1.5; CW: 1.3 vs. 1.7), but were still strongly supportive of the trisomy-driven overexpression concept.
In summary, DYRK1A was present in every analyzed structure with the highest levels in cerebral cortices, and the lowest in both white matters. Of note is the high similarity between all three cerebral cortices, particularly in the control group, indicating lack of DYRK1A involvement in diversification of functions of these brain structures. The overall increase of DYRK1A levels in the DS group was 1.43-fold, thus very close to the expected value of 1.5.
In both cohorts from the IBR Brain Bank, subjects younger than 30 years of age were underrepresented and therefore were not suitable for the study of age-related changes. Such study has become possible after inclusion of infants and young individuals, whose brain tissue was obtained from the NICHD Brain Bank. As shown in Fig. 3, which depicts data grouped by age, the levels of DYRK1A were consistently higher (DS:CTR ratios: 1.43 and 1.34, P = 0.0479) only in DS subjects of groups comprising cases older than 3 years of age. Remarkably, in the infant group, there was no difference between control and DS patients, suggesting the existence of developmentally regulated mechanism of DYRK1A expression. There was a decline with age in both controls and the DS group. Interestingly, despite a marked neuronal loss that is known to occur in elderly DS patients, the ≈ 1.5-fold increase over the control group was still preserved.
Fig. 3. DYRK1A protein levels in frontal cortex of control and DS subjects grouped by age.
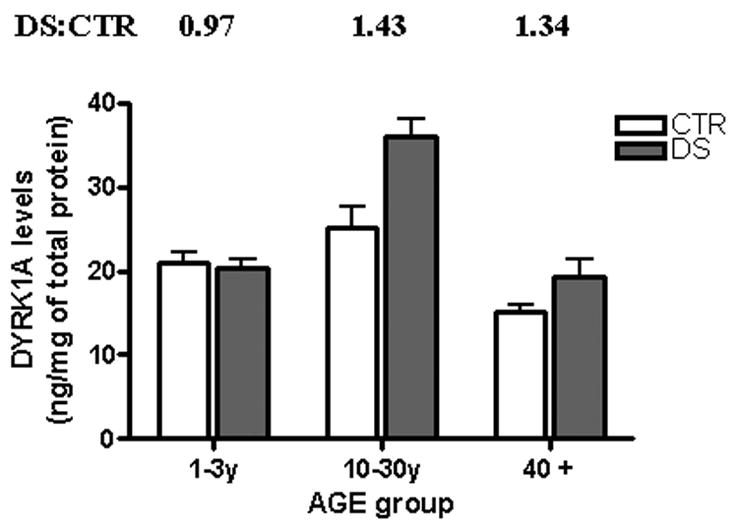
Groups 1—3 y (3 and 3) and 10—30 y (4 and 5) consisted mainly of cases received from the NICHD Brain Bank, while cases of the group 40 + (5 and 9) were from the IBR Brain Bank. All samples were prepared as described in the legend of Fig. 1 The results expressed as ng/mg of total protein in brain lysate represent mean ± SD of all individual cases within each group, where each case was independently evaluated at least three times. The difference between control and DS for all but infants group was statistically significant (P < 0.05).
The main goal of the present study was to test whether the “gene dosage effect” hypothesis, a prevailing concept in Down syndrome research [8], is applicable to DYRK1A kinase. We chose the DYRK1A gene because it is located within the DSCR, and through altered expression, it can alone produce learning and memory deficits in transgenic animals [1, 2, 4, 10], thus mimicking mental retardation, which is invariably associated with the DS phenotype. In the study that involved 12 euploid control and 17 trisomic DS subjects we evaluated the levels of DYRK1A protein by Western blot technique in six brain structures. The large size of the cohorts combined with the rigid experimental protocol repeatedly applied to individual samples in independent experiments, allowed us to establish for the first time the expression patterns of DYRK1A protein in human brain. We found DYRK1A to be present in every analyzed brain structure, with an average 1.5 – fold enhancement in the DS group. The 1.5-fold increase was also recorded in trisomic Ts65Dn mice, introduced here as a test ground of our experimental approach, in which the measured levels of DYRK1A protein are considerably less influenced by inter-individual variability, postmortem artifacts, and AD-like pathology, as it is in human brains. The influence of postmortem artifacts associated with autolysis has been substantially reduced by exclusion of cases showing signs of degradation. Inter-individual variability characterized both groups similarly. Alzheimer disease (AD)-like pathology, which DS individuals usually develop by the fourth decade of life, seems to have a rather limited influence on DYRK1A levels, because in three analyzed AD cases (data not shown), the expression of DYRK1A protein was not increased.
