Abstract
Uptake of Ca2+ by mitochondria serves as a regulator of a number of important cellular functions, including energy metabolism, cytoplasmic Ca2+ signals, and apoptosis. Recent findings reveal that the process of Ca2+ uptake by the mitochondrial uniporter is itself regulated by Ca2+ in a temporally complex manner.
The history of Ca2+ handling by mitochondria is a classic story of rags to riches (or rather riches to rags to riches) [1]. In the 1970s to early 1980s, mitochondria were thought to be prime sources of signaling Ca2+ in non-excitable cells. However, this idea fell into disfavor with the finding that, at the Ca2+ levels expected in either resting or activated cells, the relatively high Km for uptake should preclude significant Ca2+ accumulation by mitochondria [2], and ultimately with the finding that the signal for intracellular Ca2+ release, inositol trisphosphate (IP3), clearly mobilized Ca2+ from endoplasmic reticulum [3]. Biochemical studies of mitochondrial Ca2+ content and the regulation of mitochondrial Ca2+-sensitive enzymes indicated that mitochondria were more likely to be a target for Ca2+ signaling rather than a source [4,5]. When Rizzuto et al. [6] made the first direct in situ measurements of mitochondrial Ca2+, it was clear that receptor-activated Ca2+ signals caused rapid and large Ca2+ signals in the mitochondrial matrix. It soon became apparent that mitochondria are capable of accumulating Ca2+ during signaling processes because they are positioned very near to either the sites of intracellular release (by IP3, for example) or sites of entry across the plasma membrane (for example, through store-operated or voltage activated channels; for a review see [7]). Ca2+ activates several key enzymes in the mitochondrial matrix to enhance ATP production, and this provides an important mechanism for synchronizing energy production with the energy demands of Ca2+-activated processes during cell stimulation (excitation–metabolism coupling) [4,5,8]. In addition to serving as a target of Ca2+ signaling, the uptake of Ca2+ by mitochondria has important feedback effects to help shape cytosolic Ca2+ signals. This can occur through buffering of bulk cytosolic Ca2+ changes, but is most pronounced in the intimate intracellular ‘synaptic’ regions where mitochondria are in close proximity to Ca2+ release sites. Thus, rapid accumulation of Ca2+ can prevent or temper influences of Ca2+ on intracellular or plasma membrane channels [9,10]. Alternatively, in some instances mitochondrial Ca2+ uptake serves to compartmentalize Ca2+ signaling in appropriate cellular domains, a concept termed “firewall” from studies of pancreatic acinar cells [11]. In addition, under certain conditions, uptake of Ca2+ into mitochondria initiates a key step in the process of apoptosis through activation of the mitochondrial permeability transition pore, permitting escape of cytochrome c and other pro-apoptotic factors to the cytoplasm [12].
On the surface, mitochondrial Ca2+ handling seems rather simple. Uptake occurs through a channel termed a uniporter (from the Peter Mitchell nomenclature) and the rate of uptake depends upon driving force; this is considerable, as the process of electron transport in normally respiring mitochondria generates extremely negative transmembrane potentials across the inner mitochondrial membrane. This would ultimately lead to huge and potentially toxic levels of accumulated Ca2+ in the mitochondrial matrix were it not for the action of separate mitochondrial Ca2+ efflux pathways that are also coupled to the proton motive force developed by the respiratory chain. Thus, the inner mitochondrial membrane has a Ca2+/2H+ exchanger and/or a Ca2+/3Na+ exchanger analogous to that found in the plasma membrane. However, these efflux pathways can become saturated with high matrix Ca2+ loads, such that sustained rapid Ca2+ influx can still lead to mitochondrial Ca2+ overload.
