Abstract
The steroid hormone 1,25(OH)2-vitamin D3 [1,25D] has been shown to affect the growth and proliferation of primary cultures of ventricular myocytes isolated from neonatal rat hearts. The research presented here shows that the vitamin D receptor [VDR] is present in murine cardiac myocytes (HL-1 cells), and that 1,25D affects the growth, proliferation and morphology of these cells. In addition we show that 1,25D effects expression of ANP, myotrophin, and c-myc. Furthermore, 1,25D effects expression and localization of the VDR within the cell. Murine HL-1 cardiac myocytes were grown and treated with 1,25D in culture, and growth and morphology were assessed with microscopic analysis. Cells were counted and protein levels were evaluated through Western blot analysis. Subcellular localization of the VDR was determined using immunofluorescence and confocal microscopy. 1,25D was found to decrease proliferation and alter cellular morphology of the HL-1 cells. Treatment with 1,25D increased expression of myotrophin while decreasing expression of atrial natriuretic peptide [ANP] and c-myc. 1,25D treatment also increased expression and nuclear localization of the VDR in these cardiac myocytes. Thus 1,25D is an important hormone involved in modulating and maintaining heart cell structure and function.
Keywords: 1,25-dihydroxyvitamin D3; heart; HL-1 cell
INTRODUCTION
The VDR is found in heart and 1,25D affects heart cell function [1]. The mechanism of these effects remains to be established. Recent studies in our laboratory have focused on the role of the vitamin D3 endocrine system in regulating myocardial development. We previously demonstrated that cardiac myocytes contain VDR [2], and initial studies revealed that vitamin D3 deficiency increased myocardial contractility and induced hypertrophy in rats [3]. Morphometric analysis of cross sections of ventricular tissue taken from the hearts of vitamin D3-deficient rats revealed cellular hypertrophy and an increase in extracellular matrix [4]. We observed an increase in c-myc protein levels in the hearts of the vitamin D3-deficient rats suggesting that 1,25D regulates c-myc expression [5]. Additionally we showed that 1,25D directly inhibits myocyte maturation using cultured neonatal ventricular myocytes [6]. These observations indicate that 1,25D regulates several aspects of myocardial development.
In this study we analyzed the actions of 1,25D on the HL-1 cardiac myocyte cell line derived from the AT-1 mouse atrial cardiomyocyte tumor lineage. These cardiomyocytes can be serially passaged yet maintain the ability to contract and retain differentiated cardiac morphological, biochemical, and electrophysiological properties [1]. Furthermore, RT-PCR based analysis confirmed a pattern of gene expression similar to that of adult cardiomyocytes including expression of myosin, actin, connexin43, and ANP [2]. Our results show that 1,25D affects proliferation, size, and morphology of these cardiomyocytes, and regulates expression of ANP, c-myc, VDR, and myotrophin. We also show the subcellular location of the VDR and demonstrate its translocation to the nucleus in response to 1,25D.
MATERIALS AND METHODS
Preparation of HL-1 cardiomyocytes
HL-1 cells were obtained from Dr. W.C. Claycomb, Louisiana State University Medical Center, New Orleans, LA. HL-1 cardiac myocytes (passages 55–75) were maintained as previously described [7]. Vitamin D experiments were performed in either T75 flasks or Lab-Tech 2-well slides (for immunofluorescence) 24 hours after a 1:2 split of confluent cells.
Cell Number, Size, and Morphology
Myocytes were removed from the flask using a trypsin-EDTA solution (Sigma Chemical, St.. Louis, MO) and then washed and re-suspended in phosphate buffered saline [PBS]. The cells were then counted and sized using a Coulter Counter (Model ZF, Coulter Electronics, Hialeah, FL). Morphology was subjectively assessed via bright field microscopy.
