Abstract
There is convincing evidence to suggest that estrogen and inflammatory mediators play important roles in growth and progression of breast cancer. Moreover, local conversion of androgens to estrogens by aromatase (product of CYP19 gene) occurs in 70% of all breast cancers. The actions of aromatase in both the breast tumor and in surrounding adipose stromal and endothelial cells can result in high local levels of estrogen production that stimulate tumor growth. The efficacy of current endocrine therapies is predicted only if the tumor contains significant amounts of ER. Presence of PR in the tumor also is an important predictor of tumor aggressiveness and responsiveness to endocrine therapy. Immunoreactivity for aromatase in human breast tumors is highly correlated with that for cyclooxygenase 2 (COX-2), the rate-determining enzyme in prostanoid biosynthesis. COX-2 expression also is correlated with expression of HER-2/neu, an oncogene expressed in >30% of breast tumors. In this manuscript, we will review findings suggest that induction of COX-2 by inflammatory cytokines acting through NF-κB contributes to the increase in CYP19 expression and breast cancer progression, and that PR plays a dominant protective role in breast cancer cells by antagonizing NF-κB activation of COX-2.
1. Introduction
In addition to its vital role in reproduction, estrogen acting through the estrogen receptor (ER) has been implicated in the pathophysiology of a number of diseases, including cancer of the breast. Estrogen synthesis from C19-steroids is catalyzed by aromatase P450 (product of the aromatase/CYP19 gene). Aromatase is upregulated in 70% of all human breast cancers [1] and is expressed both in tumor and in surrounding adipose stromal and endothelial cells [1]. Thus, induction of aromatase within and surrounding the breast tumor can result in high local levels of estrogen production that stimulate tumor growth. In this regard, third-generation aromatase inhibitors have greater efficacy than tamoxifen in treatment of early and late-stage breast cancer [2]. However, responsiveness to such therapies is only predicted if the breast tumor contains significant amounts of ER [3]. The presence of progesterone receptor (PR) in a breast tumor also is an important predictor of responsiveness to therapy [4]. It has long been thought that this is due to the fact that PR is an estrogen-induced target gene that serves as an indicator of ER functional capacity and differentiation status of the tumor [5]. In this article we will provide support for the hypothesis that PR also serves a critical and protective role in the breast by inhibiting NF-κB activation, thereby blocking inflammatory response pathways, expression of oncogenic growth factor receptors and aromatase induction.
2. The aromatase (CYP19) gene and its tissue-specific expression
Human (h) CYP19 is a single-copy gene expressed in a number of tissues, including placenta [6], gonads [7-11], discrete nuclei of brain [12], adipose stromal cells [13] and in breast cancer epithelial and stromal cells [1]. Expression of aromatase in each of these tissues is controlled by tissue-specific promoters that lie upstream of tissue-specific first exons encoding the 5’-untranslated regions (UTRs) of the mRNAs. Thus, aromatase mRNA transcripts in gonads, brain, adipose and placenta contain different first exons (IIa, 1f, I.4/I.3 and I.1, respectively) that are alternatively spliced onto a common site just upstream of the translation initiation codon in exon II [13] (Fig. 1A). These tissue-specific promoters and first exons lie at various distances upstream of exon II (exons II - X encode the aromatase P450 protein). Sequencing of the human genome has revealed that hCYP19 encompasses ∼130 kb of DNA. The start site of transcription in placenta (exon I.1) lies ∼100,000 bp upstream of the start site of translation [14,15]. Adipose tissue has two alternative tissue-specific first exons; one is ∼70,000 bp upstream (exon I.4) [16], and the other is ∼250 bp upstream (exon I.3) [16,17] of the translation start site. The brain-specific first exon (1f) [18] lies ∼40,000 bp upstream [15], whereas in the gonads, the start site of transcription (in exon IIa) lies only 39 bp upstream of the start site of translation [19] (Fig. 1A). In human breast tumors, the start site of transcription corresponds to that used in gonads (exon IIa) or just upstream in exon I.3 [17]. Thus, the aromatase mRNAs in these different tissues have unique 5’-untranslated regions, while the sequences encoding the aromatase protein are identical.
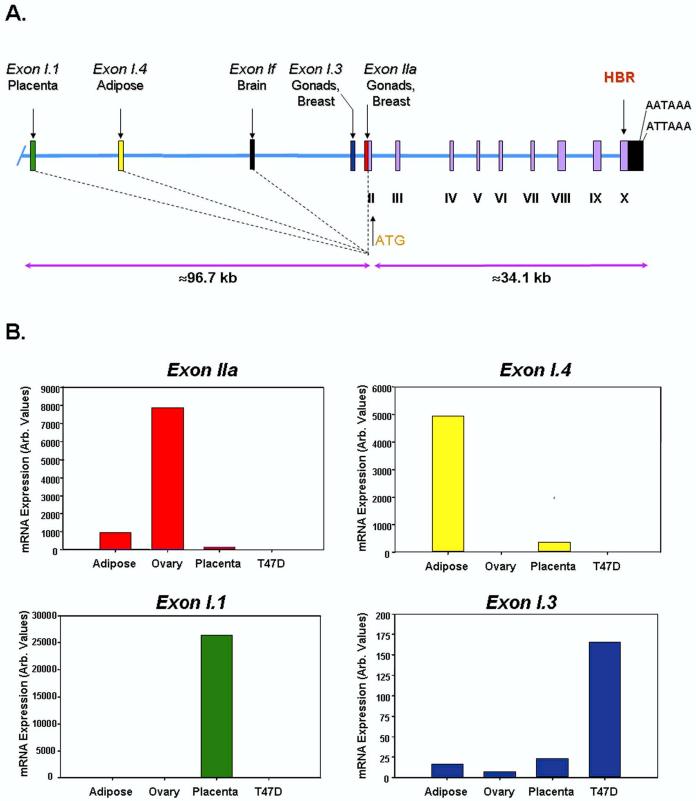
Figure 1.