In a report in a recent issue of Current Biology, Moreau et al. [13] reveal that the process of Ca2+ accumulation undergoes complex regulation by Ca2+ itself. They measured mitochondrial matrix Ca2+ concentration directly by loading the mitochondria of permeabilized mast cells (a rat basophilic leukemia line) with a fluorescent Ca2+ indicator. The uptake of Ca2+ was significantly reduced by inhibitors of calmodulin, suggesting that a Ca2+–calmodulin-mediated process is necessary for activation of the uniporter. This finding is consistent with an earlier observation that calmodulin antagonists impede the penetration of Mn2+ into mitochondria and that brief pulses of cytosolic Ca2+ can facilitate mitochondrial Ca2+ uptake [14]. Surprisingly, Moreau et al. [13] found that Ca2+ also appeared to inhibit its own uptake. Thus, uptake of Ca2+ due to addition of 100 μM Ca2+ was substantially impaired if preceded by exposure to 10 μM Ca2+. In contrast to the sensitization of mitochondrial Ca2+ uptake, the Ca2+-dependent inactivation was not sensitive to calmodulin blockers. The ability of Ca2+ to inactivate the uniporter may correspond to the phenomenon of desensitization of mitochondrial Ca2+ uptake suggested in earlier studies [15,16]. The uniporter appeared to be similarly sensitive to both activation and inhibition by Ca2+, with apparent Kds in the 10–20 μM range. This concentration range raises the standard mitochondrial problem of whether such Ca2+ concentrations are seen by the mitochondria during physiological signaling. In agreement with an earlier study [17], Moreau et al. show close associations between mitochondria and the endoplasmic reticulum, and go on to show that when Ca2+ is released from the endoplasmic reticulum by IP3 there is a much more efficient transfer of Ca2+ to the mitochondria than that seen with exogenous Ca2+ addition. Thus, in the mast cell line, Ca2+ released by IP3 is readily sensed by mitochondria, probably due to close apposition of release and uptake sites. There is ample evidence in the literature for such associations between mitochondria and the endoplasmic reticulum [18]. Interestingly, mitochondria have been shown to be somewhat mobile in cells, while the endoplasmic reticulum is relatively fixed; Ca2+ inhibits mitochondrial mobility, thus providing a mechanism to retain mitochondria at specific Ca2+ signaling sites [19].
The fact that both the activation and inactivation mechanisms have similar Ca2+ sensitivities would seem problematic for significant accumulation of Ca2+ in the mitochondrial matrix. However, when Moreau et al. [13] examined the kinetics of inactivation, it was found that inactivation occurred with a time constant of 17 seconds, while for uptake the time constant is about 6 seconds. This pattern of rapid activation and slower inactivation is reminiscent of myriad biological signaling patterns; most notably, as pointed out by Moreau et al. [13], of the biphasic regulation of Ca2+ discharge from the endoplasmic reticulum through the IP3 receptor channel. In most cell types, physiological Ca2+ signaling occurs not by sustained elevations in cytoplasmic Ca2+, but through repetitive bursts of rising intracellular Ca2+, sometimes referred to as Ca2+ oscillations, with durations ranging from less than a second to several seconds [20]. The delayed inactivation mechanism will thus permit mitochondrial matrix Ca2+ to closely track these global signals, but will prevent excessive Ca2+ accumulation if the intracellular Ca2+ elevation is prolonged (Figure 1). For example, hormone-induced cytosolic Ca2+ oscillations in hepatocytes are closely paralleled by mitochondrial Ca2+ oscillations, whereas high levels of hormone that yield a sustained cytosolic Ca2+ increase cause only a transient spike of mitochondrial Ca2+ [8]. Thus, the Ca2+-dependent activation and inactivation of mitochondrial Ca2+ uptake [13] may help to tune mitochondrial responses to oscillatory Ca2+ signals, while filtering out persistent Ca2+ elevations that would have pathological consequences. Thus, once considered a simple Ca2+ accumulating depot, mitochondria have since proven to be elegantly controlled players in the complex network of intracellular Ca2+ signaling.
Figure 1.
