Abstract
Aim
To elucidate the hormonal influences on sex differences in knee joint behavior, normal-menstruating females were compared to males on serum hormone levels and anterior knee joint laxity (displacement at 46N, 89N and 133N) and stiffness (Linear slope of ΔForce/ΔDisplacement for 46–89N and 89–133N) across the female menstrual cycle.
Methods
Twenty-two females were tested daily across one complete menstrual cycle, and 20 males were tested once per week for 4 weeks. Five days each representing the hormonal milieu for menses, the initial estrogen rise near ovulation, and the early and late luteal phases (total of 20 days) were compared to the average value obtained from males across their 4 test days.
Results
Sex differences in knee laxity were menstrual cycle dependent, coinciding with significant elevations in estradiol levels. Females had greater laxity than males on day 5 of menses, days 3–5 near ovulation, days 1–4 of the early luteal phase and days 1, 2, 4 and 5 of the late luteal phases. Within females, knee laxity was greater on day 5 near ovulation compared to day 3 of menses, and days 1–3 of the early luteal phase compared to all days of menses and day 1 near ovulation. On average, differences observed between sexes were greater than those within females across their cycle. There were no differences in anterior knee stiffness between sexes or within females across days of the menstrual cycle.
Conclusion
These results suggest sex hormones may be a primary mediator of the observed sex differences in knee laxity.
Keywords: Anterior knee displacement, Stiffness, Sex hormones, ACL injury risk factors
It is generally accepted that females have greater anterior knee joint laxity than males 1–4 which has received attention as a potential risk factor for non-contact anterior cruciate ligament (ACL) injury.5, 6 Sex differences in knee laxity appear to be restricted to adults, as similar differences have not been reported in preadolescents.7 This has led investigators to explore the influence of female endocrinology on knee joint behavior, as males and females differ substantially in the type, level, and periodic exposure of circulating sex hormones after puberty. While hormone levels remain fairly constant in males, females are exposed to rhythmic fluctuations in endogenous hormones during the course of the menstrual cycle, with the absolute levels of estrogen and progesterone varying considerably during the course of a cycle.8 Although rarely considered, testosterone levels also fluctuate somewhat across the menstrual cycle and can vary by phase (i.e. follicular vs luteal).9–11
Basic science studies have revealed that estrogen and progesterone receptors are present on the human ACL,12, 13 implying that sex hormones may directly influence the structure and composition of the ACL.12, 14, 15 Yu et al.16 found that when the ligament is exposed to increased estrogen levels, there is a dose dependent antagonist effect on fibroblast proliferation and procollagen synthesis that is attenuated within 3 to 7 days following administration. Consequently, research has examined how changes in sex hormone levels affect knee laxity in normal menstruating 17–19 and pregnant 20, 21 females. In normal menstruating females, significant increases in knee laxity have been noted in the periovulatory and mid luteal phases of the menstrual cycle compared to menses,18, 19 as defined by time periods that are thought to coincide with elevated levels of estrogen, and estrogen and progesterone respectively. In support of these findings, greater anterior knee laxity has been observed in pregnant females 20, 22 when estrogen and progesterone levels are dramatically increased.23 The hormone relaxin has also been implicated as a mediator of ligament behavior, but recent research has failed to find any relationship between relaxin and knee laxity either in normal menstruating 17 or pregnant 22 females.
While a relationship appears to exist between sex hormones and anterior knee laxity, there is much we still do not understand about the potential hormonal effects on knee joint behavior. Because previous investigations have pooled their data across multiple days to represent a particular phase of the menstrual cycle, the magnitude of change in knee joint behavior from day to day as hormones fluctuate cannot be determined. Further, it is unclear whether knee laxity remains elevated throughout these phases, or if changes are more transient in nature as hormone levels rise and fall.19 Considering that hormone levels can change as much as 400 fold over a 4-hour period near ovulation,8 and that hormonal effects on collagen may attenuate after a few days of exposure,16 it is plausible that knee joint laxity may vary considerably from day to day within a phase.
Although research has consistently found greater anterior knee laxity in females compared to males,1–4 it is unknown whether these sex differences are always present or may be dependent on the phase of the menstrual cycle. In studies examining sex differences in knee laxity, we were unable to find any that had documented or controlled menstrual cycle phase. Conversely, among studies examining menstrual cycle effects on knee laxity, we were unable to find any that used males in comparison as a control. While one study 18 tested a small cohort of males (n=8) and found no changes in knee joint displacement or stiffness across time, they reported no comparisons between sex. Hence, to further elucidate sex and hormonal influences on knee joint behavior, our purpose was to compare males and females on anterior knee joint laxity and linear stiffness at multiple days across the female menstrual cycle.