Structural organization of the human CYP19 (aromatase) gene and tissue-specific expression of CYP19 mRNA transcripts.Panel A: Exons II-X, which encode the aromatase protein, and their introns comprise a region of ∼34 kb in size. The heme-binding region (HBR) and two polyadenylation signals in the 3’-untranslated region (UTR) are encoded in exon X. The alternative first exons, I.1, I.3/I.4, If and IIa, encode the 5’-UTRs of the aromatase P450 mRNAs in the placenta, adipose tissue, brain and gonads, respectively. These tissue-specific first exons are alternatively spliced onto a common site (shown by vertical dotted line) just upstream of the ATG codon in exon II. The region containing these alternative first exons encompasses ∼100 kb. Based on the Celera Human Genome database (hcg 39857CYP19GA_x2HTBL5L7WT), the human CYP19 gene lies on chromosome 15, spanning q14→q15. [15]. Panel B: Q-RT-PCR using primers specific for the 5’-UTRs of CYP19 transcripts in ovary (Exon IIa), adipose (Exon I.4), placenta (Exon I.1), and breast cancer (Exon I.3) were used to amplify CYP19 mRNA transcripts containing these alternative first exons in human adipose tissue, ovary, placental syncytiotrophoblast and T47D breast cancer cells. These hybridize with equivalent efficiency; thus, the relative levels of CYP19 expression in each of these tissues can be compared.
The presence of unique, tissue-specific UTRs in hCYP19 transcripts is exemplified by the study shown in Figure 1B, in which quantitative reverse transcriptase-polymerase chain reaction (Q-RT-PCR) was carried out using validated primers to specifically amplify the 5’-UTRs of hCYP19 transcripts in ovary (Exon IIa), adipose (Exon I.4), placenta (Exon I.1), and breast cancer (Exon I.3). These primers were designed to hybridize with equivalent efficiency; thus, the relative levels of CYP19 expression in each of these tissues can be compared. As can be seen, exon IIa-containing transcripts were predominant in the ovary, exon I.4-containing transcripts were predominant in adipose tissue, I.1-containing transcripts predominated in placenta and I.3-containing transcripts were highest in T47D breast cancer cells. When the relative levels of exon IIa- and I.3-containing hCYP19 mRNA transcripts were compared in the T47D cells, they were found to be roughly equivalent (data not shown).
3. Regulation of hCYP19 gene expression in breast cancer
In human breast tumors and surrounding adipose stromal cells, aromatase was found to be upregulated via switching from the weak adipose-specific promoter (I.4) to the strong ovarian (IIa) [20,21] and so-called ‘breast cancer’ (I.3) [17] promoters. Promoter I.3 lies just upstream of promoter IIa; consequently, both share important response elements, including a CREB-like sequence (CLS) [22-24], two nuclear receptor response elements (NRE-A and NRE-B) [23,25,26], which likely bind the orphan nuclear receptors SF-1 and LRH-1 [23,24,26-28], and two GATA-binding sites [29,30].
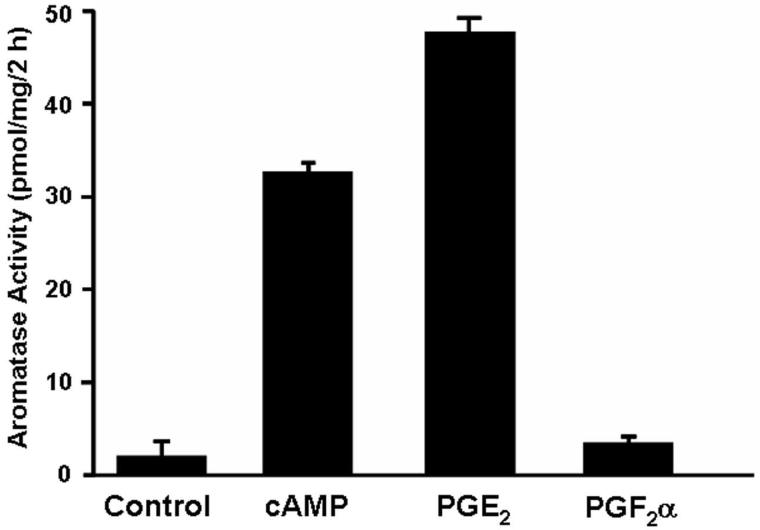
Promoter switching is likely mediated by induction of cyclooxygenase 2 (COX-2) in the breast tumor [1,31], resulting in increased prostaglandin E2 (PGE2) synthesis by breast tumor epithelial cells and infiltrating macrophages [20]. In the study shown in Figure 2, forskolin, which stimulates adenylyl cyclase and cAMP formation, and PGE2 had marked effects to stimulate aromatase activity in cultured human breast adipose stromal cells [20]. PGE2 acts via receptors that activate protein kinase A (PKA) and Ca2+/PKC pathways in the breast tumor and surrounding stromal cells. Previously, we observed that cAMP and phorbol esters acted synergistically to increase aromatase activity and expression in human adipose stromal cells [32]. The finding that PGF2α (which acts exclusively via receptors that mediate increased cytoplasmic calcium and PKC activation) had little effect on aromatase activity in these cells, supports our findings that upregulation of cAMP mediated pathways are essential for aromatase induction [33]. In this study, it was also observed that PGE2 stimulation of aromatase was associated with switching from the weak adipose-specific promoter I.4 to the strong ovarian and breast cancer promoters, IIa and I.3, respectively [20]. Similar findings regarding promoter switching were obtained by Harada and colleagues in breast cancer cells [21].
