Abstract
The aim of the study was to analyze interactions between neuronal networks mediating centrally initiated movements and reflex reactions evoked by peripheral afferents; specifically whether interneurons in pathways from group Ib afferents and from group II muscle afferents mediate actions of reticulospinal neurons on spinal motoneurons by contralaterally located commissural interneurons. To this end reticulospinal tract fibers were stimulated in the contralateral medial longitudinal fascicle (MLF) in chloralose-anesthetized cats in which the ipsilateral half of the spinal cord was transected rostral to the lumbosacral enlargement. In the majority of interneurons mediating reflex actions of group Ib and group II afferents, MLF stimuli evoked either excitatory or inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) or both EPSPs and IPSPs attributable to disynaptic actions by commissural interneurons. In addition, in some interneurons EPSPs were evoked at latencies compatible with monosynaptic actions of crossed axon collaterals of MLF fibers. Intracellular records from motoneurons demonstrated that both excitation and inhibition from group Ib and group II afferents are modulated by contralaterally descending reticulospinal neurons. The results lead to the conclusion that commissural interneurons activated by reticulospinal neurons affect motoneurons not only directly, but also by enhancing or weakening activation of premotor interneurons in pathways from group Ib and group II afferents. The results also show that both excitatory and inhibitory premotor interneurons are affected in this way and that commissural interneurons may assist in the selection of reflex actions of group Ib and group II afferents during centrally initiated movements.
INTRODUCTION
Reticulospinal (RS) neurons may contribute to the initiation, selection, or modulation of a variety of movements. These include centrally initiated voluntary movements (see Pettersson et al. 2000; Sasaki et al. 2004) as well as locomotion (see Armstrong 1986; Deliagina et al. 2002; Grillner and Dubuc 1988; Jordan 1991; Mori et al. 1989) and postural adjustments (see Mori et al. 1995; Peterson and Felpel 1971; Peterson et al. 1979; Wilson and Yoshida 1968). How they fulfill these tasks has not yet been established but activation of relevant spinal interneuronal networks by RS neurons must be essential even if direct actions on alpha motoneurons (Floeter et al. 1993; Grillner et al. 1971; Grillner and Lund 1968) would also play a role. However, very little is known about actions of RS neurons on spinal interneurons. Disynaptic excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) evoked in motoneurons by stimulation of axons of RS neurons in the cat (Floeter et al. 1993; Gossard et al. 1996; Grillner and Lund 1968; Grillner et al. 1971; Takakusaki et al. 1989) show that these actions involve both excitatory and inhibitory premotor neurons and initiation of different forms of locomotion from different parts of the brain stem both in the cat (Mori et al. 1989) and in the lamprey (Deliagina et al. 2002) links RS neurons with spinal networks responsible for them. In the lamprey it has been shown that they target premotor interneurons postulated to be involved in swimming (Ohta and Grillner 1989) and in the cat that they excite commissural neurons that are rhythmically active during fictive locomotion (Matsuyama and Mori 1998; Matsuyama et al. 2004a).
Our recent studies focused on reticulospinal actions mediated by commissural interneurons that might be involved in locomotion as well as in other kinds of movement. We have demonstrated that these interneurons contact contralateral alpha motoneurons and either excite or inhibit them (Jankowska et al. 2003). By reconstructing axonal projections of commissural interneurons we have also demonstrated that they have widespread projections to neurons located outside motor nuclei as well as to motoneurons (Bannatyne et al. 2003; Matsuyama et al. 2004b).
Potential importance of interneurons mediating actions of commissural interneurons for centrally initiated movements is indicated by recent evidence that RS neurons and commissural interneurons activated by cortical neurons provide a detour pathway that may be used to replace missing actions of damaged crossed corticospinal neurons and contribute to recovery of motor functions after stroke or other cortical or subcortical injuries (Edgley et al. 2004; Jankowska et al. 2005a,c). The restoration of lost movements might critically depend on the optimal use of the involved spinal neurons. A particularly good example of this is the much better recovery of locomotion in trained than in untrained spinal rats and cats (Rossignol et al. 2004). It should therefore be advantageous to know on which of the spinal neurons the training should focus and how contribution of these neurons, including target neurons of commissural interneurons, could be made more effective.
Only one population of premotor interneurons has so far been shown to mediate actions of commissural interneurons activated by contralaterally descending RS neurons: those mediating Ia reciprocal inhibition between flexors and extensors (“Ia interneurons”) (Jankowska et al. 2005c; see also Angel et al. 2005; Bruggencate and Lundberg 1974; Bruggencate et al. 1969). The aim of the present study was therefore to find out whether RS and commissural neurons act by premotor interneurons mediating reflex actions from group Ib tendon organ afferents (“Ib interneurons”) and group II muscle spindle afferents (“group II interneurons”), in which both Ib and group II reflex pathways play an important role in shaping patterned movements (for review see Pearson 2004). With respect to Ib interneurons, no crossed actions, whether descending or reflex, have so far been reported and it was not known whether they might contribute to centrally initiated movements mediated by RS neurons. With respect to group II interneurons it has already been established that they are affected by commissural interneurons activated by contralateral group II afferents (Arya et al. 1991; Bajwa et al. 1992) but it remained unknown whether they contribute to movements mediated by commissural interneurons activated by contralaterally descending reticulospinal neurons. Specifically we aimed at investigating whether excitatory and inhibitory commissural interneurons mediating reticulospinal actions located on one side of the spinal cord do, or do not, affect excitatory and inhibitory interneurons in pathways from group Ib and group II afferents located on the other side, as indicated in Fig. 1. Observations on coupling between reticulospinal neurons stimulated within the contralateral medial longitudinal fascicle (MLF) and group Ib and group II excited interneurons were based on intracellular records from individual interneurons (the latency of their excitation and/or inhibition from the MLF). Estimates of the effectiveness of modulation of activity of these interneurons were based on changes in the probability of activation of individual interneurons and on the degree of facilitation or depression of disynaptic EPSPs or IPSPs evoked from group I and/or group II afferents in hindlimb motoneurons after MLF stimulation. Records from motoneurons were also used to find out whether both excitatory and inhibitory interneurons mediating reflex actions of group Ib and group II afferents, or only excitatory or only inhibitory ones are affected by commissural interneurons.
FIG. 1.
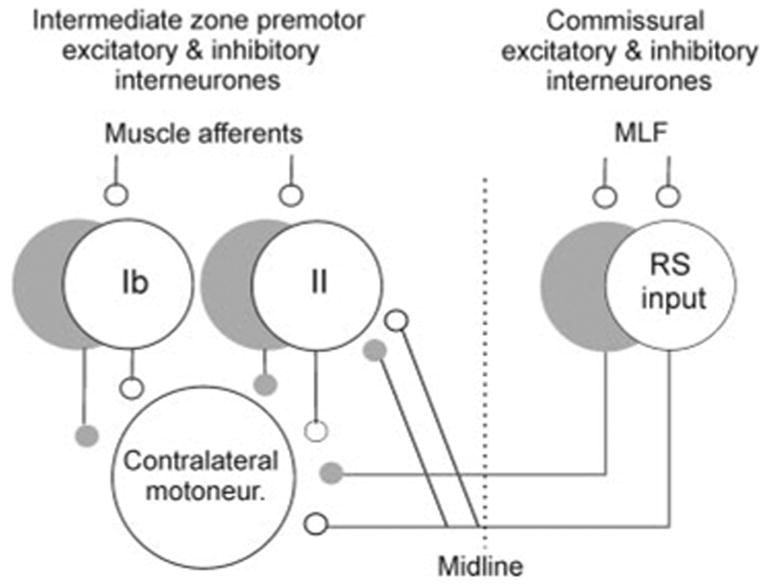
Relationhips between commissural interneurons activated by reticulospinal neurons and interneurons in disynaptic pathways between group Ib and group II afferents and motoneurons investigated in this study. White and gray cells represent excitatory and inhibitory interneurons, respectively.
METHODS
Preparation
The experiments were performed on 13 deeply anesthetized cats weighing 2.3– 4.9 kg. All experimental procedures were approved by Göteborg University Ethics Committee and followed National Institutes of Health and EU guidelines for animal care. Anesthesia was induced with sodium pentobarbital (40 mg/kg, administered intraperitoneally) and maintained with one to two intermittent doses of sodium pentobarbital (4 mg/kg, intravenous [iv]) and α-chloralose (Rhône-Poulenc Santé, France; doses of 5 mg/kg administered every 1–2 h, ≤55 mg/kg, iv). During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg · kg−1 · h−1 iv) and the animals were artificially ventilated. Additional doses of α-chloralose were given when blood pressure or heart rate, which were continuously monitored, increased, or if the pupils dilated. Mean blood pressure was kept at 100 –130 mmHg and end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml · h−1 · kg−1). The core body temperature was kept at about 37.5°C by servocontrolled infrared lamps. The experiments were terminated by a lethal dose of pentobarbital, resulting in cardiac arrest, and/or by formalin perfusion.