Materials and methods
Twenty-two normal-menstruating females [23±3.5 y, 163.4±5.6 cm, 65±11.6 kg, 24.3±3.5 body mass index (BMI)] and 20 males (23.3±2 y, 181.5±7.8 cm, 78.2±11.1 kg, 23.7±2.7 BMI), with a BMI (BMI = weight/height2) less than or equal to 30, volunteered to participate. Ethnic and racial demographics were consistent with those of the University and surrounding community (females = 14 White [1 Hispanic], 4 Asian, and 4 Black; males = 18 White [2 Hispanic]; 1 Asian and 1 Black]). A sample size of 20 subjects per group was determined a priori through pilot analysis. Inclusion criteria for females were: 1) no history of pregnancy, 2) no use of oral contraceptives or other hormone stimulating medications for 6 months, 3) non smoking behavior, 4) two healthy knees with no prior history of joint injury or surgery, 5) no medical conditions affecting the connective tissue (e.g. Marfan’s syndrome, Ehlers-Danlos disease, rheumatoid arthritis, etc.), and 6) physical activity limited to 7 hours or less per week. Inclusion criteria for males consisted of items 3–6 above. All subjects were informed of the study and associated risks, and signed an informed consent approved by the University of Virginia Health System’s Human Investigation Committee. The study was also approved by the University’s General Clinical Research Center’s Research Advisory Committee.
Procedures
All testing was performed in the University’s General Clinical Research Center. Females were tested daily across one complete menstrual cycle, and males were tested one time per week for 4 weeks. To control for diurnal fluctuations in hormone levels, testing was performed at the same general time of day. Females were tested between the hours of 8:00 a.m. – 12:00 N and males were tested between the hours of 8:00 a.m. – 10:00 a.m. Within these time blocks, repeat test sessions were scheduled as close to the same time each day as the participant’s schedule allowed.
Female participants were counterbalanced to begin and end data collection either at ovulation (detection of rise in luteinizing hormone) or the onset of menses (self-report). An independent investigator assigned the counterbalance so that the test examiner was blinded to the participant’s time in the cycle. To estimate day of ovulation, each female was provided a commercially available ovulation kit (CVS One Step Ovulation Predictor [Sensitivity 20 mIU/ml LH, Accuracy 99%]; CVS Corporation, Woondsocket, RI, USA) to use beginning on day 8 of their menstrual cycle, and they were asked to report to the research study coordinator the day the test became positive. In addition to serving as a marker to begin and end data collection, a positive test provided indirect confirmation that a normal ovulatory menstrual cycle had occurred and that the subject was not pregnant.
All subjects underwent the same data collection procedures on each day of testing. Upon arrival, 5–7 cc of venous blood were withdrawn to assay serum levels of estradiol (pg/mL), progesterone (ng/mL) and testosterone (ng/mL). Anterior knee joint laxity and stiffness were measured with the KT 2000™ knee arthrometer (MEDmetric® Corp; San Diego, CA, USA). Subjects were positioned in supine per manufacturer’s guidelines, with a thigh support placed just proximal to the popliteal fossa and the knee in ~25 degrees of flexion. The subject’s ankles were placed in the manufacturer provided foot cradle, and a Velcro strap was placed around the subject’s thighs to control rotation of the lower extremity. Once positioned, the KT-2000™ was applied to the anterior tibia of the lower extremity (side counterbalanced between participants) in proper alignment with the subject’s joint line. After instructing the subject to relax the leg muscles, a anterior-to-posterior directed force was applied to the anterior tibia to identify a stable neutral point from which measures were based. An anteriorly directed force just over 133N was then applied, using a bubble level affixed to the device to insure a direct anterior pull was achieved. Five trials were collected each day of testing.
Because of the daily data collection demands of this study, two investigators were trained to perform knee laxity measures. Both testers participated in extensive pilot testing to establish acceptable inter-tester and intra-tester reliability. When compared over 2 sets of 5 trials on 2 separate days, knee laxity measures were found to be consistent both within (ICC [2,k]=0.97, SEM=0.38 [T1]; ICC [2,k]=0.95, SEM=0.38 mm [T2]) and between (ICC [2,k]=0.92, SEM=0.50) testers.24
Data reduction and analyses
Estradiol (pg/mL) was analyzed using a double-antibody RIA Assay (DSL-4400; Diagnostic Systems Laboratories, Webster, TX, USA). Progesterone (ng/mL) and testosterone (ng/mL) levels were analyzed using chemiluminescence assays (Coat-A-count; Diagnostic Products Corporation, Los Angeles, CA, USA). Coefficient of variations (% CV) for each hormone assay ranged from 3.9–14.1% (intra-assay) and 2.8–16.3% (inter-assay) for estradiol, 3.4–10% (intra-assay) and 3.8–12% (inter-assay) for progesterone, and 3.4–10% (intra-assay) and 3.8–12% (inter-assay) for testosterone.
The continuous raw force and displacement data from the KT 2000™ between 0–133N were exported into Microsoft Excel® (Microsoft Corp.; Redmond, WA, USA) to calculate knee laxity and stiffness measures. Laxity was recorded as the amount of anterior tibial displacement (mm) each at 46, 89 and 133N. Stiffness was recorded as the linear slope of the line (ΔForce/ΔDisplacement) between 46–89N and between 89–133N. The average value of the middle three trials for each variable and day was used for data analyses.