Figure 2.
Confluent monolayers of human adipose stromal cells were cultured for 24 h in the absence (control) or presence of forskolin (25 μM) (cAMP), PGE2 or PGF2α (1μM each) and aromatase activity was assay by the incorporation of tritium from [3H]androstenedione into water. Modified from Y. Zhao, V.R. Agarwal, C.R. Mendelson, E.R. Simpson. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene, Endocrinology 137 (1996) 5739-5742 [20], with permission. Copyright 2006, The Endocrine Society.
Many of the same transcription factors that mediate cAMP stimulation of hCYP19 expression in ovarian granulosa cells likely cause its upregulation in breast cancer. Cyclic AMP/PKA induction of promoter IIa activity in ovarian granulosa cells is mediated by CREB transcription factor, which binds to the CREB-like sequence (CLS) [15] and by the orphan nuclear receptors steroidogenic factor-1 (SF-1) and liver receptor homologue-1 (LRH-1), which bind to NRE-A and NRE-B sites [26-28]. The hCYP19 exon IIa 5’-flanking region also contains enhancers that bind the transcription factors GATA3 and 4 [30,34], which are expressed in some breast cancer cell lines and act synergistically with LRH-1 in a PKA-dependent manner to increase promoter IIa activity in transfected breast cancer cells [34]. In a panel of breast tumors, LRH-1 was found to be upregulated 4-5 fold in the tumor and surrounding stromal cells, as compared to normal tissues [35]. Furthermore, in cultured human adipose stromal cells, LRH-1 and aromatase expression were found to be induced by treatment with PGE2 [35]. Notably, LRH-1 is localized to human chromosome 1q32.1, a region amplified in primary breast tumors and breast cancer cell lines [35]. LRH-1 has been suggested to contribute to intestinal tumorigenesis by serving as a potent coactivator of β-catenin-mediated induction of the cyclin D1 and c-Myc promoters and as a direct transcriptional activator of the cyclin E1 promoter in intestinal epithelial cells [36]. The orphan nuclear receptor, estrogen-related receptor (ERR)α, which is structurally and functionally similar to the classical ERs but does not bind estrogen, was also found to interact with the NRE-A element and to induce aromatase expression via promoter I.3 [37]. ERRα expression in human breast tumors is significantly associated with risk of recurrence and adverse clinical outcome [38]. Presence of ERRα also is correlated with markers of tumor aggressiveness, such as absence of ERα and increased HER-2/neu [39]. Thus, a number of the transcription factors that are known to increase CYP19 gene expression are found to be upregulated in malignant breast tumors and in breast cancer cell lines.
4. COX-2 and breast cancer
There is convincing evidence that COX-2, the rate-determining and highly regulated enzyme in prostanoid biosynthesis, is overexpressed in breast cancer [40]. COX-2 upregulation is associated with increased aromatase [1] and enhanced expression of the tumorigenic marker, HER-2/neu. HER-2/neu and COX-2 appear to exist in a positive feedback loop, whereby HER-2/neu increases COX-2 transcription and elevated COX-2, via PGE2, induces HER-2/neu expression [41]. In a study of >6,000 women, use of nonsteroidal anti-inflammatory drugs was found to reduce the risk of breast cancer [42]. Furthermore, COX-2 inhibition decreased aromatase expression in human breast cancer cells in culture [43]. Consequently, clinical trials of COX-2 inhibitors alone and in conjunction with aromatase inhibitors are in progress. It is, therefore, evident that activation of inflammatory response pathways in the breast can lead to induction of COX-2 and aromatase, as well as growth factor receptors, such as HER-2/neu, which promote tumorigenesis.
5. NF-κB and breast cancer
The transcription factor NF-κB is a critical mediator of the inflammatory response and of cell survival, and plays an important role in cellular transformation and oncogenesis. The NF-κB family is comprised of the RelA (p65), RelB and c-Rel proteins, which are synthesized in their mature forms [44]. Other family members include NF-κB1 (p100) and NF-κB2 (p105), which are synthesized as larger precursors that are proteolytically processed at their C-terminal ends to form p50 and p52, respectively [44]. NF-κB is typically comprised of a heterodimer of p50 and p65 proteins [44]. In the inactive state, p50/p65 heterodimers are retained in the cytoplasm in a complex with IκBα or IκBβ. Upon stimulation of the cell by a variety of signals, including proinflammatory cytokines and bacterial LPS, there is activation of a specific IκB kinase (IKKβ), resulting in the phosphorylation, ubiquitination and proteolytic degradation of IκB [44]. This permits release of NF-κB heterodimer, its translocation to the nucleus and transcriptional activation of target genes involved in the inflammatory response, immunoregulation, anti-apoptosis and in enhanced cell proliferation [45].