Western Blot Analysis
Trypsinized cells were removed from flasks and centrifuged at 3000 RPM for 2 minutes to obtain a pellet, which was then re-suspended in an SDS-leupeptin sample buffer. This whole cell lysate was then boiled for 15 minutes and subjected to a standard Bradford protein determination. Equal amounts of protein were loaded onto either 12.5% or 15% Tris-HCl Criterion pre-cast gels (Bio-Rad), along with BenchMark pre-stained Protein Ladder (Invitrogen). Electrophoresis and blotting were carried out in the typical fashion [1].
The primary antibodies used were rabbit anti-mouse VDR (sc-1008; Santa Cruz Bio; 1:200), rabbit anti-mouse ANP (AB 5490; Chemicon; 1:500), mouse monoclonal anti-myotrophin (V11220, BD Transduction Laboratory; 1:1000), rabbit anti-mouse c-myc (sc-764, Santa Cruz Bio; 1:100). The secondary antibodies used were HRP conjugated goat anti-rabbit (AP 132P, Chemicon; 1:1000) for VDR, ANP, and c-myc. HRP conjugated goat anti-mouse (A 2304, Sigma; 1:1000), and bovine anti-goat Ab (sc-2350, Santa Cruz Bio; 1:250) for myotrophin.
Immunofluorescence
Immunofluorescent staining of HL-1 cells was carried out in a method similar to that previously described [8]. Briefly, cells grown on 2-well slides (Lab-Tek) were washed with PBS and fixed with 3.7% formaldehyde, 0.05% gluteraldehyde, and 0.5% Triton X100. Following blocking for 10 minutes in 10% goat serum/PBS the primary antibody was applied in 5% goat serum/PBS. After a one-hour incubation the slides were washed with PBS, incubated for 30 minutes with the FITC conjugated secondary antibody, and washed again. The cells were then stained for five minutes with DAPI (Invitrogen) and cover slips were mounted with Prolong Gold anti-fade reagent (Invitrogen). Slides were analyzed with Olympus FV-500 on a Olympus iX 81 confocal microscope.
RESULTS
Effects of 1,25D on Myocyte Size, Proliferation, and Morphology
Figure 1A shows that concentrations of 1,25D from 1nM to 100nM significantly decreased proliferation. Maximal effects were seen at 30nM with a 48% inhibition of growth compared to control (p<0.0001). There was a slight but significant increase in cell size for those cells treated with 10nM 1,25D (p<0.05) (Fig. 1B). Photomicrograph figures 1C and D show the altered morphology that we typically see in 1,25D treated cells. These data indicate that treatment with 1,25D leads to hypoplasia, slight hypertrophy, and altered morphology of these dividing cardiomyocytes.
Figure 1. Effect of 1,25D on cell growth.
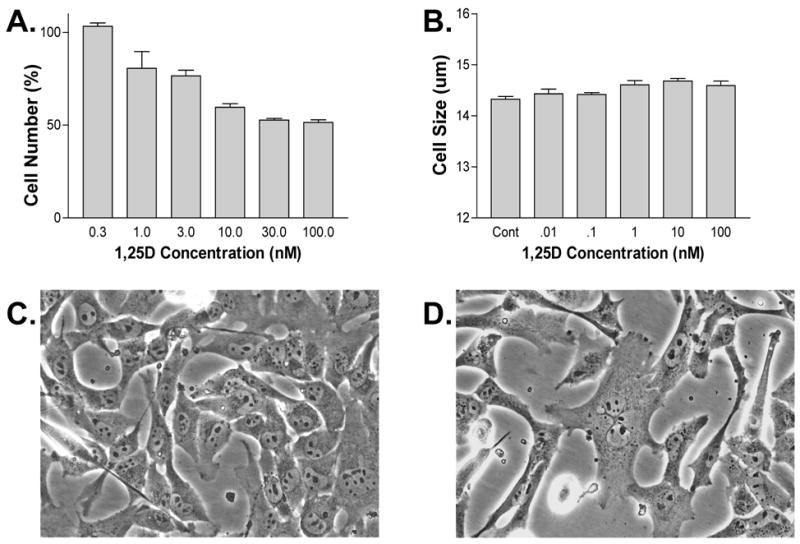
A-B) Cells treated for 3 days with varying concentrations of 1,25D show reduced proliferation and slight hypertrophy relative to control. C-D) Photomicrograph of cells treated for 2 days with (C) control v. (D) 100nM 1,25D.