Because of the substantial variability in cycle length and hormone phasing between female subjects, data for all females were aligned at the onset of menses, the initial rise in estradiol near ovulation, the initial rise in progesterone at the onset of the early luteal phase, and the initial decline in progesterone levels to identify the late luteal phase. We aligned the data in this manner so that knee laxity and stiffness data were representative of a similar hormonal milieu across all subjects. The onset of menses was defined as the 1st day of menstrual bleeding, per self-report. The initial rise in estradiol near ovulation was defined for each subject as the day when their estradiol level exceeded 2 standard deviations of their average level during the first 5 days of menses. The initiation of the early luteal phase was defined as the day when progesterone exceeded 2 ng/mL.25 The start of the late luteal phase was defined as the day that progesterone levels initially declined (1st day after peak level). Once data were aligned using these time points, we were able to identify 5 consecutive days for each defined phase (20 total days) that were sufficiently representative of all females (i.e. no more than 3 subjects were missing values for any given day). In the few cases where data were not available on a particular subject for one of the latter days of the early or late luteal phases (due to a short luteal phase), the series mean was used.
As hormone and knee joint behavior was not expected to change over time for the males, a single representative value for each variable across the 4 test days was used to compare against each of the measurement time points for females. To confirm there were no significant differences in males across the 4 test days, a repeated measures ANOVA with one within variable (days) was run on each measure prior to obtaining the combined average value.
Three separate repeated measures ANOVA were used to compare serum levels of estradiol, progesterone and testosterone between sex and within females across their menstrual cycle. To quantify and compare differences in knee joint laxity at anterior directed loads of 46N, 89N and133N, we used a repeated measures ANOVA with one between (sex) and two within (anterior load, days) to compare between sex and within females across the 20 represented days of the menstrual cycle. A similar analysis was used to quantify and compare differences in knee joint stiffness at low (46–89N) and high (89–133) force ranges. Posthoc testing consisted of repeated contrasts within and simple main effects testing between to further explore significant findings. Bonferroni corrections were used for multiple contrasts. However, given the number of multiple contrasts across days, this method was too conservative to assess differences in knee laxity within females. Hence, the least significant difference pairwise multiple comparison test was used as the follow-up analysis for these data. All data were analyzed using the SPSS Statistical Software Package version 11.0 (Allegiant Technologies, Inc.). Alpha level was set a priori at P<0.05.
Results
Means (±SD) for serum hormone levels, and knee laxity and stiffness values for females across the 20 days and for males (average of the 4 test days) are reported in Table I. No significant differences were found across the 4 test days for males for estradiol (F[3,60]=0.06, P=0.98, β=0.06), progesterone (F[3,60]=1.033, P=0.385, β=0.267), testosterone (F[3,60]=1.88, P=0.143, β=0.463), knee laxity (F[3,6]=0.037, P=0.99, β=0.056), or knee stiffness (F[3,60]=0.51, P=0.677, β=0.148). Given the stability of these measures across repeated tests, the average value for each variable across the 4 test days was then compared to each of the 20 measurement time points for females. Because the same value is repeated across all 20 test days for males, statistical results for within subject differences by days and for days by sex in the following ANOVA models are identical, as any differences in either of these results are solely due to changes within females. Hence, the interaction between sex and days are reported as representative of both.
Table I.
Means (±SD) for serum hormone levels, knee laxity and knee stiffness values for females and males.
| Females (No.=22) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | 01 | 02 | 03 | 04 | 05 | EL1 | EL2 | EL3 | EL4 | EL5 | LL1 | LL2 | LL3 | LL4 | LL5 | Males Mean* |
|
| Estradiol (pg/mL) | |||||||||||||||||||||
| 50 (14) |
46 (19) |
56 (12) |
57 (14) |
59 (174) |
99 (19) |
111 (35) |
131 (50) |
158 (65) |
175 (51) |
95 (30) |
96 (29) |
106 (40) |
120 (31) |
129 (31) |
138 (39) |
125 (35) |
106 (36) |
81 (25) |
64 (21) |
55 (10) |
|
| Progesterone (ng/mL) | |||||||||||||||||||||
| 1 (0.5) |
0.9 (0.4) |
0.8 (0.4) |
0.9 (0.3) |
0.8 (0.3) |
0.8 (0.3) |
0.7 (0.3) |
0.7 (0.2) |
0.9 (0.3) |
1.2 (0.4) |
2.7 (0.7) |
4.6 (1.5) |
7.2 (1.9) |
9.9 (3) |
12.7 (3.7) |
11.1 (4.9) |
8.8 (4.4) |