There is increasing evidence to suggest that NF-κB plays an important role in normal mammary gland development and in breast carcinogenesis. Increased levels of IKK activity, nuclear NF-κB and NF-κB DNA-binding activity have been observed in human breast cancer cell lines and in breast cancer specimens (see [45] for review). On the other hand, a dominant-negative mutation of IKKβ in mouse mammary tumor cells inhibited NF-κB activation and decreased tumorigenic potential of the cells [46]. In a recent study of human breast tumors, NF-κB activation was increased in ER(-) as compared to ER(+) tumors and was predominately upregulated in ER(-)/HER-2/neu(+) tumors [46]. Additionally, increased NF-κB p50 DNA-binding activity was found to be associated with poor clinical outcome in ER(+) breast tumors [47]. Furthermore, increased levels and DNA-binding activity of NF-κB proteins RelB and p52 were observed in mouse mammary tumors induced by treatment with 7,12-dimethylbenz(a) anthracene (DMBA) [48].
Evidence also exists for a cooperative interaction between NF-κB- and ER-mediated inflammatory response pathways in the breast. Estrogen action to stimulate breast cancer growth is mediated, in part, by nongenomic mechanisms involving interaction of ERα with growth factor receptors and signaling cascades that originate at the plasma membrane. This involves activation of mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase pathways, with enhanced estrogen-independent phosphorylation and activation of nuclear and membrane-associated ERα [49]. Interestingly, the coactivator SRC-3 (AIB1), which is upregulated in metastatic human breast cancer, is phosphorylated in response to estradiol-17β, MAPK [50], and HER-2/neu tyrosine kinase [51]. Phosphorylation of SRC-3 causes its nuclear translocation, increased interaction with ER and the resulting enhancement of ER transcriptional activity [50]. SRC-3 also is phosphorylated by IKK in response to inflammatory cytokines, which causes its nuclear translocation; SRC-3 acts in concert with IKK to enhance NF-κB-mediated gene expression [52]. The finding that ERα stimulation of target gene transcription in MCF-7 cells is dependent upon SRC-3 phosphorylation by MAPK and IKK signaling pathways [50] suggests that SRC-3 may serve as a potentially harmful integrator of ER- and NF-κB-mediated inflammatory signaling pathways in the breast.
6. PR and breast cancer
It has long been appreciated that the presence of PR in a breast tumor is an independent predictor for benefit from adjuvant endocrine therapy and of disease-free survival [53-55]. Breast tumors that are PR(-) have a much higher proliferation rate and are more likely to manifest increased expression of the tumorigenic prognostic indicators, HER-2/neu and EGFR, than PR(+) tumors [56-59]. PR exists as three major isoforms, PR-A (94 kDa), PR-B (114 kDa) [60] and PR-C (60 kDa) [61]. PR-B and PR-A both bind to progesterone response elements (PREs) in DNA; however, PR-A lacks one of three transcriptional activation domains that are present in PR-B [62]. In this regard, PR-A can act as a potent dominant-negative repressor of PR-B in a cell- and promoter-specific context [63]. PR-A also may inhibit PR-B transcriptional activity by competing for limiting amounts of coactivators. PR-A and PR-B are present in roughly equivalent amounts in normal breast tissue and are distributed in luminal cells throughout the intralobular ducts and alveoli [64]. Interestingly, in invasive breast tumors the ratio of PR-A to PR-B has been reported to increase [65]. However, studies of gene targeted mice where PR-A or PR-B were independently ablated suggest that both isoforms function in a tissue-specific manner as distinct transcriptional activators [66-68]. In cell types and gene contexts where PR-A does not antagonize PR-B, coactivators may not be limiting [63].
The third PR isoform, PR-C, is an N-terminally truncated form of PR, which lacks the DNA binding domain and two activation (AF) domains near the aminoterminus, but contains the ligand-binding domain [61]. PR-C inhibits PR-B transcriptional activity in transfected cells [69]. Since PR-C cannot bind DNA but binds progesterone [70], PR-C may inhibit PR function by sequestering progesterone and coactivators away from the PR-B-isoform. PR-C also binds to PR-B, thereby reducing the capacity of PR-B to interact with PREs in progesterone-responsive genes [71]. Interestingly, we recently observed that PR-C expression was increased in T47D breast cancer cells as a result of increased NF-κB activation and binding to the PR gene promoter [69].