A laminectomy exposed the lumbar (L) and low thoracic (Th) segments of the spinal cord. The spinal cord was hemisected on the left-hand side in the Th12 or L2 segment (after removing the dorsal columns) at the beginning of the experiment. A number of left hindlimb nerves were transected and mounted on stimulating electrodes. Subcutaneous cuff electrodes were used for nerves accessed in the iliac fossa: quadriceps (Q) and sartorius (Sart) nerves. The remaining nerves including the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), gastrocnemius-soleus (GS), plantaris (Pl), flexor digitorum and hallucis longus (FDL), and deep peroneal (DP) were mounted on pairs of silver hook electrodes in a paraffin oil pool (at 36–37°C) created by skin flaps.
The caudal part of the cerebellum was exposed by a craniotomy and a tungsten electrode (impedance 40–150 kΩ) was placed in the right medial longitudinal fascicle (MLF) at the level of the inferior olive. The electrode was inserted at an angle of 35° from vertical (with the tip directed rostrally). The initial target was at Horsley–Clarke coordinates P10, L0.6, H −5, the final position of the electrode being adjusted on the basis of records of descending volleys from the surface of the lateral funiculus at the Th13 or L2 levels. The electrode was left at a site from which distinct descending volleys were evoked at stimulus intensities of ≤20 μA. At the end of the experiments this site was marked with an electrolytic lesion (0.4-mA constant current for 15 s). The location (Fig. 2) was subsequently verified on 100-μm-thick frontal sections of the brain stem, cut in the plane of insertion of the electrode using a freezing microtome and counterstained with cresyl violet.
FIG. 2.
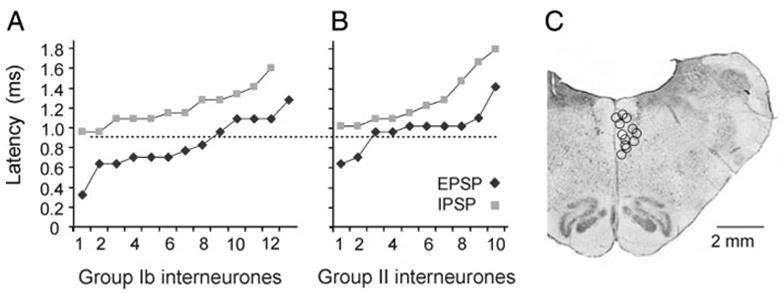
Minimal latencies of excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) evoked from medial longitudinal fascicle (MLF) and MLF stimulation sites. A and B: minimal latencies of EPSPs and IPSPs evoked in 18 group Ib interneurons and in 15 group II interneurons, respectively, ranked in the ascending order. When both EPSPs and IPSPs were evoked in the same neuron latencies of both were measured, with the resulting 25 data points for Ib interneurons and 20 data points for group II interneurons. Horizontal dotted line separates EPSPs that were most likely evoked monosynaptically from the disynaptically evoked EPSPs and IPSPs. Latencies were measured from the positive peak of the first descending volleys after MLF stimuli (indicated by the first arrow in Fig. 3J). C: location of the stimulating electrodes in the right MLF in all of the experiments of this series. It is indicated on the transverse section of the medulla from one of these experiments in the plane of insertion of the electrodes.
Stimulation and recording
Peripheral nerves were stimulated by pairs of silver electrodes with constant voltage stimuli (0.2-ms duration, intensity expressed in multiples of threshold, T, for the most sensitive fibers in the nerve). For activation of reticulospinal tract fibers, three to four constant-current cathodal stimuli (0.2 ms, 50–200 μA, at 300 or 400 Hz) were applied by a tungsten electrode inserted into the right MLF as described previously (Edgley et al. 2004; Jankowska et al. 2003; Matsuyama and Jankowska 2004) against a large electrode inserted into a neck muscle. Near maximal stimuli applied in the MLF were expected to activate a large proportion of ponto- and medullary reticulospinal tract fibers (see Jankowska et al. 2003). These stimuli would also activate vestibulospinal tract fibers arising from the medial vestibular nucleus that run in the MLF. However, these fibers do not project as far caudally as the lumbar segments (Nyberg-Hansen and Mascitti 1964), so the effects in the lumbar segments can be attributed to reticulospinal fibers.
Descending volleys were recorded monopolarly from the surface of the lateral funiculi at Th 13 or L2 levels during placement of the MLF electrode and from the cord dorsum at a midlumbar level during recordings from the interneurons and motoneurons.
Glass micropipettes filled with 2 M solution of potassium citrate or with 2 M solution of sodium chloride were used for intracellular recording from interneurons and motoneurons or for extracellular records from interneurons, respectively. Both the motoneurons and interneurons were recorded from the left side.
Records from interneurons were restricted to neurons that did not project rostral to the lumbar segment, as indicated by lack of anti-dromic activation by stimuli (0.2 ms, ≤1 mA) applied to the left and right lateral funiculi at the Th13 level a few millimeters caudal of the level of the hemisection.
Analysis
Both the original data and averages of 10 to 40 PSPs recorded in interneurons and motoneurons were stored on-line. The latencies and the amplitudes of these potentials were estimated from the averaged records but individual records were also taken into account. In motoneurons changes in the PSPs evoked by conditioning stimuli were estimated by comparing the areas of the averaged potentials within a selected time window, after having subtracted PSPs evoked by test and conditioning stimuli alone from PSPs evoked by their joint application. A software sampling and analysis system designed by E. Eide, T. Holmström, and N. Pihlgren (Göteborg University) was used to this end. Differences between samples of neurons were assessed for statistical significance using Student’s t-test (for paired and unpaired data).
Changes in extracellularly recorded responses of individual interneurons were estimated from peristimulus time histograms (PSPHs) and cumulative sums of responses evoked by 20 consecutive submaximal test stimuli applied alone, or after conditioning stimuli, taking into account both the number of responses evoked by these stimuli and their latencies (Jankowska et al. 1997).
Criteria of identification of neurons recorded from and of classification of PSPs evoked in them
Ib interneurons were identified by responses evoked by stimuli maximal for group I afferents at latencies <2 ms and by their location in the intermediate zone, within the areas where distinct field potentials were evoked from group I afferents. The inhibitory subpopulation of these interneurons was identified by their antidromic activation from the lateral funiculus at the border between the L3 and L4 segments (Hongo et al. 1983a,b; Jankowska 1992). The sample of Ib interneurons included 26 intracellularly and 31 extracellularly recorded interneurons with monosynaptic input from group I afferents from the Q, GS, Pl, and FDL nerves that are the main source of actions of Ib afferents on motoneurons (Eccles et al. 1957); they were located in the L5 and L6 segments. Three of the intracellularly recorded and five of the extracellularly recorded interneurons were antidromically activated from the lateral funiculus at the border between the L3 and L4 segments. Seventeen other interneurons were antidromically activated from GS motor nucleus, making it likely that they affected motoneurons in this and/or neighboring nuclei, but providing no indications as to whether they were excitatory or inhibitory. Stimuli in the GS motor nuclei were applied at the border between the L7 and S1 segments (by a 50- to 200-MΩ tungsten electrode against a larger electrode in a back muscle); the location was identified by recording field potentials from GS. Antidromic activation was evidenced by short and stable latency of the responses (0.4–1.1 ms from L3 and 0.6–1.3 ms from the GS motor nucleus) and/or by their collision with the synaptically evoked responses (see RESULTS).
Group II interneurons were identified by their dominating input from group II afferents (at stimulus intensities supramaximal for group I afferents) and their location within the areas of the largest intermediate zone field potentials evoked from group I and group II afferents (Edgley and Jankowska 1987). The sample included 20 intracellularly recorded and 16 extracellularly recorded interneurons with input from Q and FDL located in the L4 and L5 segments; 13 of the former and all of the latter were antidromically activated from the GS motor nuclei.
Motoneurons were identified by antidromic activation after stimulation of a muscle nerve, the ventral roots being left intact.
PSPs of group Ib origin were classified as those evoked at thresholds not exceeding thresholds of activation of group II afferents (generally <1.6T; Jack 1978) in motoneurons in which no group Ia reciprocal inhibition is evoked (Eccles et al. 1957). PSPs evoked at latencies 1.1–1.9 ms from the afferent volleys were considered as induced disynaptically. The areas of these PSPs were measured within the time windows of 1.6–2.5 ms from their onset, depending on the slope.
PSPs of group II origin were classified as those evoked at thresholds 1.8–2.5T. PSPs evoked at latencies 2.2– 4 ms from group I afferent volleys were considered as compatible with disynaptic coupling in view of longer conduction time along group II than along group I afferents (on the average about 0.8 ms for afferents of the Q and Sart nerves and about 1.2 ms for afferents of more distal nerves, e.g., DP and FDL) but no differentiation between the disynaptically and trisynaptically mediated actions was attempted. The areas of group II PSPs were measured within the time windows of 2–3 ms.