8.3 (4.5) |
5.3 (2.7) |
3.2 (2.1) |
0.8 (0.2) |
|
| Testosterone (ng/mL) | |||||||||||||||||||||
| 30 (16) |
34 (17) |
33 (15) |
33 (15) |
36 (15) |
37 (14) |
39 (21) |
33 (10) |
38 (14) |
42 (15) |
48 (19) |
42 (15) |
45 (16) |
43 (14) |
43 (14) |
41 (14) |
34 (10) |
36 (14) |
30 (12) |
27 (08) |
515 (177) |
|
| Laxity (mm) | |||||||||||||||||||||
| 46N | 2.1 (1.1) |
2.3 (1.2) |
2.1 (1.2) |
2.4 (1.2) |
2.5 (1.4) |
2.1 (1.2) |
2.3 (1.3) |
2.5 (1.3) |
2.4 (1.2) |
2.6 (1.4) |
2.7 (1.4) |
2.7 (1.3) |
2.98 (1.5) |
2.4 (1.2) |
2.3 (1.2) |
2.5 (1.3) |
2.6 (1.3) |
2.4 (1.3) |
2.5 (0.7) |
2.5 (1.1) |
1.6 (1.1) |
| 89N | 4 (1.6) |
4.1 (1.8) |
3.8 (1.5) |
4.1 (1.7) |
4.2 (1.7) |
4 (1.8) |
4.2 (1.6) |
4.2 (1.6) |
4.3 (1.5) |
4.4 (1.6) |
4.7 (1.6) |
4.7 (1.5) |
4.7 (1.8) |
4.3 (1.6) |
4.2 (1.6) |
4.3 (1.7) |
4.3 (1.7) |
4.1 (1.6) |
4.3 (1.2) |
4.3 (1.5) |
3.3 (1.4) |
| 133N | 5.0 (1.6) |
5.1 (1.8) |
4.8 (1.6) |
5 (1.7) |
5.1 (1.7) |
5.1 (1.7) |
5.2 (1.7) |
5.2 (1.7) |
5.3 (1.6) |
5.4 (1.7) |
5.7 (1.8) |
5.8 (1.6) |
5.7 (2) |
5.2 (1.6) |
5.1 (1.6) |
5.3 (1.9) |
5.4 (1.9) |
5.2 (1.7) |
5.3 (1.3) |
5.2 (1.6) |
4.2 (1.8) |
| Stiffness (N/mm) | |||||||||||||||||||||
| 46–89N | 32 (17) |
35 (25) |
34 (20) |
32 (16) |
32 (14) |
35 (22) |
29 (14) |
32 (18) |
30 (17) |
29 (11) |
27 (15) |
29 (16) |
31 (19) |
32 (19) |
30 (16) |
31 (15) |
34 (18) |
33 (14) |
30 (15) |
33 (14) |
32 (20) |
| 89–133N | 54 (25) |
55 (28) |
54 (24) |
52 (26) |
55 (17) |
56 (33) |
49 (27) |
53 (26) |
54 (32) |
52 (20) |
52 (23) |
51 (24) |
54 (25) |
55 (21) |
55 (16) |
54 (24) |
51 (17) |
548 (18) |
52 (14) |
54 (17) |
60 (22) |
Represents the average value obtained for each male across 4 test days. M1–M5: days of menses; O1–O5: days of the initial estradiol rise near ovulation; EL1–EL5: days of the early luteal phase (rise in progesterone <2 ng/mL); LL1–LL5: days of the late luteal phase (decline in progesterone levels).
Estradiol
Females had significantly greater estradiol levels than males (F[1,40]=114.8, P<0.0001), but these sex differences were dependent on day of the female menstrual cycle (F[19,760]=29.9, P<0.0001). Posthoc analyses revealed females had higher serum estradiol levels than males on days 1–5 of the initial estrogen rise near ovulation, days 1–5 of early luteal phase, and days 1–4 of late luteal, but not for the days of menses or day 5 of late luteal when female estradiol levels are expected to be at or near nadir. Serum estradiol levels within females remained consistent during days 1–5 of menses, then increased significantly during the 5 days representing the initial estrogen rise near ovulation. While there were no differences in serum estradiol between days 1–3 of the initial estrogen rise, estradiol levels were greater on day 4 compared to day 1, and day 5 compared to days 1–2. Estradiol levels during days 1–4 of the early luteal phase were significantly lower than day 5 of the initial estrogen rise, but remained higher than all days of menses as expected. Estradiol levels then rose again on day 5 of early luteal, remained elevated through day 2 of late luteal, then began to decrease significantly on day 3 of late luteal. Values remained elevated above days of menses until day 5 of the late luteal.
Progesterone
Females had significantly greater serum progesterone levels than males (F[1,40]=19.4, P<0.0001), but these sex differences varied by day of the female menstrual cycle (F[19,760]=75.3, P<0.0001). Females had greater serum progesterone levels than males on day 1 of menses, day 5 of the initial estrogen rise at ovulation, and through all days of the early luteal and late luteal phases. Within females across days, there were no differences in progesterone levels between days 1–5 of menses and days 1–4 of the initial estrogen rise at ovulation. Progesterone levels increased somewhat on day 5 compared to days 2 and 3 of the initial estrogen rise at ovulation, then increased steadily from days 1–5 of the early luteal phase. Progesterone values then leveled off through the 3rd day of the late luteal when they began to decrease steadily until day 5 of late luteal. While day 5 of late luteal was still greater than days 1–5 of menses and the estrogen rise at ovulation, it was significantly lower than most days of the early (days 3–5) and late (days 1–4) luteal phases, which was expected with normal female physiology.
Testosterone
Males had significantly greater serum testosterone levels than females (F[1,40]=160, P<0.0001), and these sex differences varied by day of the female menstrual cycle (F[19,760]=4.247, P<0.0001). Posthoc comparisons confirmed males had higher serum testosterone levels than females across all test days, but revealed a transient spike in serum testosterone levels within females during days of the early luteal phase. Day 1 of the early luteal phase was higher than days 1 and 3 of menses, and all days of the early luteal phase were significantly higher than days 4 and 5 of the late luteal phase.