7. PR as a potential inhibitor of NF-κB-mediated breast tumorigenesis
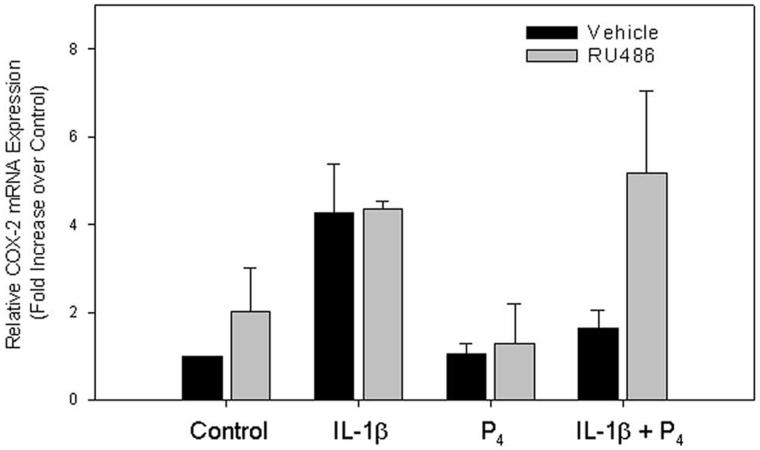
In recent studies, we observed that progesterone caused pronounced inhibition of IL-1β induction of COX-2 mRNA levels in cultured T47D human breast cancer (Fig. 3) and myometrial cells [72]. An antagonistic effect of progesterone on IL-1β-induced COX-2 expression also was observed in studies of human amnion epithelial cells, LUS fibroblasts [73], and in human fetal lung type II cells [74]. The finding that the inhibitory effects of progesterone in T47D cells were blocked by the PR antagonist, RU486 (Fig. 3), suggests that this effect is mediated by the PR.
Figure 3.
RU486 Blocks the Progesterone-induced Suppression of COX-2 mRNA in T47D Cells. T47D cells were incubated for 6 h the absence or presence of RU486 (10-7M) with and without IL-1β (10 ng/ml), progesterone (10-7 M), or both. RNA was isolated and the expression of COX-2 was analyzed by Q-RT-PCR. The data are expressed as fold increase over vehicle control cells. The bars represent the mean ± S.E.M. of values from three independent experiments. From D.B. Hardy, B.A. Janowski, D.R. Corey, C.R. Mendelson. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol. Endocrinol. (2006), in press [72], with permission. Copyright 2006, The Endocrine Society.
The human COX-2 gene contains two well-characterized NF-κB response elements in its promoter that bind NF-κB in vitro and in vivo [75-77]. In studies using human myometrial cells in culture, we found using chromatin immunoprecipitation (ChIP) that the effects of IL-1β to stimulate recruitment of NF-κB p65 to both proximal and distal NF-κB elements of the COX-2 promoter were markedly diminished by co-incubation with progesterone [72]. These findings suggest that PR either directly interacts with NF-κB to block its binding to DNA, or inhibits NF-κB activation and translocation to the nucleus. A mutual antagonism between the PR and the p65 subunit of NF-κB has also been reported in COS-1 and HeLa cells, in which activation of NF-κB by TNF-α repressed PR transcriptional activity, while PR also repressed p65-mediated transcription [78]. Also, in amnion epithelial cells, PR overexpression repressed NF-κB transcriptional activity, while IL-1β activated NF-κB and inhibited PR transcriptional activity [79]. Based on findings that PR and p65 interact in vitro [78], it was suggested that mutual repression resulted from formation of an inactive complex of these proteins on the DNA that was incapable of interacting with essential coregulators.
While the mechanisms for such antagonistic effects of PR and NF-κB on expression of specific genes are incompletely defined, there is abundant evidence to suggest that inflammatory and immunosuppressive actions of glucocorticoids are mediated by glucocorticoid receptor (GR) antagonism of NF-κB transactivation via several cell type- and promoter-specific mechanisms [80]. These include: (1) direct [81] or indirect (through CREB-binding protein [CBP]) [82] interaction between the N-terminus of p65 and DNA-binding domain of GR; (2) competition between GR and NF-κB for limiting amounts of the coactivators, CBP/p300 and steroid receptor coactivator (SRC)-1 [83] and/or; (3) glucocorticoid/GR induction of IκBα gene expression [84]. An action of progesterone to inhibit NF-κB activation by induction of IκBα expression was previously observed in human myometrial cells [72], macrophage cell lines [85] and in T47D cells [86]. Since PR is structurally very similar to GR, it is likely that similar mechanisms of NF-κB crossrepression apply to both nuclear receptors.