Effects of 1,25D on Gene Expression
Western blot analysis of gene product expression is shown in Figure 2. VDR antibody recognized bands at 51 and 64 kd which were both induced by treatment with 1,25D. This effect was achieved within one hour and was maximal at 48hrs (data not shown). The maximal dose effect was achieved at the highest dose tested here, 100nM, with the effect decreasing as the dose decreased. Expression of myotrophin was also increased by 1,25D while that of ANP and proto-oncogene c-myc were both decreased.
Figure 2. Western blot of HL-1 gene products.
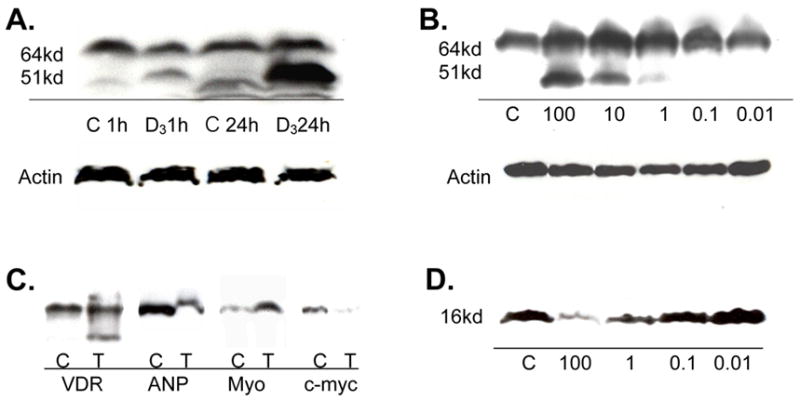
A) Time dependent expression of VDR at 1 hr and 24 hrs when treated with 100nM 1,25D (D3) v. control (C). B) Dose dependent expression of VDR at 24 hrs when treated with 0.01 to 100 nM 1,25D v. control. C) Expression of VDR, ANP, myotrophin, and c-myc relative to control when treated with 100nM 1,25D for 24 hrs. D) Dose dependent expression of ANP when treated with 0.01 to 100 nM 1,25D v. control.
Effects of 1,25D on Receptor Localization
The ligand binding assay shown in Figure 3 reveals saturable and specific binding of 1,25D, confirming the presence of the VDR in the HL-1 cytosol. Immunostaining and confocal microscopy results show increased expression, and localization to the nucleus, of VDR in response to 1,25D treatment.
Figure 3. VDR Ligand Binding Assay in HL-1 Cytosol.
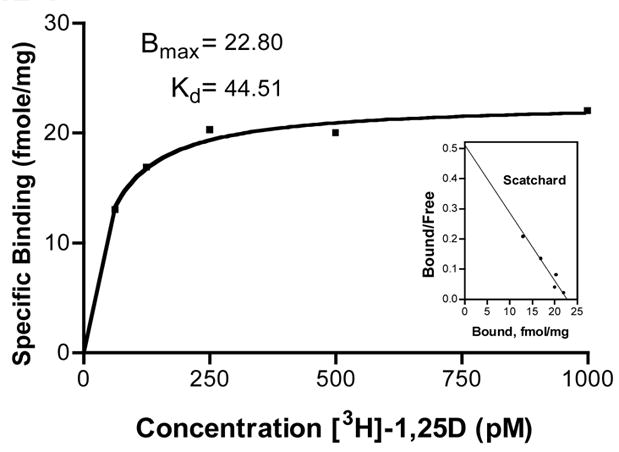
HL-1 cells 24 hours post 1:2 split of confluent flask were assayed as previously described [2]. Scatchard transformation of data shown as inset.