Knee laxity
Females had significantly greater knee laxity than males (F[1,40]=5.55, P=0.023), and these sex differences varied by day of the female menstrual cycle (F[19,760]=1.839, P=0.016) (Figure 1). Females had greater laxity than males on day 5 of menses, days 3–5 of the initial estrogen rise, days 1–4 of the early luteal phase and days 1, 2, 4 and 5 of late luteal. Within females across days of the menstrual cycle, knee laxity was greater on day 5 of the estrogen rise at ovulation compared to day 3 of menses, and days 1–3 of the early luteal phase were greater than all days of menses and day 1 of the initial estrogen rise at ovulation. Day 3 of the early luteal phase was also greater than days 2 and 4 during the initial estrogen rise. Knee laxity then decreased significantly from day 3 to day 4 of the early luteal, returning to levels more consistent with menses. However, days 1 and 2 of late luteal remained elevated above day 3 of menses. As expected, knee laxity increased significantly from 46N (mean±SE, 2±0.16) to 89 N (3.8±0.22) to 133N (4.7±0.25) (F[2,80]=250.9, P<0.0001), but there was no difference in knee laxity between the 3 loads tested when compared between sex (F[2,80]=0.193, P=0.825, β=0.079) or between sex by days of the menstrual cycle (F[38,1520]=0.741, P=0.876, β=0.814). For qualitative purposes only, Figure 2 provides a graphical representation comparing changes in serum hormone levels and knee laxity values across the 20 days. Each value was converted to Z-scores to allow equivalent scaling.
Figure 1.
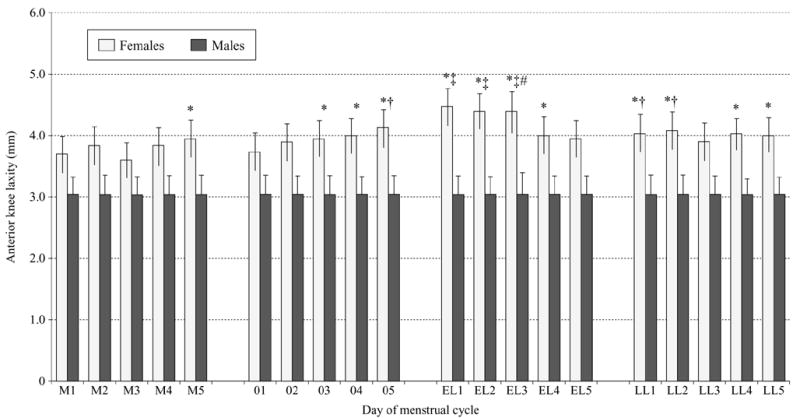
Comparison of knee laxity values between males and females by day of the menstrual cycle (P=0.026). M1–M5: days of menses; O1–O5: days of the initial estradiol rise near ovulation; EL1–EL5: days of the early luteal phase (rise in progesterone <2 ng/mL); LL1–LL5: days of the late luteal phase (decline in progesterone levels). *Females greater than males. †Greater than day 3 of menses. ‡Greater than days 1–5 of menses and day 1 of initial estrogen rise at ovulation. #Greater than days 2 and 4 during the initial estrogen rise.
Figure 2.
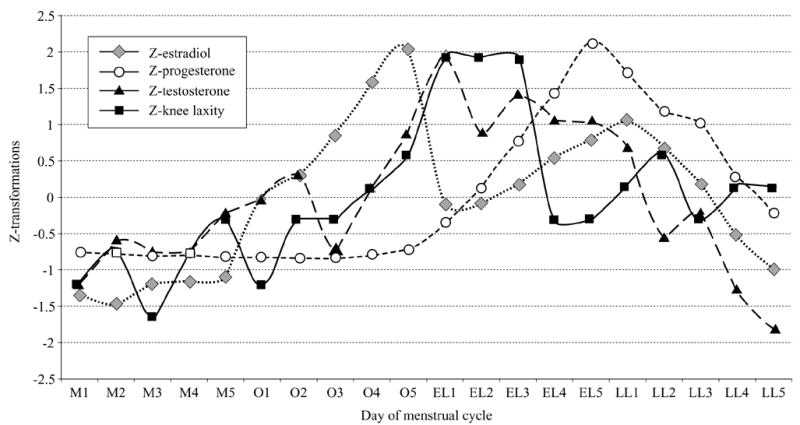
Graphic representation of changes in serum hormone levels and knee laxity values in females across the 20 days of the menstrual cycle. Each value was converted to Z-scores to allow equivalent scaling. M1 – −M5 =: days of menses; O1 – −O5 =: days of the initial estradiol rise near ovulation; EL1 – −EL5 =: days of the early luteal phase (rise in progesterone <2 ng/mL); LL1–LL5=: days of the late luteal phase (decline in progesterone levels).
Knee stiffness
In contrast to the knee laxity findings, we found no difference in knee stiffness by sex (F[1,40]=0.62, P=0.436, β=0.12), or between sex by day of the menstrual cycle (F[19,76]=0.623, P=0.891, β=0.483). Stiffness was greater at the higher force range of 89–133N (mean(SE, 56.8(3.2) compared to the lower force range of 46–89N (mean(SE, 31.8(2.6), but the difference in stiffness by force range was not affected by sex (F[1,40]=1.733, P=0.196, (=0.25) or sex by days of the menstrual cycle (F[19,760]=0.493, P=0.966, β=0.375).