8. PR acts in a ligand-independent manner to inhibit NF-κB activation and COX-2 expression in human breast cancer cells
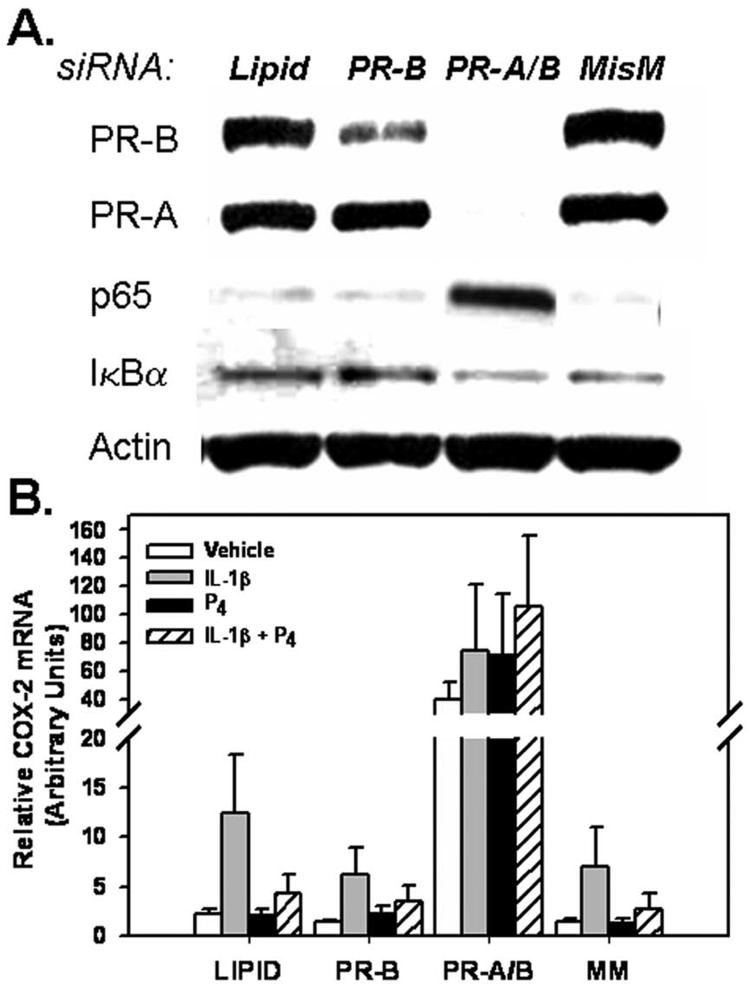
To further define the mechanisms for PR-mediated inhibition of NF-κB activation and COX-2 expression, T47D cells, which express PR-A, PR-B and PR-C isoforms, were transfected with a siRNA specific for full-length PR (PR-B), with siRNAs for both PR-B and the truncated PR-A, or with an PR-B siRNA mismatch, as control. Cells incubated with the transfection reagent in the absence of siRNA also were studied, as control (Lipid). After PR knockdown was achieved (Fig. 4A), the cells were incubated for 6 h in the absence or presence of IL-1β, progesterone or the two agents in combination, and COX-2 mRNA levels were analyzed by Q-RT-PCR (Fig. 4B). As observed in studies shown in Figure 3, in control cells transfected with PR-B siRNA mismatch or with transfection reagent alone, IL-1β stimulated COX-2 expression, while progesterone diminished this induction (Fig. 4B). Reduction of PR-B protein levels using siRNA specific for PR-B mRNA did not alter basal COX-2 mRNA levels or the ability of progesterone to impair IL-1β-induced increases in COX-2 expression, as compared to cells incubated with transfection reagent alone or transfected with a siRNA mismatch. By contrast, complete ablation of PR-A plus PR-B using siRNA directed against both PR sub-types greatly enhanced both basal and IL-1β stimulated COX-2 mRNA (Fig. 4B). This also was associated with a pronounced increase in nuclear NF-κB p65 protein levels (Fig. 4A). Furthermore, in the T47D cells lacking PR-A and PR-B, progesterone was unable to diminish COX-2 expression (Fig. 4B). Moreover, none of the PR siRNA treatments had an effect on basal expression of IκBα protein (Fig. 4A).
Figure 4.
Knockdown of PR-A and PR-B markedly enhances expression of COX-2 mRNA in T47D cells. T47D cells were transfected with siRNA targeted against PR-B alone or with siRNAs against both PR-B and PR-A for 5 days. Cells were cultured for 6 h in the absence or presence of IL-1β (10 ng/ml) with or without progesterone (10-7 M). Cell lysate proteins were analyzed for levels of PR, NF-κB p65, IκBα, and β-actin by immunoblotting (Panel A). RNA was isolated and the expression of COX-2 (Panel B) mRNA transcripts were analyzed by Q-RT-PCR. Data are the mean ± SEM of values from four independent experiments and are expressed as arbitrary units. From D.B. Hardy, B.A. Janowski, D.R. Corey, C.R. Mendelson. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol. Endocrinol. (2006) in press [72], with permission. Copyright 2006, The Endocrine Society).
These intriguing findings indicate that PR has a dominant ligand-independent action to inhibit NF-κB activation and COX-2 expression. Whereas, siRNA directed against PR-B alone had no effect on NF-κB activation or on COX-2 mRNA levels, complete knockdown of PR-A plus PR-B enhanced NF-κB p65 nuclear translocation and caused a >30-fold increase in COX-2 mRNA levels. These effects were observed in the absence of exogenous progesterone treatment, and prevented progesterone-mediated inhibition of COX-2 expression. Collectively, these findings suggest that PR plays a dominant protective role in human breast cancer by blocking induction of COX-2. The effect of PR to inhibit NF-κB activation likely occurs by direct interaction with p65 [78]. This ligand-independent mechanism may be of great importance in breast tissues of postmenopausal women, where PR may serve a protective role in the presence of negligible circulating levels of progesterone. On the other hand, progesterone clearly is required for PR induction of IκBα expression. Studies are ongoing to determine whether abrogation of PR expression in breast cancer cells results in a coordinate increase in expression of aromatase, HER-2/neu and on cellular proliferation.