Monosynaptic EPSPs of MLF origin were classified as those evoked at latencies <0.9 ms with respect to the positive peak of the first of two components of the descending volleys induced by MLF stimuli (Jankowska et al. 2005b). When EPSPs or IPSPs were evoked at a latencies 0.9–1.8 ms from the first components of MLF volleys they were classified as evoked disynaptically, by direct actions of commissural interneurons. Excitation or inhibition evoked at longer latencies could be evoked by two or three interneurons in series, commissural interneurons acting by other interneurons or being activated di- or polysynaptically (see following text). As reported previously, the first components of MLF volleys (indicated by the first arrow in Fig. 3K) reflect action potentials in reticulospinal tract fibers, whereas the second components reflect activation of commissural interneurons that are monosynaptically excited by them and display a marked temporal facilitation (Jankowska et al. 2003). The latter volleys will be referred to as “relayed” or “commissural” volleys. Any effects attributable to actions of commissural interneurons should thus be closely related to these volleys.
FIG. 3.
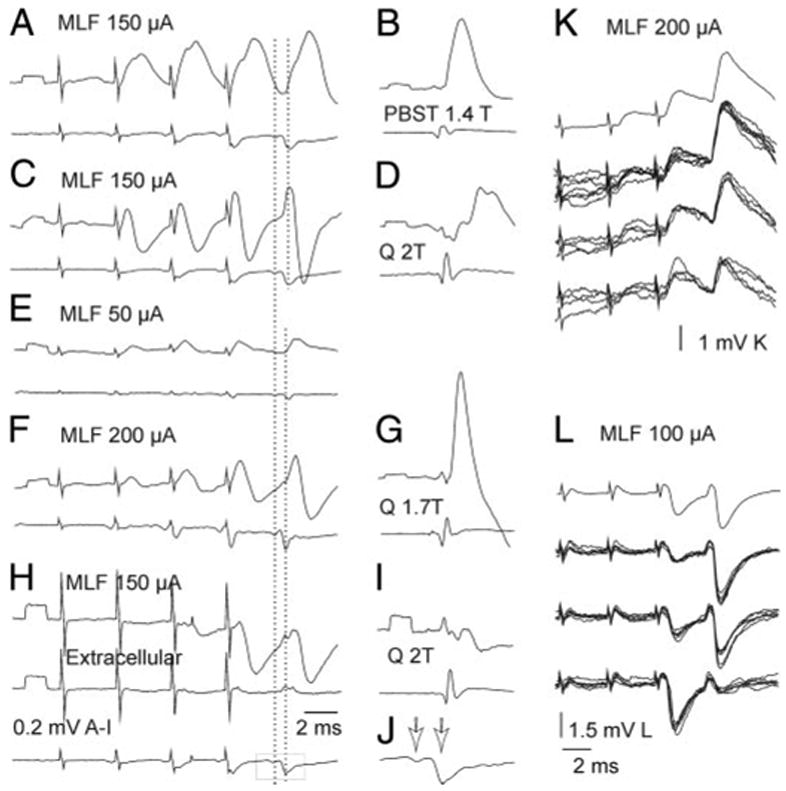
EPSPs and IPSPs evoked from MLF in Ib interneurons. A–B, C–D, E–G, and H–I: intracellular averaged records from 4 Ib interneurons projecting to motor nuclei (top traces), records from the cord dorsum (bottom traces) and extracellular records from outside the last interneuron (middle trace in H). A and C: monosynaptic EPSPs of MLF origin, followed by disynaptic EPSPs (arrows in A) or IPSPs. E and F: EPSPs classified as evoked disynaptically, followed by IPSPs at higher stimulus intensities. H: disynaptic IPSPs. J: twice expanded MLF volleys boxed in H; arrows indicate the direct and relayed components. Dashed lines indicate positive peaks of the 2 components. EPSPs with the onset between these peaks were considered as evoked monosynaptically. K and L: averaged (top) and single records from 2 additional interneurons. Those of a similar size are superimposed separately.
RESULTS
Actions of commissural interneurons on group Ib and group II interneurons
INTRACELLULARLY RECORDED POSTSYNAPTIC POTENTIALS OF MLF ORIGIN IN IB INTERNEURONS
MLF stimuli evoked short-latency EPSPs and/or IPSPs in 18/26 of Ib interneurons, including interneurons antidromically activated from the L3 segments (n = 2) and from the GS motor nuclei (n = 13). EPSPs were evoked in six of these interneurons (Fig. 3, A and K), EPSPs followed by IPSPs in seven (Fig. 3, C, F, and L), and IPSPs in five (Fig. 3H). In the remaining eight interneurons no distinct PSPs of MLF origin were found, although EPSPs of 1.5–5 mV from group I afferents were evoked in all of them.
EPSPs were evoked at latencies of 0.83 ± 0.07 ms (mean ± SE) and IPSPs at latencies of 1.2 ± 0.05 ms from the positive peak of the first MLF volleys. Figure 2A shows that latencies of all of the IPSPs and of some of the EPSPs were ≥0.9 ms and therefore compatible with disynaptic linkage from MLF by commissural interneurons. However, some EPSPs were evoked at latencies coinciding with the onset of the second (relayed) volley from the MLF or were only 0.1– 0.3 ms delayed with respect to it. As illustrated in Fig. 3, the onset of such EPSPs preceded the onset of IPSPs evoked either in the same (A and F) or other (H) interneurons. These unexpectedly short latencies suggest that these EPSPs were evoked monosynaptically by crossed axon collaterals of reticulospinal neurons.
Amplitudes of EPSPs evoked in Ib interneurons were usually small. Even after the third or fourth stimulus they were only 0.6 ± 0.08 mV (>1 mV in only two interneurons). The size of EPSPs evoked from the MLF must have been affected by the depolarization of the interneurons after their penetration, although they were usually much smaller than EPSPs evoked from group I afferents (see, e.g., Fig. 3, A and B, or F and G).
Amplitudes of IPSPs depended on the degree of depolarization of the interneurons to an even greater extent, which were considerably increased with the depolarization that followed the penetration of the neurons, or passage of 5–20 nA of depolarizing constant current. In neurons with membrane potential of 30 – 40 mV, amplitudes of IPSPs evoked from the MLF were up to about 2 mV.
INTRACELLULARLY RECORDED POSTSYNAPTIC POTENTIALS OF MLF ORIGIN IN GROUP II INTERNEURONS
EPSPs, EPSPs followed by IPSPs, or only IPSPs were evoked from the MLF. They were evoked in similar proportions (5:5:5) of the 20 interneurons tested, with examples in Fig. 4, A, C, and E. EPSPs were evoked at a mean minimal latency of 0.99 ± 0.07 ms and IPSPs at a mean minimal latency of 1.28 ± 0.09 ms from the MLF volleys. As shown in Fig. 2B, the latencies of these PSPs were like those in Ib interneurons and compatible with both monosynaptic and disynaptic coupling. EPSPs evoked at latencies <0.9 ms, illustrated in Fig. 4A, were classified as evoked monosynaptically. The remaining EPSPs and IPSPs displayed both longer latencies and marked temporal facilitation (Fig. 4, A, C, and E and later components of EPSPs in A), which is characteristic of disynaptically evoked PSPs.
FIG. 4.
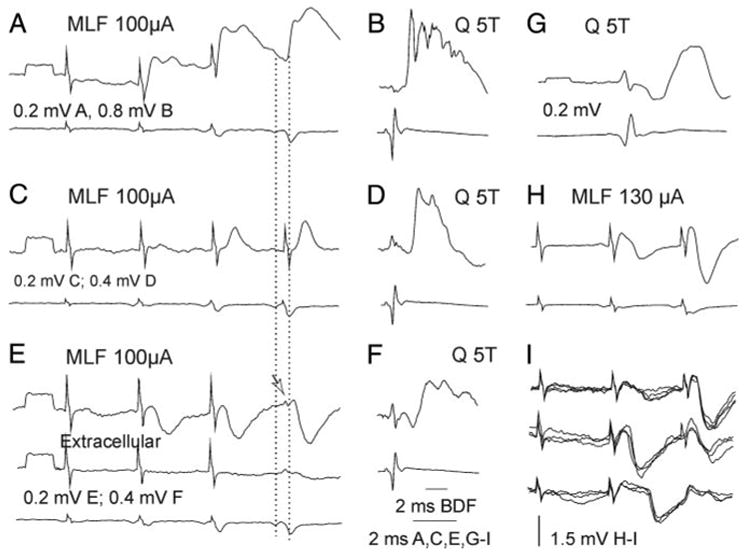
Examples of EPSPs and IPSPs evoked from MLF in group II interneurons. A–B, C–D, E–F, and G–H: averaged intracellular records from 4 interneurons with input from Q group II afferents (top traces), simultaneous records from cord dorsum (bottom traces), and extracellular records (middle trace in E). A: EPSPs of MLF origin, classified as evoked monosynaptically, followed by disynaptic EPSPs (arrows). C and H: disynaptic EPSPs followed by disynaptic IPSPs. E: disynaptically evoked IPSPs. Dashed lines indicate peaks of the direct and relayed MLF volleys. Arrow indicates terminal potential preceding the IPSP. I, examples of single sweep records of IPSPs adding to the average records in H; those of a similar size are superimposed.