Discussion
Our primary findings were that sex differences in knee laxity were cycle dependent, with the observed differences being consistent across the three loads tested. Increased laxity in females appeared to coincide with days of the menstrual cycle when estradiol and progesterone were significantly elevated compared to males (Figure 1). On average, observed differences between sex were greater than differences within females across the cycle. These results suggest sex hormones may be a primary mediator of the observed sex differences. Sex differences in passive knee joint behavior were limited to knee laxity, as no differences were found in anterior knee stiffness either between sex or within females across the 20 measured time points of their menstrual cycle.
Sex differences in knee laxity across the menstrual cycle
While several studies have identified sex differences in knee laxity 1–4 as well as differences in knee laxity across the female menstrual cycle,18, 19 we are unaware of any previous research that has examined whether sex differences are menstrual cycle dependent. We observed consistently higher average knee laxity values in females compared to males at all time points, but these differences were not statistically different in the days of menses where estrogen and progesterone levels were similar between sex. As knee laxity gradually increased in women once estradiol levels began to rise, significant differences were then apparent between males and females during the periovulatory, early and late luteal test days.
Mean differences within women across days of the cycle were smaller in magnitude, and were only apparent in the early luteal, and to a lesser extent, late luteal phase of the cycle. These findings appear to be somewhat consistent with previous studies,18, 19 but direct comparisons are difficult due to study designs. Heitz 19 measured laxity as well as hormone levels on days 1 (phase 1), 10–13 (phase 2), and 20–23 (phase 3) of 7 normal menstruating females. They found laxity was greater in phase 2 (periovulatory) and 3 (mid luteal) compared to phase 1 (menses), with non significant increases from phase 2 to phase 3 observed. However, data for laxity, estrogen and progesterone were pooled for each phase, and they used day of the cycle to predict cycle phase for all females tested. Deie et al.18 showed similar results, using basal metabolic and weekly serum estradiol and progesterone levels to predict cycle phase, once again pooling data by phase.
In contrast, we used actual serum hormone levels to define the phase in the cycle specific for each female, and monitored daily changes. We chose this method as the day of ovulation 26–28 and length of the follicular and luteal phases 9, 26, 29 can vary considerably among women. This variability was clearly evident in our subjects even though we recruited subjects with consistent overall cycles lengths of 28–32 days. For example, while days 11–15 were selected on average to represent the initial rise near ovulation, the actual days selected for each individual female based on her serum hormone levels ranged from days 6–10 to days 16–20. This is consistent with known female physiology where variability in the follicular phase is seen far more frequently than variability in the luteal phase. The selection of these variable time frames was further confirmed by evidence of a positive ovulation test consistently occurring within the selected days for each female. Hence, it likely the time points defining each phase vary somewhat between our work and previous studies, and may explain why we found no difference in the periovulatory phase while others have.
Daily measures of both knee laxity and hormone levels also provides a better sense of the transient nature of knee laxity changes and the hormone(s) that may be responsible for these changes. Heitz 19 concluded that it was impossible to determine from their data whether increased knee laxity in the mid-luteal phase was due to estrogen peaks near ovulation, or the combined rise in estrogen and progesterone, as no measures were taken on days 14–19 between hormonal peaks. Based on our observations of Figure 2, it appears the increase in knee laxity in the early and late phases are transient, and may be more of a delayed response to elevate estradiol levels, as knee laxity appears to decrease once progesterone begins to rise. The relationship between hormone changes and changes in knee laxity, with the potential of a delayed response, is explored in depth in a related article.30
Laxity vs stiffness
Passive joint characteristics at the knee are often measured clinically by quantifying the amount of anterior displacement of the tibia on the femur within a range of posterior-to-anterior directed forces applied to the posterior tibia. Knee joint laxity has been defined as the combination of joint hypermobility and musculotendinous flexibility,31 and is typically measured as the amount of anterior tibial displacement relative to the femur at a given force (e.g. 133N). In an effort to better understand the strain characteristics of the joint, measurements of anterior knee stiffness (or terminal knee stiffness) have also been employed by measuring the linear slope of the line (ΔForce/ΔDisplacement) at both lower 32, 33 and upper limits 18, 32, 33 of clinically measured force ranges. Measures of anterior knee stiffness are thought to provide more information on joint behavior than displacement at a single force, as it provides continuous recordings of force and displacement data across a specified range of forces.32
To date, sex differences in anterior knee stiffness have not been reported in the literature. In previously cited studies that have demonstrated sex differences in knee laxity, measures of knee stiffness were not recorded. However, our findings of no changes of linear stiffness in females across the menstrual cycle are consistent with those of Deie et al.,18 and are further supported by other studies demonstrating no net change in measures of knee stiffness secondary to hormonal influences in either human 34 or animal models.35, 36 While Romani et al.34 found a significant correlation between anterior knee stiffness and estradiol levels near ovulation, their results revealed no mean difference from one measurement time point to the next. These findings suggest that hormone-mediate increases in knee laxity are not associated with significant alterations in the mechanical properties of the ligamentous structures.35, 36
Conclusions
Our purpose was to comprehensively quantify sex differences in knee laxity across multiple days of the female menstrual cycle, using actual serum hormone levels to define cycle phase. The unique finding of our study is that sex differences are cycle dependent, with differences between males and females being greatest when females are in the early luteal phase of their menstrual cycle. Hence, it appears that female sex hormones may be a primarily mediator of these sex differences. What is yet unknown however is the consequence of these hormone-mediated increases in knee laxity on knee joint function and injury risk, either between males and females, or within females across their menstrual cycle. Research is now needed to determine to what extent mild to moderate increases in knee laxity affect neuromuscular and biomechanical function during weight-bearing tasks. Understanding how hormone-mediated increases in knee laxity affect joint biomechanics and resultant neuromuscular control of the knee will help to further elucidate the potential role that knee laxity and female sex hormones may play in ACL injury risk.