9. Conclusions
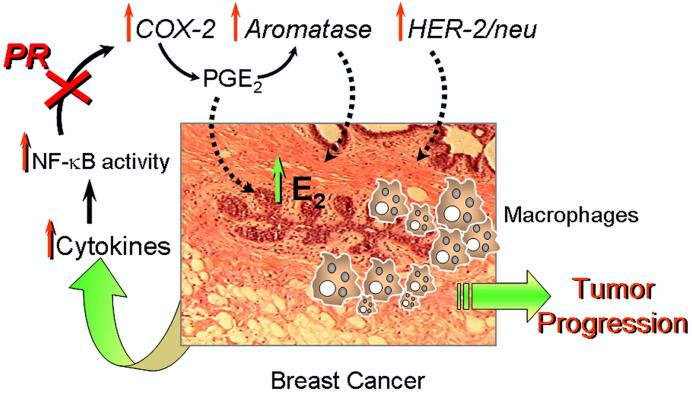
As discussed above, it is well recognized that human breast cancer is associated with an inflammatory response, involving increased macrophage invasion, with resulting enhanced cytokine production, NF-κB activation and increased expression of COX-2. As outlined in Figure 5, we propose that the increased levels of prostaglandins within the tumor and stroma, in turn, upregulates expression of the genes encoding aromatase and HER-2/neu. The elevated levels of estrogen formed within the tumor and surrounding stromal cells, acts together with the increased prostaglandins and HER-2/neu to further enhance the inflammatory response and tumor cell proliferation, leading to tumor progression. We further propose that the progesterone receptor serves a crucial role in blocking breast tumor formation and progression by its action to inhibit NF-κB activation via ligand-dependent ( i.e. induction of IκBα expression) and independent (i.e. direct interaction) mechanisms. In this manner, the presence of increased levels of PR in breast epithelial cells inhibits inflammatory response pathways and tumor formation and progression by preventing induction of aromatase and oncogenic tyrosine kinase-linked growth factor receptors. Conversely, a decline in PR expression and/or an impairment of PR function in the breast may facilitate the initiation of tumor growth and progression.
Figure 5.
Progesterone/PR serves a protective role in the breast by inhibiting NF-κ B activation of inflammatory response pathways. Human breast cancer is associated with an inflammatory response, with increased macrophage invasion, enhanced cytokine production, NF-κB activation and increased expression of COX-2. The increased production of prostaglandins, in turn, upregulate expression of the genes encoding aromatase and HER-2/neu. The elevated levels of estrogen formed within the tumor and surrounding stromal cells, act together with the increased prostaglandins and HER-2/neu to further enhance the inflammatory response and tumor cell proliferation, leading to tumor progression. On the other hand, we also postulate that the progesterone receptor serves a crucial role in blocking breast tumor formation and progression by its action to inhibit NF-κB activation.
Acknowledgments
Our research was supported by NIH grants 2 R01 DK031206 and 5 P01 HD011149. Daniel B. Hardy, Ph.D., is the recipient of a postdoctoral fellowship (PDF 0600877) from the Susan G. Komen Breast Cancer Foundation.
Footnotes
Supported by NIH grants 2 R01 DK031206 and 5 P01 HD011149. DBH is funded by a postdoctoral fellowship from the Susan G. Komen Breast Cancer Foundation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citableform. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, DeJong PC, Blankenstein MA, Nortier JW, Slee PH, van de Ven J, van Gorp JM, Elbers JR, Schipper ME, Blijham GH, Thijssen JH. Aromatase and COX-2 expression in human breast cancers. J. Steroid Biochem. Mol. Biol. 2001;79:41–47. doi: 10.1016/s0960-0760(01)00131-5. [DOI] [PubMed] [Google Scholar]
- [2].Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- [3].Chang J, Powles TJ, Allred DC, Ashley SE, Makris A, Gregory RK, Osborne CK, Dowsett M. Prediction of clinical outcome from primary tamoxifen by expression of biologic markers in breast cancer patients. Clin. Cancer Res. 2000;6:616–621. [PubMed] [Google Scholar]
- [4].Clark GM, McGuire WL, Hubay CA, Pearson OH, Marshall JS. Progesterone receptors as a prognostic factor in Stage II breast cancer. N. Engl. J. Med. 1983;309:1343–1347. doi: 10.1056/nejm198312013092240. [DOI] [PubMed] [Google Scholar]
- [5].Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978;103:1742–1751. doi: 10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- [6].Fournet-Dulguerov N, MacLusky NJ, Leranth CZ, Todd R, Mendelson CR, Simpson ER, Naftolin F. Immunohistochemical localization of aromatase cytochrome P-450 and estradiol dehydrogenase in the syncytiotrophoblast of the human placenta. J. Clin. Endocrinol. Metab. 1987;65:757–764. doi: 10.1210/jcem-65-4-757. [DOI] [PubMed] [Google Scholar]
- [7].Steinkampf MP, Mendelson CR, Simpson ER. Regulation by follicle-stimulating hormone of the synthesis of aromatase cytochrome P-450 in human granulosa cells. Mol. Endocrinol. 1987;1:465–471. doi: 10.1210/mend-1-7-465. [DOI] [PubMed] [Google Scholar]
- [8].Krasnow JS, Hickey GJ, Richards JS. Regulation of aromatase mRNA and estradiol biosynthesis in rat ovarian granulosa and luteal cells by prolactin. Mol. Endocrinol. 1990;4:13–12. doi: 10.1210/mend-4-1-13. [DOI] [PubMed] [Google Scholar]
- [9].Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol. Reprod. 1998;58:919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- [10].Carreau S. Germ cells: a new source of estrogens in the male gonad. Mol. Cell Endocrinol. 2001;178:65–72. doi: 10.1016/s0303-7207(01)00411-7. [DOI] [PubMed] [Google Scholar]