Amplitudes of EPSPs and IPSPs evoked in group II interneurons were within the same ranges as for Ib interneurons. They were of 0.44 ± 0.1 and 0.68 ± 0.2 mV, respectively, in averaged records. EPSPs >1 mV were seen in only one interneuron and IPSPs of such size in two interneurons. EPSPs and IPSPs recorded in individual traces were more often 1–2 mV and appeared as events of a few constant amplitudes (Fig. 4I), suggesting that only a small number of commissural interneurons were responsible for them. In the illustrated neuron they were also of different shapes and were evoked at different latencies.
CONSEQUENCES OF SYNAPTIC ACTIONS FROM MLF ON GROUP IB AND GROUP II INTERNEURONS
In view of small sizes of EPSPs and IPSPs evoked by commissural interneurons, other tests were needed to estimate their functional consequences. To this end, effects of conditioning stimulation of MLF on the probability of activation of 31 group Ib and 16 group II interneurons were analyzed when these interneurons were extracellularly recorded.
Effects of MLF conditioning stimuli on Ib interneurons were tested on responses evoked by submaximal stimulation of group I afferents (when the neurons were activated in 10–75% of trials). MLF stimuli were between 50 and 200 μA and the conditioning–testing intervals 0.5–1.5 ms (between the relayed components of the MLF volleys and group I components of the afferent volleys). Responses of Ib interneurons were facilitated and/or depressed, matching effects of MLF stimulation found in intracellular records. Figure 5, A and B illustrates an increase in the probability of activation of one of these interneurons, in which it almost doubled, when group I stimuli were applied after MLF stimuli. The increase is easy to see by comparing the cumulative sums of the responses as well as the original PSTHs. Effects for all individual interneurons are plotted in Fig. 5, E and F.
FIG. 5.
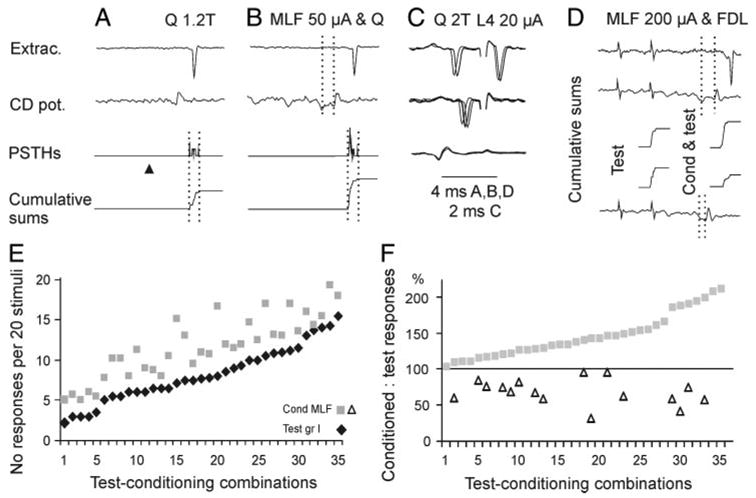
Modulation of activation of Ib interneurons. A and B, top to bottom: examples of responses of an interneuron, cord dorsum potentials, peristimulus time histograms (PSTHs), and cumulative sums of responses, evoked by quadriceps (Q) stimulation alone and after conditioning stimulation of the MLF. Number of responses evoked by 20 stimuli was increased from 10 to 17. Dotted lines in top of B indicate conditioning–testing interval and those in the bottom, the time windows within which the counts were made. C: collision test for antidromic activation of the same interneuron from the lateral funiculus at the border between the L3 and L4 segments (20 μA), extracellular records (4 superimposed single traces, top and middle traces), and records from the cord dorsum. Note that the L4 stimuli (shock artifacts truncated) stopped to activate the neuron when the interval between the synaptically evoked spikes and these stimuli was reduced (middle traces). D: records from another Ib interneuron projecting to the L3/L4 segment. Top traces: responses of the interneurons and cord dorsum potentials when test stimuli were preceded by conditioning stimuli, at a conditioning testing interval indicated by the dotted lines. Bottom trace: cord dorsum potentials at a shorter conditioning–testing interval. Middle traces: cumulative sums of responses evoked by the same test when applied alone and when preceded by conditioning MLF stimuli at the 2 intervals. Note facilitation at a longer interval and depression at the shorter one. E: mean number of facilitated responses of 31 interneurons to nerve stimuli alone (ranked in the increasing order) and when these were preceded by conditioning MLF stimulation (35 test-conditioning combinations). F: plot of both facilitated (filled squares) and depressed (open triangles) conditioned responses in percentage of the test responses. Data are ranked from the weakest to the most potent facilitation from MLF.
Activation of most of the interneurons was facilitated. At optimal stimulus parameters the mean increase was from 8.2 ± 0.9 to 12.3 ± 1.2 responses per 20 stimuli (151%; the difference was statistically significant at P < 0.001) and their latencies shortened by 0.1 ± 0.06 ms.
The dominating facilitation was found in 15/31 of the interneurons, including four interneurons projecting to the L4 segment. Activation of 16 other interneurons was facilitated under some experimental conditions (gray squares in Fig. 5F) but depressed in others (open triangles), and both differences were statistically significant at P < 0.001. In one interneuron conditioning 100-μA stimuli, applied at the depth from which maximal descending volleys were evoked, were followed by facilitation (from 6 to 11.3 responses), whereas the same intensity stimuli applied 1–2 mm more dorsally induced a depression (from six to 2.5 responses). In the two situations there was no statistically significant difference between effects of the test stimuli and the highly significant difference between effects of the conditioned stimuli (P < 0.05 and P > 0.005, respectively). In four neurons facilitation or no effect was evoked at one conditioning–testing interval (0.6; 1, 1.5, 0.4 ms after the last relayed MLF volley), whereas depression appeared at other intervals, about 0.4 – 0.8 ms longer or shorter, with other stimulus parameters remaining the same. Again there were no statistically significant differences between effects of the test stimuli and significant differences between those of conditioned stimuli at different intervals, as illustrated in Fig. 6D. In the remaining interneurons the variability in effects of the MLF stimulation could not be associated with any single factor and was only noted.
FIG. 6.
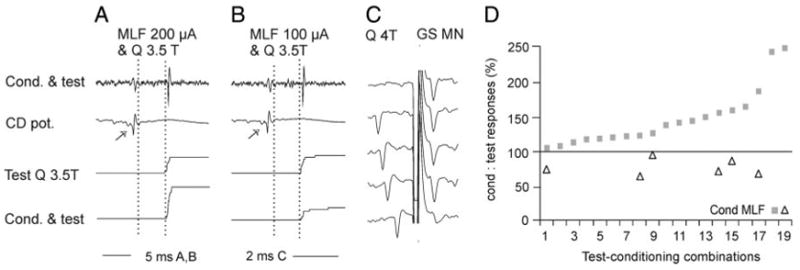
Modulation of activation of group II interneurons. A and B: data for an interneuron antidromically acticated from the gastrocnemius-soleus (GS) motor nucleus. Each panel shows (i) an example of records from the interneuron, (ii) records of afferent volleys after stimulation of the ipsilateral Q and of MLF, and (iii) cumulative sums of responses evoked by 20 consecutive test stimuli applied alone or preceded by conditioning stimuli. Conditioning stimuli in A were of 200 μA and in B of 100 μA; note that they were followed by an increase and a decrease in the number of responses, respectively. Dashed lines indicate the estimated time of arrival of afferent volleys in group II afferents and the shortest latencies of the test and conditioned responses. Arrows indicate the last descending relayed conditioning volleys. C: summary of effects from MLF on the whole sample of 16 interneurons (19 test-conditioning combinations). They are expressed in percentage increases, or decreases in the number of responses evoked by 2 or more series of 20 consecutive stimuli when these were preceded by stimulation of the MLF, compared with the number of responses evoked when the test stimuli were applied alone at optimal parameters.
Group II interneurons tested in the same way included 16 interneurons with input from Q and/or Sart, FDL, and DP (total of 19 test-conditioning combinations). The conditioning–testing intervals were 0.4 –1.8 ms between the relayed MLF volleys and group I components of the afferent volleys; they corresponded to about 1.2- to 2.6-ms intervals with respect to group II components of the afferent volleys.