DISCUSSION
Since the discovery of the VDR in heart much has been learned about the effects of 1,25D on this organ, however, much remains to be learned about the mechanism of these effects. Lack of vitamin D has been linked to heart disease including ischemic disease, heart failure, and hypertension [9]. The cardiac disease associated with end stage renal disease, as well as several known mechanisms of heart failure including increased collagen deposition, hypertrophy, and hyperplasia, have been linked to 1,25D deficiency [10, 11]. In the present study we used the HL-1 cell as a model for elucidating the effects of 1,25D on heart cells.
1,25D has previously been shown to inhibit proliferation of other cell lines and this study finds similar results in the HL-1 cell [4]. Individual cell size increased and shape changed when exposed to 1,25D (Fig. 1). Given that true cardiomyocytes do not divide, it is unclear whether or not this same effect would be observed in vivo.
Western blot analysis for VDR revealed specific staining of a 64kd and a 51kd protein (Fig. 2). The 51kd protein was the major inducible form when exposed to 1,25D, however, expression of both proteins increased following 24 hr treatment, and expression both was blocked by VDR specific peptide. These size variants could represent an active and inactive form of the VDR. Given the immunostaining results in which VDR increased primarily in the nucleus following 1,25D treatment, and remained relatively unchanged in the cytoplasm, it is possible that the 51kd protein is the nuclear form of the VDR.
Myotrophin has been linked to the pathogenesis of cardiac hypertrophy as well as normal cardiomyocyte development, and is a potential compensatory mechanism for congestive heart failure [12]. 1,25D increases expression of myotrophin in the HL-1 cell indicating that it may be necessary for normal cardiac development and prevention of heart failure.
Much attention has been paid to ANP, and the other natriuretic peptides, in the cardiovascular literature over the past few years. ANP functions as part of a counter-regulatory system to the renin-angiotensin system and its production is stimulated by stress applied to the cell [13]. The reduction of ANP seen with 1,25D treatment may be due to a direct effect of the hormone or could be mediated through secondary effects of the hormone that reduce stress on the cell.
Expression of proto-oncogene c-myc is related to cell growth and differentiation. Our data is consistent with prior studies that show 1,25D decreases c-myc levels. This is a possible mechanism for the inhibition of proliferation seen when c-myc is overexpressed in myocytes [5].
Taken together these results suggest that the HL-1 cardiac myocyte cell line is a reasonable tool for studying heart disease processes, that VDR is present in these cells, and that 1,25D has direct effects on the heart. Through action of the VDR several of the key processes involved in the development of heart failure are interrupted or potentially reversed. The VDR holds promise as a target for possible therapeutic interventions for heart disease.
Figure 4. Immunofluorescent analysis of VDR.