While it may be impractical to examine the effects of knee laxity on neuromuscular and biomechanical function across multiple days of the menstrual cycle, the daily measures obtained in this study may prove useful to clinicians and researchers in selecting appropriate days to examine these complex relationships. We have also confirmed that healthy, active females who report a normal 28–32 day menstrual cycle may still vary considerably in the timing and phasing of hormone concentration changes across the cycle. This is important in that it demonstrates the need to use actual serum hormone levels rather than a particular day(s) of the menstrual cycle to accurately define cycle phase and the time points where knee laxity is likely to change within each female. When data for each female were aligned using consistent serum hormone profiles, our findings suggest that knee laxity is least around the 3rd day of menses after progesterone and estradiol return to their nadirs, and is greatest in the days following peak estradiol levels when progesterone levels initially begins to rise. Investigators may wish to consider similar methods to define cycle phase and use these critical time points when examining the consequence of hormone mediated increases in knee laxity on sex differences in knee joint function and injury risk.
Acknowledgments
The authors wish to thank Christopher R. Carcia Ph.D., P.T., for his assistance with data collection.
Footnotes
This data was presented in part as a Free Communication at the 50th Annual Meeting of the American College of Sports Medicine, May 2003.
This research was supported by NIAMS/NIH RO3 AR 47178, and in part by a grant to the University of Virginia, General Clinical Research Center, NIH MO1 RR00847. Support was also provided by NICHD/NIH through cooperative agreement (U54 HD28934) as part of the Specialize Cooperative Centers Program in Reproductive Research.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy or the Department of Defense.
References
- 1.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24:427–36. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 2.Rosene JM, Fogarty TD. Anterior tibial translation in collegiate athletes with normal anterior cruciate ligament integrity. J Athl Train. 1999;34:93–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Rozzi SL, Lephart SM, Gear WS, Fu FH. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27:312–9. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 4.Trimble MH, Bishop MD, Buckley BD, Fields LC, Rozea GD. The relationship between clinical measurements of lower extremity posture and tibial translation. Clin Biomech. 2002;17:286–90. doi: 10.1016/s0268-0033(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 5.Loudon JK, Jenkins W, Loudon KL. The relationship between static posture and ACL injury in female athletes. J Orthop Sports Phys Ther. 1996;24:91–7. doi: 10.2519/jospt.1996.24.2.91. [DOI] [PubMed] [Google Scholar]
- 6.Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994;29:343–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JM, Mackenzie W, Kolstad K, Sandifer E, Jawad AF, Galinat B. Objective evaluation of knee laxity in children. J Pediatr Orthop. 2000;20:259–63. [PubMed] [Google Scholar]
- 8.Guyton AC. Female physiology before pregnancy, and the female hormones. In: Guyton AC, editor. Textbook of medical physiology. 8. Philadelphia: W.B. Saunders Company; 1991. pp. 899–914. [Google Scholar]
- 9.Dawood MY, Saxena BB. Plasma testosterone and dihydrotestosterone in ovulatory and anovulatory cycles. Am J Obstet Gynecol. 1976;126:430–5. doi: 10.1016/0002-9378(76)90632-3. [DOI] [PubMed] [Google Scholar]
- 10.Mathor MB, Achado SS, Wajchenberg BL, Germek OA. Free plasma testosterone levels during the normal menstrual cycle. J Endrocrinol Invest. 1985;8:437–41. doi: 10.1007/BF03348533. [DOI] [PubMed] [Google Scholar]
- 11.Oka K, Hirano T, Noguchi M. Changes in the concentration of testosterone in serum during the menstrual cycle, as determined by liquid chromatography. Clin Chem. 1988;34:557–60. [PubMed] [Google Scholar]
- 12.Liu SH, Al-Shaikh RA, Panossian V, Finerman GM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. Orthop Res Soc. 1996;14:526–33. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- 13.Sciore P, Smith S, Frank CB, Hart DA. Detection of receptors for estrogen and progesterone in human ligaments and rabbit ligaments and tendons by RT-PCR. 43rd Annual Meeting of the Orthopaedic Research Society. Orthopaedic Research Society; San Francisco, CA, USA. 1997. pp. 51–9. [Google Scholar]
- 14.Hamlet WP, Liu SH, Panossian V, Finerman GA. Primary immuno-localization of androgen target cells in the human anterior cruciate ligament. J Orthop Res. 1997;15:657–63. doi: 10.1002/jor.1100150505. [DOI] [PubMed] [Google Scholar]