MLF stimuli facilitated activation of all interneurons of this sample; the mean number of responses per 20 stimuli increased from 10 ± 0.9 to 13 ± 1.1 (130%, at P < 0.001) and their latency shortened by 0.24 ± 0.05 ms. However, when weaker or stronger MLF or test stimuli were used and when the conditioning–testing intervals were changed, activation of six of these interneurons was depressed (to 75%). Facilitation and depression are illustrated in Fig. 6, A and B, respectively. The data for the whole sample are plotted in Fig. 6C.
Modulation of synaptic actions of group Ib and group II afferents on motoneurons
We could not a priori predict whether moderate increases and/or decreases in the probability of activation of individual interneurons by group Ib and group II afferents described in the preceding section would essentially modify their actions on motoneurons. Effects of conditioning stimuli applied in the MLF were therefore tested on PSPs evoked from these afferents in motoneurons. The disadvantage of these tests was that they required additional tests to sort out which effects of conditioning stimuli were secondary to changes at a mo-toneuronal level and which at a premotoneuronal level. In addition, when PSPs evoked from peripheral nerves overlapped with PSPs evoked by commissural interneurons (at intervals expected to be most effective for facilitation of activation of the interneurons; about 1 ms from the descending volleys after the third or fourth MLF stimuli), their areas could not be directly measured. To circumvent the latter problem, sums of records after the test and conditioning stimuli alone were subtracted from the records after joint application of these stimuli and the difference was used as a measure of the facilitation or depression of the test PSPs. The procedure is illustrated in Fig. 7.
FIG. 7.
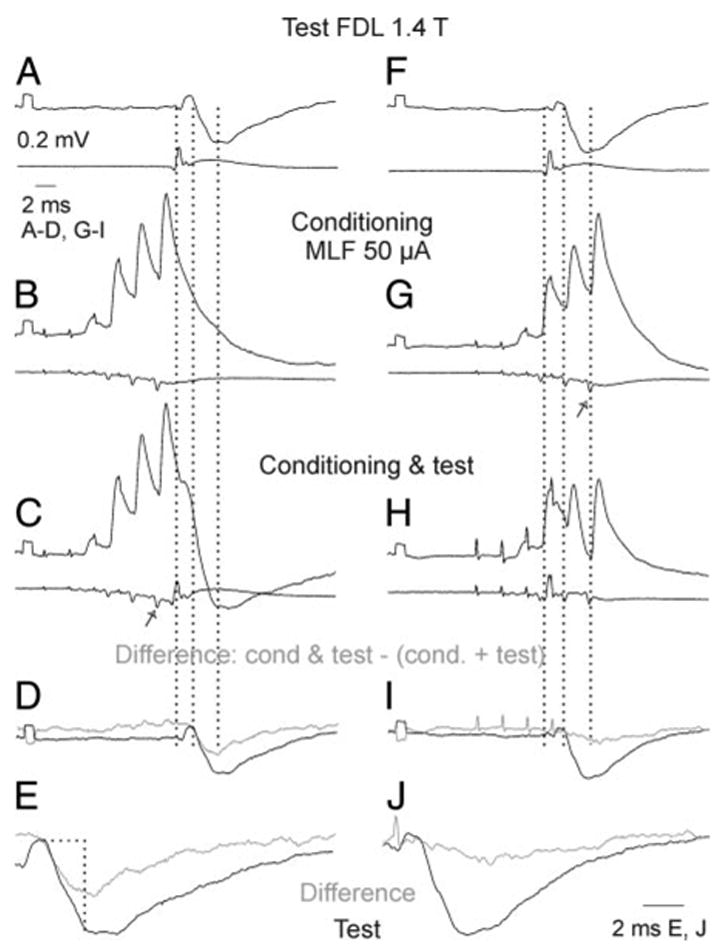
Facilitation of Ib IPSPs recorded in a GS motoneuron by conditioning stimulation of the contralateral MLF. Pairs of records in A–C and F–H are from a GS motoneuron and from the cord dorsum. A and F: PSPs evoked by test stimuli alone. B and G: PSPs evoked by conditioning stimuli alone. C and H: PSPs after both these stimuli. D–J: differences between the conditioned and test IPSPs (gray) superimposed on the test IPSPs (black), at the same scale and twice expanded. Areas that were measured are enclosed by the horizontal and vertical lines in E. Test and conditioning stimuli were timed in such a way that group I volleys indicated by the first dotted line in A–C and F–H were preceded by the last relayed MLF volley (arrow in C) in left panels or were preceded by the second MLF volley in right panels. Consequently the Ib IPSPs were superimposed on the decay phase of EPSPs evoked from MLF in C and coincided with these EPSPs in H. Second and third dotted lines indicate onset of the IPSPs and the end of the time windows used for the measurements of the areas of the IPSPs. Calibrations in A are for all records except E and J. Note that the relayed volleys reflecting activation of commissural interneurons were marginal after the first stimulus and increased after the successive stimuli. All records are averages of 20 single records.
MODULATION OF SYNAPTIC ACTIONS FROM IB AFFERENTS
Figure 7 shows that an IPSP of group Ib origin recorded in a GS motoneuron after stimulation of the MLF (C) was larger than the test IPSP (A). To quantify its increase, the sum of records in A and B was subtracted from the record C, and the net difference (D and E, gray traces) compared with the original IPSP. In the illustrated case the area within the rise time (2 ms) of the difference trace amounted to 80% of the area of the test IPSP. However, the increase might have been caused by the depolarization of the motoneuron during EPSPs evoked from the MLF. To estimate the relative role of such depolarization the interval between the test and conditioning stimuli was shortened in the series of records in Fig. 7, F–J, so that the IPSP was induced during the maximal depolarization of the motoneuron, but the afferent volley coincided with the commissural volley after the second rather than the more effective fourth MLF stimulus. The resulting increase in the IPSP (I and J) was then only marginal (by 7% of the test area). Similarly marginal increases were seen in four other motoneurons when the conditioning–testing intervals were reduced in the same way, indicating that the major facilitation of the IPSPs occurred at a premotoneuronal level and that only additional effects were secondary to the depolarization of the motoneurons.
The procedure illustrated in Fig. 7, A–E was used to estimate effects of conditioning MLF stimulation on Ib IPSPs evoked in 57 hindlimb motoneurons, including GS, Q, PL, FDL, ABSM, DP, and PBST motoneurons. In several of these, Ib IPSPs were evoked from more than one nerve, the total number of the test-conditioning combinations amounting to 78 (see Table 1). The test stimuli were applied to group I afferents of the PL, FDL, GS, Q, and ABSM nerves.
TABLE 1.
Summary of effects of conditioning stimulation of MLF on IPSPs and/or EPSPs evoked in hindlimb motoneurons from group Ib and group II afferents
| Increase
|
Decrease
|
Number
|
||||||
|---|---|---|---|---|---|---|---|---|
| Test PSPs | Mean + SE | Range | % MN | Mean + SE | Range | % MN | MN | Tests |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Group Ib IPSPs | 43 ± 4%*** | 3 to 161% | 86 | −18 ± 4%*** | −2 to −33% | 14 | 57 | 78 |
| Group Ib EPSPs | −43 ± 22%*** | −32 to −100% | 100 | 3 | 8 | |||
| Group II IPSPs | 28 ± 2%*** | 2 to 100% | 92 | −19 ± 3%*** | −2 to −61% | 24 | 49 | 80 |
| Group II EPSPs | 27 ± 3%*** | 3 to 105% | 48 | −24 ± 4%*** | −2 to −64% | 70 | 23 | 45 |
Increases and decreases in the areas of the early parts of the PSPs listed in column 1 are expressed as mean percentage differences between the test and conditioned PSPs (columns 2 and 5) and their ranges (columns 3 and 6). The areas were measured within time windows of 1.5–2 and 2–3 ms from the onset of the PSPs evoked from group Ib and group II afferents, respectively. Differences between test and conditioned PSPs were statistically significant at P < 0.001. In columns 4 and 7 are percentages of motoneurons in which the PSPs were increased or decreased by at least 10%, respectively. The decreases were often seen in the same motoneurons in which IPSPs or EPSPs evoked by group II afferents were facilitated when they were evoked at somewhat different stimulus intensities or from different nerves, or at different conditioning-testing intervals (see text for the reasons). The sums of percentages of motoneurons in which they were seen thus exceed 100%. In columns 8 and 9 are numbers of motoneurons tested and numbers of conditioning-test combinations. This table includes data for combinations in which intervals between the afferent and descending volleys (after the last of the conditioning stimuli) did not exceed 3 ms.