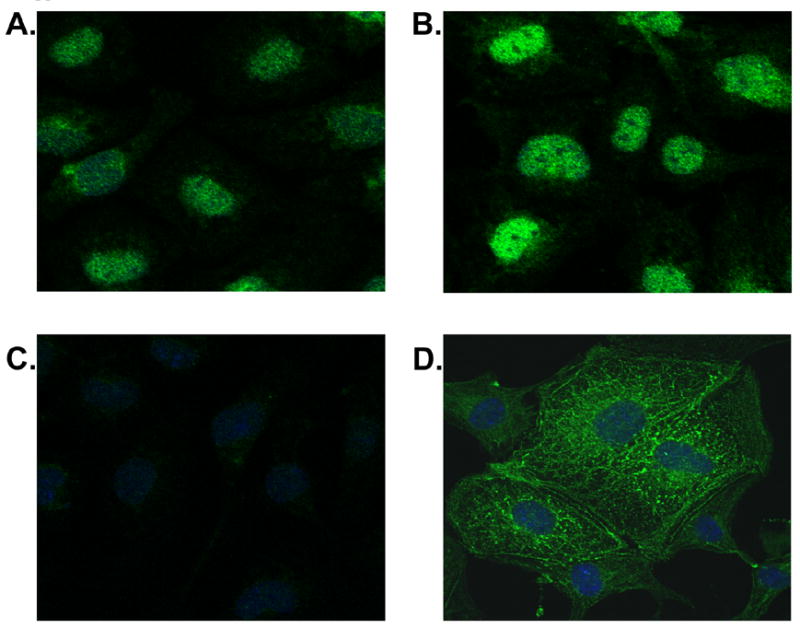
Confocal images of HL-1 cells treated with 100 nM 1,25D (B) v. control (A) for 24 hrs. To ensure specific staining a VDR specific peptide, SC1008P (Santa Cruz), was pre-incubated with primary VDR Ab (C). Adequate Ab penetration and cell architecture preservation is evidenced by actinin staining (D). All digital images are 0.35 um sections captured via Olympus FV500. VDR primary Ab is SC1008 with FITC conjugated secondary AP307F (Chemicon). Actinin primary Ab is A7811 with FITC conjugated secondary A8711 (both Sigma).
Footnotes
THIS WORK WAS SUPPORTED BY THE NATIONAL INSTITUTES OF HEALTH GRANT # 5 RO1-HL074894-02
References
- 1.O’Connell T, Simpson R. Immunochemical identification of the 1,25-dihydroxyvitamin D3 receptor protein in human heart. Cell Biol Int. 1996;20(9):621–624. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 2.Simpson R, Thomas G, Arnold A. Identification of 1,25-dihydroxyvitain D3 receptors and activities in muscle. J Biol Chem. 1985;260(15):8882–8891. [PubMed] [Google Scholar]
- 3.Weishaar R, Simpson R. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79(6):1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connell T, Barry J, Jarvis A, Sommerman M, Simpson R. 1,25-dihydroxyvitamin D3 regulation of cardiac myocyte proliferation and hypertrophy. Am J Physiol. 1997;272(4):H1751–1758. doi: 10.1152/ajpheart.1997.272.4.H1751. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell T, Simpson R. 1,25-dihydroxyvitamin D3 regulation of myocardial growth and c-myc levels in the rat heart. Biochem Biophys Res Commun. 1995;213(1):59–65. doi: 10.1006/bbrc.1995.2098. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell T, Giacherio D, Jarvis A, Simpson R. Inhibition of cardiac myocyte maturation by 1,25-dihydroxyvitamin D3. Endocrinology. 1995;136(2):482–488. doi: 10.1210/endo.136.2.7835280. [DOI] [PubMed] [Google Scholar]
- 7.White S, Constantin P, Claycomb W. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286(3):H823–829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 8.Huhtakangas J, Olivera C, Bishop J, Zanello L, Norman A. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18(11):2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 9.Holick M. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11):2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 10.Levin A, Li Y. Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int. 2005;68(5):1973–1981. doi: 10.1111/j.1523-1755.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Connell T, Simpson R. 1,25-dihydroxyvitamin D3 and cardiac muscle structure and function, Calcium Regulating Hormones and Cardiovascular Function. CRC Press. 1995:191–211. [Google Scholar]
- 12.McMurray J, Hiller C. The rise and fall of myotrophin in heart failure. J Am Coll Cardiol. 2003;42(4):726–727. doi: 10.1016/s0735-1097(03)00761-7. [DOI] [PubMed] [Google Scholar]
- 13.Clerico A, Recchia F, Passino C, Emdin M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am J Physiol Heart Circ Physiol. 2006;290(1):H17–H29. doi: 10.1152/ajpheart.00684.2005. [DOI] [PubMed] [Google Scholar]