- 15.Yu WD, Hatch JD, Panossian V, Finerman GA, Liu SH. Effects of estrogen on cellular growth and collagen synthesis of the human anterior cruciate ligament: an explanation for female athletic injury. 43rd Annual Meeting of the Orthopaedic Research Society; Rider Dickerson, Inc., San Francisco, CA, USA. 1997. p. 397. [Google Scholar]
- 16.Yu WD, Liu SH, Hatch JD, Panossian V, Finerman GA. Effect of estrogen on cellular metabolism of the human anterior cruciate ligament. Clin Orthop Rel Res. 1999;366:229–38. doi: 10.1097/00003086-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Arnold C, Van Bell C, Rogers V, Cooney T. The relationship between serum relaxin and knee joint laxity in female athletes. Orthopedics. 2002;25:669–73. doi: 10.3928/0147-7447-20020601-18. [DOI] [PubMed] [Google Scholar]
- 18.Deie M, Sakamaki Y, Sumen Y, Urabe Y, Ikuta Y. Anterior knee laxity in young women varies with their menstrual cycle. Int Orthop. 2002;26:154–6. doi: 10.1007/s00264-001-0326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heitz NA. Hormonal changes throughout the menstrual cycle and increased anterior cruciate ligament laxity in females. J Athl Train. 1999;343:144–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Charlton WPH, Coslett-Charlton LM, Ciccotti MG. Correlation of estradiol in pregnancy and anterior cruciate ligament laxity. Clin Orthop Rel Res. 2001;1:165–70. doi: 10.1097/00003086-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Schauberger CW, Rooney BL, Goldsmith L, Shenton D, Silva PD, Schaper A. Peripheral joint laxity increases in pregnancy but does not correlate with serum relaxin levels. Am J Obstet Gynecol. 1996;174:667–71. doi: 10.1016/s0002-9378(96)70447-7. [DOI] [PubMed] [Google Scholar]
- 22.Shambaugh JP, Klein A, Herbert JH. Structural measures as predictors of injury in basketball players. Med Sci Sports Exerc. 1991;23:522–7. [PubMed] [Google Scholar]
- 23.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. Am J Obstet Gynecol. 1972;112:1095–100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 24.Perrin DH, Shultz SJ, Sander TC, Carcia CR. Reliability of ligament compliance and tibial displacement measures obtained from two knee arthrometers. Med Sci Sports Exerc. 2002;34:S147. [Google Scholar]
- 25.Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112:1043–6. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- 26.Landgren BM, Unden AL, Deczfalusy E. Hormonal profile of the cycle in 68 normal menstruating women. Acta Endocrinol. 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 27.Rossmanith WG, Schenkel B, Benz R. Role of androgens in the regulation of the human menstrual cycle. Gynecol Endocrinol. 1994;8:151–9. doi: 10.3109/09513599409072449. [DOI] [PubMed] [Google Scholar]
- 28.Smith KD, Rodriguez-Rigau LJ, Tcholakian RK, Steinberger E. The relation between plasma testosterone levels and the lengths of phases of the menstrual cycle. Fertil Steril. 1979;32:403–7. doi: 10.1016/s0015-0282(16)44295-0. [DOI] [PubMed] [Google Scholar]
- 29.Nestour EL, Marraoui J, Lahlou N, Roger M, Ziegler DD, Bouchard P. Role of estradiol in the rise in follicle-stimulating hormone levels during the luteal-follicular transition. J Clin Endocrinol Metab. 1993;77:439–42. doi: 10.1210/jcem.77.2.8345049. [DOI] [PubMed] [Google Scholar]
- 30.Shultz SJ, Sander TC, Kirk SE, Johnson M, Perrin DH. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–74. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: Risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–50. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee: the contributions of the supporting structures. J Bone Joint Surg. 1976;58-A:583–95. [PubMed] [Google Scholar]
- 33.Eagar P, Hull ML, Howell SM. A method for quantifying the anterior load-displacement behavior of the human knee in both the low and high stiffness region. J Biomech. 2001;34:1655–60. doi: 10.1016/s0021-9290(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 34.Romani W, Patrie J, Curl LA, Flaws JA. The correlations between estradiol, estrone, estriol, progesterone, and sex hormone-binding globulin and anterior cruciate ligament stiffness in healthy, active females. J Womens Health (Larchmt) 2003;12:287–98. doi: 10.1089/154099903321667627. [DOI] [PubMed] [Google Scholar]
- 35.Hart DA, Reno C, Frank CB, Shrive NG. Pregnancy affects cellular activity, but not tissue mechanical properties, in the healing rabbit medial collateral ligament. J Orthop Res. 2000;18:462–71. doi: 10.1002/jor.1100180320. [DOI] [PubMed] [Google Scholar]
- 36.Strickland SM, Belknap TW, Turner SA, Wright TM, Hannafin JA. Lack of hormonal influences on mechanical properties of sheep knee ligaments. Am J Sports Med. 2003;31:210–5. doi: 10.1177/03635465030310020901. [DOI] [PubMed] [Google Scholar]