In motoneurons in which conditioning MLF stimuli evoked EPSPs, or a mixture of EPSPs and IPSPs (Jankowska et al. 2003), the predominant effect was facilitation of Ib IPSPs. The degree of facilitation varied depending on intervals between the conditioning and test stimuli and the intensity of these stimuli. The facilitation was usually most pronounced when the test stimuli were submaximal; when the conditioning stimuli were maximal (about 100–150 μA); and when the group I volleys were preceded by the third, fourth, or fifth descending volley by 0.5–3 ms. The much smaller difference between the conditioned and test IPSPs evoked by stronger (H) than by weaker (D) test stimuli (gray records) is illustrated in Fig. 8, A–H. When optimal parameters of the stimuli were used, Ib IPSPs were on the average increased by 43 ± 0.4% but ≤ 161% (Table 1, columns 2 and 3). No systematic differences were found in the degree of the facilitation depending on the kind of the motoneurons tested or the source of the IPSPs.
FIG. 8.
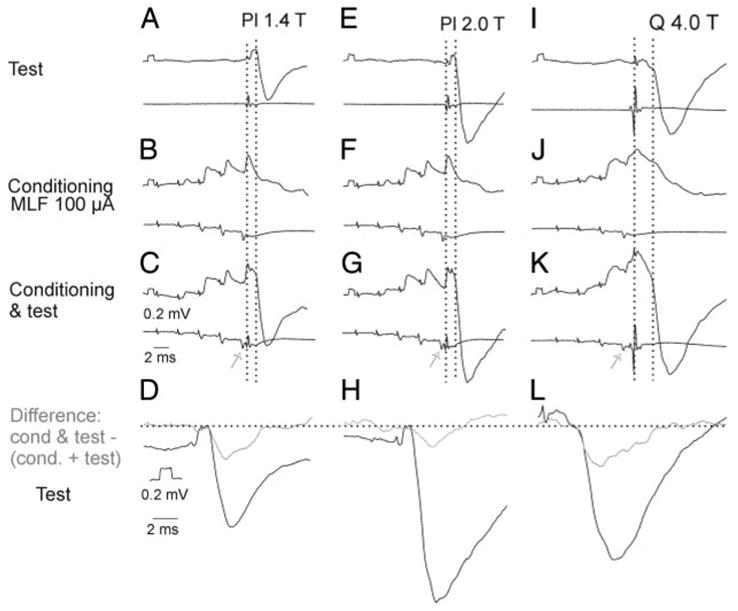
Facilitation of IPSPs evoked from group Ib and II afferents. A–H: records from a GS motoneuron arranged as in Fig. 7. A–C: PSPs evoked by stimuli submaximal for group I afferents alone, by conditioning stimuli alone, and by test stimuli preceded by conditioning stimuli. D: differences between the conditioned and test Ib IPSPs (gray), obtained as in Fig. 7, superimposed on the original IPSPs (black), both twice expanded. Horizontal dotted line provides a reference level for deviations between them. Test and conditioning stimuli were timed in such a way that the last relayed conditioning MLF volley (arrows in C, G, and K) preceded group I volleys indicated by the first group of vertical dotted lines by 0.6 ms. Second group of vertical dotted lines indicates the onset of the IPSPs. E–H: records from the same motoneuron when stronger test stimuli were used. Note in D and H that the difference between the test and conditioned IPSPs was larger in the case of IPSPs evoked by weaker stimuli. I–L: similar records from a plantaris (Pl) motoneuron illustrating facilitation of IPSPs evoked from group II afferents. Voltage and time calibrations in D are for records in D, H, and L. Calibrations in C are for all other records.
In eight motoneurons in which the predominant effect of MLF stimulation was inhibition, the Ib IPSPs were as a rule smaller than the sum of IPSPs evoked by separate MLF and peripheral stimuli, as illustrated in Fig. 9E and indicated in Table 1 (columns 5 and 6). This was in particular the case when IPSPs evoked by the conditioning and test stimuli coincided. One explanation of this effect would be occlusion of effects mediated by the same interneurons when stimuli applied to peripheral nerves were due to activate them during the refractory period after their activation by MLF stimuli. As occlusion would be an expression of excitation of Ib interneurons by commissural interneurons activated by reticulospinal neurons and depression by occlusion cannot be classified as a reflecting inhibition. Inhibitory commissural interneurons could nevertheless contribute to the depression of Ib IPSPs recorded in motoneurons because MLF stimuli evoked IPSPs in individual Ib interneurons and they decreased the probability of activation of these interneurons without a preceding activation. However, the relative contribution of occlusion and of genuine inhibition to the depression of Ib IPSPs could not be determined because IPSPs of MLF origin were found in practically all motoneurons in which the depression occurred. Furthermore, even when the depression was associated with EPSPs of MLF origin, these EPSPs were most often followed by IPSPs (see Jankowska et al. 2003). The depression was found in GS, Pl, Q, and PBST motoneurons and involved IPSPs evoked from ABSM, Pl, FDL, and GS group I afferents.
FIG. 9.
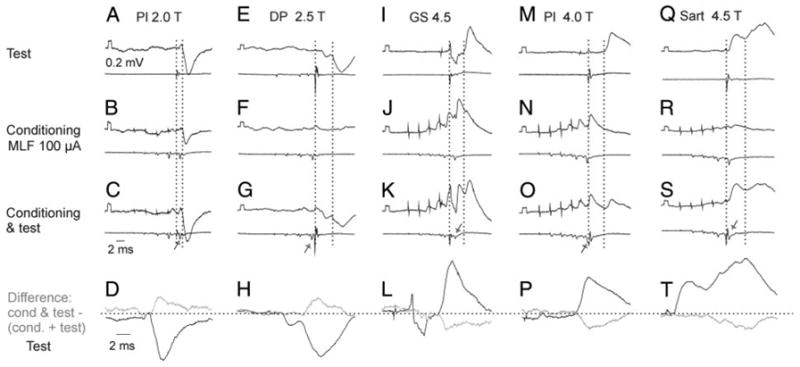
Examples of depression of PSPs evoked from group Ib and group II afferents after MLF stimulation. A–D, E–H, I–L, M–P, and Q–T: records from 2 GS, 2 posterior biceps and semitendinosus (PBST), and one deep peroneal (DP) motoneuron, respectively. Same format as in Fig. 8. Intervals between the last relayed MLF volleys (arrows) and group I volleys in C, G, K, O, and S were −1.1, 0.9, −1.3, 0.3, and −0.8 ms, respectively. They would correspond to intervals of about 0.1, 2.1, −0.1, 1.5, and 0 ms with respect to afferent volleys in the fastest conducting group II afferents in the stimulated nerves that are delayed with respect to group I volleys. Note the depression of the second component (of group II origin) of the PSP in H and T, but not of the first component, which may be attributed to Ia afferents (Ia reciprocal inhibition from DP to GS motoneuron in E–H; monosynaptic Ia EPSP, as judged by its segmental latency of 0.8 ms in Q–T).
EPSPs of Ib origin were found in only three motoneurons. In all these motoneurons, and in all eight conditioning–testing combinations, the size of the EPSPs was strongly reduced (Table 1, columns 5 and 6). However, we could not determine the relative contribution of occlusion and of genuine inhibition to their depression because all of the test EPSPs overlapped with EPSPs evoked by conditioning stimuli at the optimal conditioning testing intervals.
MODULATION OF SYNAPTIC ACTIONS OF GROUP II INTERNEURONS
Effects of MLF stimulation on PSPs evoked from group II afferents were analyzed in 49 motoneurons, including GS, FDL, PL, Q, ABSM, PBST, and DP motoneurons. The latencies of these PSPs were 2–3.8 ms from group I volleys from Q and Sart and 2.5–4.2 ms from PBST, DP, GS, Pl, and FDL. Considering that the arrival of nerve impulses in the fastest group II afferents is delayed with respect to group I afferents by 0.6–0.9 ms and 1.2–2 ms from branches of the femoral and sciatic nerves, respectively, latencies of these PSPs would correspond to latencies of 1–3 ms from group II volleys. Latencies of some of the tested IPSPs and EPSPs would thus be compatible with disynaptic coupling (see Jankowska et al. 2005b) but the other ones could be evoked trisynaptically. The effects of conditioning stimuli were analyzed within time windows of 2–3 ms from the onset of these PSPs, depending on their slope, and likewise could involve both di- and trisynaptically evoked components.
As shown in Table 1 (columns 2 and 3), IPSPs and EPSPs of group II origin were facilitated from MLF to a smaller degree than Ib IPSPs. Marked facilitation was nevertheless also seen, with an example in Fig. 8, I–L.