The gene dosage–predicted increase was preserved across a wide range of ages, with the exception of infants 1 to 3 years of age, in which control and DS groups did not significantly differ. This could be the reflection of well-documented delay in development of DS infants [3, 18, 24, 26], particularly in dendritization process, in which DYRK1A has been shown to be involved. [13,27]. DYRK1A must also play an important role in adult brain, where, as shown in this study, it is ubiquitously present in gray and white matter irrespective of their location within the brain. For example, the identification of dynamin 1 [5] and amphiphysin 1 [17] as DYRK1A substrates, suggests a role in regulating endocytosis at synapsis. It is tempting to speculate that DYRK1A acting through the gene dosage effect on processes such as neuritogenesis and synaptogenesis may be in part responsible for sustaining the mental retardation status of individuals with DS throughout their entire life.
The gene dosage effect hypothesis and the input of genes located in the triplicated DSCR on chromosome 21 into DS phenotype, have been challenged by some authors [6, 20, 21]. However, to rule in or out triplication of a particular gene as a causative factor for a given phenotype, one needs to examine its expression at the protein level in relevant to its function tissue or organ. This critical requirement had not been satisfactorily elaborated for most of the genes from the DSCR, including DYRK1A. In the only study involving DYRK1A [6], the authors measured levels of DYRK1A protein in fetal DS brains, and found no elevation over the euploid age matched controls. However, the Western blot patterns of DYRK1A protein described by these authors were so discrepant from the characteristic triplet of bands observed by us and other groups [4, 9, 16, 19], that it raises serious concerns regarding the specificity of DYRK1A detection in this study. Thus, our findings of DYRK1A protein overexpression in a gene-dosage dependent manner in DS brain, the organ to which most of its functions have been assigned, fulfill this critical requirement for gene dosage-effect hypothesis, confirming, along with the studies discussed above, the relevance of DYRK1A gene triplication in the mental retardation of individuals with DS.
Acknowledgments
Supported in parts by funds from the New York State Office of Mental Retardation and Developmental Disabilities and grants R01 HD38295 (YWH), R01 HD43960 (JW) from the National Institutes of Health, and Tosinvest Sanita, Italy (SP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn K, Jeong H, Choi H, Ryoo S, Kim Y, Goo J, Choi S, Han J, Ha I, Song W. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol Dis. 2006;22:463–472. doi: 10.1016/j.nbd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Altafaj X, Dierssen M, Baamonde C, Marti E, Visa J, Guimera J, Oset M, Gonzalez JR, Florez JR, Fillat C, Estivill X. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down’s syndrome. Hum Mol Gene. 2001;10:1915–1923. doi: 10.1093/hmg/10.18.1915. [DOI] [PubMed] [Google Scholar]
- 3.Becker LE, Armstrong DL, Chan F. Dendritic atrophy in children with Down’s syndrome. Ann Neurol. 1986;20:520–526. doi: 10.1002/ana.410200413. [DOI] [PubMed] [Google Scholar]
- 4.Branchi I, Bichler Z, Minghetti L, Delabar JM, Malchiodi-Albedi F, Gonzales MC, Chettouh Z, Nicolini A, Chabert C, Smith DJ, Rubin EM, Migliore-Samour D, Alleva E. Transgenic mouse in vivo library of human Down syndrome critical region 1: Association between Dyrk1A overexpression, brain development abnormalities, and cell cycle protein alteration. J Neuropath Exp Neurol. 2004;63:429–440. doi: 10.1093/jnen/63.5.429. [DOI] [PubMed] [Google Scholar]
- 5.Chen-Hwang M-C, Chen H-R, Elzinga M, Hwang Y-W. Dynamin is a minibrain kinase/dual specificity Yak1-related kinase 1A substrate. J Biol Chem. 2002;277:7597–17604. doi: 10.1074/jbc.M111101200. [DOI] [PubMed] [Google Scholar]
- 6.Cheon MS, Bajo M, Kim SH, Claudio JO, Stewart AK, Patterson D, Kruger WD, Kondoh H, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: Challenging the gene dosage effect hypothesis (Part II) Amino Acids. 2003;24:119–125. doi: 10.1007/s00726-002-0337-1. [DOI] [PubMed] [Google Scholar]
- 7.Davisson MT, Schmidt C, Keson A. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 8.Epstein CJ. The consequences of chromosome imbalance: Principles, mechanisms, and models. Cambridge University Press; New York: 1986. [Google Scholar]