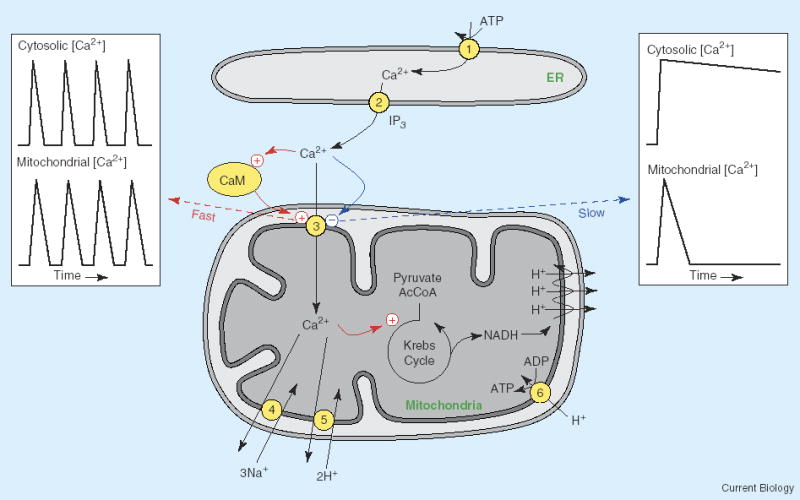
Regulation of mitochondrial Ca2+ uptake.
Close apposition between mitochondria and endoplasmic reticulum (ER) Ca2+ release sites facilitates Ca2+ uptake into the mitochondria. A fast activation of the uniporter, mediated by calmodulin (CaM), enhances mitochondrial Ca2+ accumulation. A slower Ca2+-dependent inactivation of the uniporter serves to limit mitochondrial Ca2+ uptake during a prolonged rise of cytosolic Ca2+. The fast activation allows the mitochondrial matrix [Ca2+] to closely follow changes in the cytosolic [Ca2+], as occurs during cytosolic Ca2+ oscillations (left inset). The slower inactivation process limits mitochondrial Ca2+ uptake when the elevation of cytosolic Ca2+ is more sustained (right inset). (1) SERCA Ca2+ pump, (2) IP3 receptor Ca2+ release channel, (3) Ca2+ uniporter, (4) mitochondrial Ca2+/Na+ exchanger, (5) mitochondrial Ca2+/H+ exchanger, (6) ATP synthetase. The mitochondrial respiratory chain is shown as 3 cycles of proton pumping from the matrix.
References
- 1.Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein MP, Kendrick NC, Fried RC, Ratzlaff RW. Calcium metabolism at the mammalian presynaptic nerve terminal: Lessons from the synaptsome. In: Cowan MW, Ferrendelli JA, editors. Society for Neuroscience Symposia, Vol II Approaches to the Cell Biology of Neurons. Bethesda, MD: Society for Neuroscience; 1977. pp. 172–194. [Google Scholar]
- 3.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial store in pancreatic cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–68. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 4.Shears SB, Kirk CJ. Determination of mitochondrial calcium content in hepatocytes by a rapid cellular fractionation technique. Vasopressin stimulates mitochondrial Ca2+ uptake. Biochem J. 1984;220:417–421. doi: 10.1042/bj2200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denton RM, McCormack JG. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985;249:E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- 6.Rizzuto R, Simpson AWM, Brini M, Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 7.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hajnóczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 9.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajnóczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 11.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, pergranular and subplasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 13.Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 14.Csordas G, Hajnoczky G. Plasticity of mitochondrial calcium signaling. J Biol Chem. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- 15.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J Biol Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 16.Maechler P, Kennedy ED, Wang H, Wollheim CB. Desensitization of mitochondrial Ca2+ and insulin secretion responses in the beta cell. J Biol Chem. 1998;273:20770–20778. doi: 10.1074/jbc.273.33.20770. [DOI] [PubMed] [Google Scholar]
- 17.Csordás G, Thomas AP, Hajnóczky G. Quasi-synaptic calcium transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabadkai G, Simoni AM, Bianchi K, De SD, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca(2+) signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas AP, Bird G StJ, Hajnóczky G, Robb-Gaspers LD, Putney JW., Jr Spatial and temporal aspects of cellular calcium signalling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]