When IPSPs and EPSPs evoked from group II afferents were depressed by conditioning stimuli, the degree of depression was generally like that of Ib IPSPs and no systematic differences were found to be related to the kind of the motoneurons tested or the source of the IPSPs. As in the case of Ib IPSPs, the relative contribution of occlusion and of genuine inhibition at a premotoneuronal level to this depression could not be defined because the depression of EPSPs and IPSPs of group II origin was as a rule seen in motoneurons in which conditioning stimuli themselves evoked EPSPs and IPSPs, respectively. Furthermore, the effects of conditioning stimuli often changed from depressive to facilitatory when either the intensity of the test stimuli or conditioning–testing intervals were changed. However, in several cases inhibition appeared to be more likely. For instance when EPSPs from group II afferents were evoked during EPSPs of MLF origin (at intervals as short as those in Fig. 9K), the relative contribution of occlusion might be more important than that of inhibition. However, when group II EPSPs were evoked at longer intervals between MLF and group II stimuli (as in Fig. 9O), at which EPSPs of MLF origin were subject to temporal facilitation, the relative contribution of inhibition should be more decisive. Records in Fig. 9, Q–S provide another example of a decrease of group II EPSPs that could hardly be explained by occlusion in view of only negligible EPSPs after MLF stimuli. Whatever the mechanism of the depression, when it occurred, it was restricted to the IPSPs (Fig. 9H) or EPSPs (Fig. 9T) of group II origin and did not involve earlier group Ia components, thus increasing the confidence that it occurred at a premotoneuronal level.
DISCUSSION
The results of this study show that commissural interneurons activated by reticulospinal neurons have direct contacts with interneurons mediating reflex actions from group Ib tendon organ afferents and group II muscle spindle afferents and modulate actions of these interneurons. Interneurons with input from group Ib tendon organ afferents and group II muscle spindle afferents may thus be added to the list of ipsilaterally acting premotor interneurons involved in bilateral coordination of movements by commissural interneurons activated by contralaterally descending reticulospinal neurons. Previously investigated premotor interneurons include Ia inhibitory interneurons affected by commissural interneurons with input from reticulospinal as well as vestibulospinal neurons or from group II and other high-threshold muscle, skin, and joint afferents (Bruggencate and Lundberg 1974; Bruggencate et al. 1969). They also include Renshaw cells reported to be excited or inhibited by as yet unspecified commissural neurons (Nishimaru et al. 2004) and commissural interneurons themselves found to be contacted by other likewise unspecified commissural interneurons located on the other side of the gray matter (Birinyi et al. 2003). For reviews of involvement of these interneurons in actions mediated by ipsilaterally descending corticospinal, rubrospinal, vestibulospinal, and reticulospinal tract fibers see Baldissera et al. (1981) and Jankowska (1992).
Coupling between commissural interneurons and group Ib and group II interneurons
The conclusion that there exists a monosynaptic coupling between commissural interneurons and interneurons in pathways from group Ib and group II afferents to motoneurons—i.e., a disynaptic coupling between contralateral reticulospinal tract fibers in the MLF and interneurons of groups Ib and II—is based on intracellular records from individual interneurons. As shown in the first section of RESULTS, several EPSPs and IPSPs of MLF origin were evoked at similar latencies. Latencies of 1.3–1.8 ms from the first components of the MLF descending volleys were within the same range as of EPSPs and IPSPs evoked in motoneurons that fulfilled criteria of disynaptically evoked PSPs (Jankowska et al. 2003). Terminal potentials (Munson et al. 1980) that tightly followed direct descending volleys and preceded EPSPs and IPSPs (as in Fig. 2K) further support the conclusion that inhibitory as well as excitatory commissural interneurons have direct actions on interneurons of groups Ib and II. Some EPSPs that were evoked at latencies about 0.3 ms shorter (1.0 –1.3 ms) were classified as evoked disynaptically, despite such short latencies, because they fulfilled the temporal facilitation criterion of disynaptically evoked PSPs. The reason was that the shorter latencies of PSPs evoked in interneurons than in motoneurons might be accounted for by different segmental levels of location of motoneurons (most in the L7 segment) and of the interneurons (in the L4 –L6 segments).
The shortest-latency components of a number of EPSPs were nevertheless even shorter (≤0.9 ms) and their onset preceded the onset of the relayed MLF volleys or coincided with its raising phase. They could thus be attributed to monosynaptic actions of crossed axon collaterals of reticulospinal tract fibers (Matsuyama et al. 1993, 1999), especially because the temporal facilitation involved primarily only later components of these EPSPs. Such early components were found in 8/13 EPSPs in Ib interneurons and in 2/10 EPSPs in group II interneurons. The proportion of interneurons directly contacted by crossed axon collaterals of reticulospinal tract neurons indicated by these observations [10/23 (38%)] would thus be higher than that of motoneurons [14/108 (13%); Jankowska et al. 2003].
Commissural interneurons affect both inhibitory and excitatory interneurons
Table 1 and Figs. 7–9 show that both EPSPs and IPSPs of group Ib and group II origin recorded in motoneurons were affected by conditioning MLF stimulation. Our results thus show that actions of both excitatory and inhibitory interneurons in reflex pathways from group Ib and group II afferents are modulated by commissural interneurons activated by reticulospinal neurons and that both excitatory and inhibitory sub-populations of these neurons are involved in coordinating motor activity on both sides of the body. Even if some facilitatory effects may be attributed to monosynaptic actions of crossed axon collaterals of reticulospinal tract fibers and some facilitatory and/or depressive effects were secondary to PSPs evoked in motoneurons, our observations indicate that they might be responsible for only a fraction of crossed actions that we found in the present study and that their bulk must reflect actions of commissural interneurons at the level of group Ib and group II interneurons.
Functional consequences of actions of commissural interneurons
It was a recurring finding that activation of extracellularly recorded interneurons could be either facilitated or depressed by commissural interneurons and that commissural interneurons had opposite actions on PSPs of group Ib or group II origin evoked in motoneurons. Excitatory and inhibitory commissural interneurons thus appear to provide two parallel lines of crossed actions and the final outcome of these actions must depend on different proportions of excitatory and inhibitory commissural interneurons activated under different behavioral situations. The variability in modulation of synaptic actions of group Ib and group II afferents on motoneurons by RS neurons would also indicate that the balance between actions of excitatory and inhibitory commissural interneurons on premotor interneurons might depend on very small changes in the timing of activation of reticulospinal neurons with respect to the peripheral stimuli, and perhaps also on subpopulations of reticulospinal neurons recruited.
The degree of facilitation and/or depression of synaptic actions of group Ib and group II afferents on motoneurons by RS neurons found in the present study was most likely underestimated because of both the anesthesia and our experimental paradigm. For instance, we had to use intervals that were longer than the likely optimal intervals between conditioning and testing stimuli because IPSPs or EPSPs evoked from group Ib and group II afferents in motoneurons usually coincided with EPSPs and/or IPSPs evoked from the MLF. Despite this the effects of MLF stimulation indicate that reticulospinal neurons and commissural interneurons may considerably increase the flexibility in the use of interneurons activated by afferents of groups Ib and II in different behavioral situations. They might, for example, contribute to the switching from Ib inhibition of extensor motoneurons under resting conditions to Ib excitation during locomotion (Angel et al. 1996, 2005; Gossard et al. 1994; Guertin et al. 1995; McCrea et al. 1995). They might also be involved in the selection of the most appropriate crossed reflex actions of group II afferents together with the descending monoaminergic tract neurons (Aggelopoulos and Edgley 1995; Aggelopoulos et al. 1996; Arya et al. 1991) and in the resetting of the locomotor and scratch cycles by group Ib afferents (Conway et al. 1987; Perreault et al. 1999) and group II afferents (Perreault et al. 1995; Stecina et al. 2005). Lack of modulatory actions of reticulospinal neurons and commissural interneurons might also contribute to the dramatic changes in actions of group Ib or group II afferents after lesions at different levels of the brain stem and after spinalization (Holmqvist and Lundberg 1959, 1961; Lundberg 1982).
Theoretically, subpopulations of RS neurons and commissural interneurons could also favor group Ib and group II actions on particular motor nuclei and contribute to different motor synergies because stimuli applied at different sites in the brain stem activate different groups of muscles on both sides of the body and different postural and locomotor reactions (Mori et al. 1989). However, our experimental conditions did not allow us to explore this possibility, both because we stimulated axons of any RS neurons running in the MLF and because the reduced and anesthetized preparations that we used are not suitable for such analysis. We can only conclude that postsynaptic actions evoked in any motor nuclei by group Ib and/or group II afferents from any muscles could be modified by RS neurons by commissural interneurons. Even though inhibition from these afferents dominates in extensors and excitation in flexors (Eccles and Lundberg 1959; Eccles et al. 1957), EPSPs and IPSPs of group Ib and group II origin evoked in either extensor or flexor motoneurons appeared to be affected in a similar way. Investigation of a potential selection of interneurons that affect particular combinations of motoneurons would require another experimental approach.