- 9.Ferrer I, Barrachina M, Puig B, de Lagran MM, Marti E, Avila J, Dierssen M. Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, Down syndrome, Pick disease, and related transgenic models. Neurobiol Disease. 2005;20:392–400. doi: 10.1016/j.nbd.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Fotaki V, Dierssen M, Alcantara S, Martinez S, Marti E, Casa C, Visa J, Soriano E, Estivill X, Arbones ML. Dyrk 1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol Cell Biol. 2002;22:6636–6647. doi: 10.1128/MCB.22.18.6636-6647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guimera J, Casas C, Pucharcos C, Solans A, Domenech A, Planas AM, Ashley J, Lovett M, Estivill X, Pritchard MA. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum Mol Genet. 1996;9:1305–1310. doi: 10.1093/hmg/5.9.1305. [DOI] [PubMed] [Google Scholar]
- 12.Guimera J, Casas C, Estivill X, Pritchard M. Human minibrain homologue (MNBH/DYRK1): Characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics. 1999;57:407–418. doi: 10.1006/geno.1999.5775. [DOI] [PubMed] [Google Scholar]
- 13.Hämmerle B, Carnicero A, Elizalde C, Ceron J, Martinez S, Tejedor FJ. Expression patterns and subcellular localization of the Down syndrome candidate protein MNB/DYRK1A suggest a role in late neuronal differentiation. Eur J Neurosci. 2003;17:2277–2286. doi: 10.1046/j.1460-9568.2003.02665.x. [DOI] [PubMed] [Google Scholar]
- 14.Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, Carpenter N, Daumer C, Dignan P, Disteche C, Graham JM, Jr, Hugdins L, McGillivray B, Miyazaki K, Ogasawara N, Park JP, Pagon R, Pueschel S, Sack G, Say B, Schuffenhauer S, Soukup S, Yamanaka T. Down syndrome phenotypes: The consequences of chromosomal imbalance. Proc Natl Acad Sci USA. 1994;91:4997–5001. doi: 10.1073/pnas.91.11.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu DP, Schmidt C, Billings T, Davisson MT. Quantitative PCR genotyping assay for the Ts65Dn mouse model of Down Syndrome. BioTechniques. 2003;35:1170–1180. doi: 10.2144/03356st02. [DOI] [PubMed] [Google Scholar]
- 16.Marti E, Altafaj X, Dierssen M, de la Luna S, Fotaki V, Alvarez M, Perez-Riba M, Ferrer I, Estivill X. Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res. 2003;964:250–263. doi: 10.1016/s0006-8993(02)04069-6. [DOI] [PubMed] [Google Scholar]
- 17.Murakami N, Xie W, Lu RC, Chen-Hwang MC, Wieraszko A, Hwang YW. Phosphorylation of amphyphysin 1 by minibrain kinase/dual-specificity tyrosine-phosphorylation-regulated kinase,1A, a kinase implicated in Down syndrome. J Biol Chem. 2006;281:23712–23724. doi: 10.1074/jbc.M513497200. [DOI] [PubMed] [Google Scholar]
- 18.Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspectives. Genes Brain Behav. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 19.Okui M, Ide T, Morita K, Funakoshi E, Ito F, Ogita K, Yoneda Y, Kudoh J, Shimizu N. High-level expression of the Mnb/Dyrk1A gene in brain and heart during rat early development. Genomics. 1999;62:165–171. doi: 10.1006/geno.1999.5998. [DOI] [PubMed] [Google Scholar]
- 20.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific Down syndrome phenotypes. Science. 2004;306:687–690. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro BL. The Down syndrome critical region. J Neural Trans Suppl. 1999;57:293–303. doi: 10.1007/978-3-7091-6380-1_3. [DOI] [PubMed] [Google Scholar]
- 22.Shindoh N, Kudoh J, Maeda H, Yamaki A, Minoshima S, Shimizu Y, Schimizu N. Cloning of a human homolog of the Drosophila minibrain/rat Dyrk gene from “the Down syndrome critical region” of chromosome 21. Biochem Biophys Res Commun. 1996;225:92–99. doi: 10.1006/bbrc.1996.1135. [DOI] [PubMed] [Google Scholar]
- 23.Song WJ, Sternberg LR, Kasten-Sportes C, Van Keuren ML, Chung S-H, Slack AC, Miller DM, Glover TW, Chiang PW, Lou L, Kurnit DM. Isolation of human and murine homologues of the Drosophila minibrain gene: human homolog maps to 21q22.2 in the Down syndrome “critical region”. Genomics. 1996;38:331–339. doi: 10.1006/geno.1996.0636. [DOI] [PubMed] [Google Scholar]
- 24.Takashima S, Fida K, Mito K, Arina M. Dendritic and histochemical development and aging in patients with Down syndrome. J Intellect Disabil Res. 1994;38:265–273. doi: 10.1111/j.1365-2788.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 25.Wegiel J, Kuchna I, Nowicki K, Frackowiak J, Dowjat K, Silverman WP, Reisberg B, de Leon M, Wisniewski T, Adayev T, Chen-Hwang MO, Hwang YW. Cell type- and brain structure-specific patterns of distribution of minibrain kinase in human brain. Brain Res. 2004;1010:69–80. doi: 10.1016/j.brainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Wisniewski KE. Down syndrome children often have brain with maturation delay, retardation of growth, and cortical dysgenesis. Am J Med Gen Suppl. 1990;7:274–281. doi: 10.1002/ajmg.1320370755. [DOI] [PubMed] [Google Scholar]
- 27.Young EJ, Ahn YS, Chung KC. Protein kinase Dyrk1A activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells. J Biol Chem. 2001;276:39812–39824. doi: 10.1074/jbc.M104091200. [DOI] [PubMed] [Google Scholar]