Contribution of RS neurons and commissural interneurons to movement coordination will be hampered when input to these neurons is reduced after various central injuries, cortical or subcortical. To improve the recovery of motor functions after these injuries it might therefore be useful to increase the relative contribution of peripheral input to activation of both the commissural interneurons and interneurons by which they affect motoneurons and any activity-dependent plasticity in their operation. On the basis of the present study and previous studies it might therefore be useful to include enhanced activation of spinal neuronal networks in the rehabilitation procedures, not only by such means as locomotion or dorsal column stimulation, but also under conditions when afferents of groups Ia, Ib, and II are specifically activated.
Acknowledgments
We thank Dr. I. Hammar for comments on the manuscript and R. Larsson for invaluable assistance.
Present address of A. Cabaj: Department of Neurophysiology, Nencki Institute of Experimental Biology, 02– 093 Warsaw, Poland.
Footnotes
GRANTS
The study was supported by National Institute of Neurological Disorders and Stroke Grant NS-40863 and Swedish Research Council Grant 15393-01A.
References
- Aggelopoulos NC, Burton MJ, Clarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. Neuroscience. 1996;16:723–729. doi: 10.1523/JNEUROSCI.16-02-00723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggelopoulos NC, Edgley SA. Segmental localisation of the relays mediating crossed inhibition of hindlimb motoneurones from group II afferents in the anaesthetized cat spinal cord. Neurosci Lett. 1995;185:60 – 64. doi: 10.1016/0304-3940(94)11225-8. [DOI] [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jimenez T, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol. 1996;494:851– 861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel MJ, Jankowska E, McCrea DA. Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol. 2005;563:597– 610. doi: 10.1113/jphysiol.2004.076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM. Supraspinal contributions to the initiation and control of locomotion in the cat. Prog Neurobiol. 1986;26:273–361. doi: 10.1016/0301-0082(86)90021-3. [DOI] [PubMed] [Google Scholar]
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol. 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S, Edgley SA, Harrison PJ. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol. 1992;455:205–217. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Handbook of Physiology. The Nervous System. Motor Control. Am Physiol Soc. sect 1, vol II. Bethesda, MD: 1981. Integration in spinal neuronal systems; pp. 509–595. [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Weber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol. 2003;461:429 – 440. doi: 10.1002/cne.10696. [DOI] [PubMed] [Google Scholar]
- Bruggencate GT, Burke R, Lundberg A, Udo M. Interaction between the vestibulospinal tract, contralateral flexor reflex afferents and la afferents. Brain Res. 1969;14:529 –532. doi: 10.1016/0006-8993(69)90131-0. [DOI] [PubMed] [Google Scholar]
- Bruggencate GT, Lundberg A. Facilitatory interaction in transmission to motoneurones from vestibulospinal fibres and contralateral primary afferents. Exp Brain Res. 1974;19:248 –270. doi: 10.1007/BF00233233. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643– 656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Brain Res Rev. 2002;40:166 –177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions in motoneurones caused by impulses in Golgi tendon afferents. J Physiol. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Arch Ital Biol. 1959;97:199 –221. [Google Scholar]
- Edgley SA, Jankowska E. Field potentials generated by group II muscle afferents in the middle lumbar segments of the cat spinal cord. J Physiol. 1987;385:393– 413. doi: 10.1113/jphysiol.1987.sp016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. Neuroscience. 2004;24:7804 –7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter MK, Sholomenko GN, Gossard JP, Burke RE. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res. 1993;92:407– 419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Floeter MK, Degtyarenko AM, Simon ES, Burke RE. Disynaptic vestibulospinal and reticulospinal excitation in cat lumbosacral motoneurons: modulation during fictive locomotion. Exp Brain Res. 1996;109:277–288. doi: 10.1007/BF00231787. [DOI] [PubMed] [Google Scholar]
- Grillner S, Dubuc R. Control of locomotion in vertebrates: spinal and supraspinal mechanisms. Adv Neurol. 1988;47:425– 453. [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Convergent effects on alpha motoneurones from the vestibulospinal tract and a pathway descending in the medial longitudinal fasciculus. Exp Brain Res. 1971;12:457– 479. doi: 10.1007/BF00234243. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. The origin of a descending pathway with monosynaptic action on flexor motoneurones. Acta Physiol Scand. 1968;74:274 –284. doi: 10.1111/j.1748-1716.1968.tb04236.x. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist B, Lundberg A. On the organization of the supraspinal inhibitory control of interneurones of various spinal reflex arcs. Arch Ital Biol. 1959;97:340 –356. [Google Scholar]
- Holmqvist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiol Scand Suppl. 1961;186:1–15. [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol. 1983a;342:145–159. doi: 10.1113/jphysiol.1983.sp014844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983b;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB. Studies in Neurophysiology. Cambridge, UK: Cambridge Univ. Press; 1978. Some methods for selective activation of muscle afferent fibres; pp. 155–176. [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. Neuroscience. 2005a;25:7401–7405. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005b;565:645– 658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo Lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur J Neurosci. 1997;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. Neuroscience. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Matsuyama K. Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol. 2005c;568:617– 628. doi: 10.1113/jphysiol.2005.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM. Neurobiological Basis of Human Locomotion. Tokyo: Japan Scientific Societies Press; 1991. Brainstem and spinal cord mechanisms for the initiation of locomotion; pp. 3–20. [Google Scholar]
- Lundberg A. Brain Stem Control of Spinal Mechanisms. Amsterdam: Elsevier Biomedical Press; 1982. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways; pp. 179–225. [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol. 2004;91:1183–1192. doi: 10.1152/jn.00896.2003. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi Y, Takakusaki K, Mori S, Kimura H. Termination mode and branching patterns of reticuloreticular and reticulospinal fibers of the nucleus reticularis pontis oralis in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1993;17:9 –21. doi: 10.1016/0168-0102(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413– 430. [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res. 2004a;143:239 –249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori S. Lumbar interneurons involved in the generation of fictive locomotion in cats. Ann NY Acad Sci. 1998;860:441– 443. doi: 10.1111/j.1749-6632.1998.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004b;474:546 –561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Iwakiri H, Homma Y, Yokoyama T, Matsuyama K. Neuro-anatomical and neurophysiological bases of postural control. Adv Neurol. 1995;67:289 –303. [PubMed] [Google Scholar]
- Mori S, Sakamoto T, Ohta Y, Takakusaki K, Matsuyama K. Site-specific postural and locomotor changes evoked in awake, freely moving intact cats by stimulating the brainstem. Brain Res. 1989;505:66 –74. doi: 10.1016/0006-8993(89)90116-9. [DOI] [PubMed] [Google Scholar]
- Munson JB, Fleshman JW, Sypert GW. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980;44:713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Kiehn O. Rhythmicity and commissural inputs to Renshaw cells in mice. Soc Neurosci Abstr. 2004:883.821. [Google Scholar]
- Nyberg-Hansen R, Mascitti TA. Sites and mode of termination of fibers of the vestibulospinal tract in the cat. J Comp Neurol. 1964;122:369 –387. doi: 10.1002/cne.901220307. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Grillner S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J Neurophysiol. 1989;62:1079 –1089. doi: 10.1152/jn.1989.62.5.1079. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Perreault MC, Angel MJ, Guertin P, McCrea DA. Effects of stimulation of hindlimb flexor group II afferents during fictive locomotion in the cat. J Physiol. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault MC, Enriquez-Denton M, Hultborn H. Proprioceptive control of extensor activity during fictive scratching and weight support compared to fictive locomotion. Neuroscience. 1999;19:10966 –10976. doi: 10.1523/JNEUROSCI.19-24-10966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW, Felpel LP. Excitation and inhibition of reticulospinal neurons by vestibular, cortical and cutaneous stimulation. Brain Res. 1971;27:373–376. doi: 10.1016/0006-8993(71)90264-2. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Blagovechtchenski E, Perfiliev S, Krasnochokova E, Lundberg A. Recovery of food-taking in cats after lesions of the corticospinal (complete) and rubrospinal (complete and incomplete) tracts. Neurosci Res. 2000;38:109 –112. doi: 10.1016/s0168-0102(00)00143-7. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Langlet C, Barthelemy D, Chau C, Giroux N, Brustein E, Marcoux J, Leblond H, Reader TA. Determinants of locomotor recovery after spinal injury in the cat. Prog Brain Res. 2004;143:163–172. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Stecina K, Quevedo J, McCrea DA. Parallel reflex pathways from flexor muscle afferents evoking resetting and flexion enhancement during fictive locomotion and scratch in the cat. J Physiol. 2005;569:275–290. doi: 10.1113/jphysiol.2005.095505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Vestibulospinal and reticulospinal effects on hindlimb, forelimb, and neck alpha motoneurons of the cat. Proc Natl Acad Sci USA. 1968;60:836 – 840. doi: 10.1073/pnas.60.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]