- [11].Lanzino M, Catalano S, Genissel C, Ando S, Carreau S, Hamra K, McPhaul MJ. Aromatase messenger RNA is derived from the proximal promoter of the aromatase gene in Leydig, Sertoli, and germ cells of the rat testis. Biol. Reprod. 2001;64:1439–1443. doi: 10.1095/biolreprod64.5.1439. [DOI] [PubMed] [Google Scholar]
- [12].Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol. Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- [13].Simpson ER, Zhao Y, Agarwal VR, Michael MD, Bulun SE, Hinshelwood MM, Graham-Lorence S, Sun T, Fisher CR, Qin K, Mendelson CR. Aromatase expression in health and disease. Recent Prog. Horm. Res. 1997;52:185–213. [PubMed] [Google Scholar]
- [14].Means GD, Mahendroo MS, Corbin CJ, Mathis JM, Powell FE, Mendelson CR, Simpson ER. Structural analysis of the gene encoding human aromatase cytochrome P-450, the enzyme responsible for estrogen biosynthesis. J. Biol. Chem. 1989;264:19385–19391. [PubMed] [Google Scholar]
- [15].Kamat A, Hinshelwood MM, Murry BA, Mendelson CR. Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol. Metab. 2002;13:122–128. doi: 10.1016/s1043-2760(02)00567-2. [DOI] [PubMed] [Google Scholar]
- [16].Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. J Biol. Chem. 1993;268:19463–19470. [PubMed] [Google Scholar]
- [17].Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. J. Steroid Biochem. Mol. Biol. 1996;59:163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- [18].Sasano H, Takashashi K, Satoh F, Nagura H, Harada N. Aromatase in the human central nervous system. Clin. Endocrinol. (Oxf) 1998;48:325–329. doi: 10.1046/j.1365-2265.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- [19].Means GD, Kilgore MW, Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific promoters regulate aromatase cytochrome P450 gene expression in human ovary and fetal tissues. Mol. Endocrinol. 1991;5:2005–2013. doi: 10.1210/mend-5-12-2005. [DOI] [PubMed] [Google Scholar]
- [20].Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- [21].Harada N, Honda S. Molecular analysis of aberrant expression of aromatase in breast cancer tissues. Breast Cancer Res. Treat. 1998;49(Suppl 1):S15–S21. doi: 10.1023/a:1006076101178. [DOI] [PubMed] [Google Scholar]
- [22].Michael MD, Michael LF, Simpson ER. A CRE-like sequence that binds CREB and contributes to cAMP-dependent regulation of the proximal promoter of the human aromatase P450 (CYP19) gene. Mol. Cell. Endocrinol. 1997;134:147–156. doi: 10.1016/s0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- [23].Fitzpatrick SL, Richards JS. Cis-acting elements of the rat aromatase promoter required for cyclic adenosine 3′,5′-monophosphate induction in ovarian granulosa cells and constitutive expression in R2C Leydig cells. Mol. Endocrinol. 1993;7:341–354. doi: 10.1210/mend.7.3.8387157. [DOI] [PubMed] [Google Scholar]
- [24].Fitzpatrick SL, Richards JS. Identification of a cyclic adenosine 3′,5′-monophosphate-response element in the rat aromatase promoter that is required for transcriptional activation in rat granulosa cells and R2C Leydig cells. Mol. Endocrinol. 1994;8:1309–1319. doi: 10.1210/mend.8.10.7854348. [DOI] [PubMed] [Google Scholar]
- [25].Michael MD, Kilgore MW, Morohashi K, Simpson ER. Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 (CYP19) gene in the ovary. J. Biol. Chem. 1995;270:13561–13566. doi: 10.1074/jbc.270.22.13561. [DOI] [PubMed] [Google Scholar]
- [26].Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol. Cell. Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- [27].Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144:3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- [28].Liu DL, Liu WZ, Li QL, Wang HM, Qian D, Treuter E, Zhu C. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol. Reprod. 2003;69:508–517. doi: 10.1095/biolreprod.102.011767. [DOI] [PubMed] [Google Scholar]
- [29].Bouchard MF, Taniguchi H, Viger RS. Protein kinase A-dependent synergism between GATA factors and the nuclear receptor, liver receptor homolog-1, regulates human aromatase (CYP19) PII promoter activity in breast cancer cells. Endocrinology. 2005;146:4905–4916. doi: 10.1210/en.2005-0187. [DOI] [PubMed] [Google Scholar]
- [30].Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142:977–986. doi: 10.1210/endo.142.3.7995. [DOI] [PubMed] [Google Scholar]
- [31].Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxy-genases: enzymes in breast cancer. J. Steroid Biochem. Mol. Biol. 2003;86:501–507. doi: 10.1016/s0960-0760(03)00380-7. [DOI] [PubMed] [Google Scholar]
- [32].Evans CT, Corbin CJ, Saunders CT, Merrill JC, Simpson ER, Mendelson CR. Regulation of estrogen biosynthesis in human adipose stromal cells. Effects of dibutyryl cyclic AMP, epidermal growth factor, and phorbol esters on the synthesis of aromatase cytochrome P-450. J. Biol. Chem. 1987;262:6914–6920. [PubMed] [Google Scholar]
- [33].Mendelson CR, Corbin CJ, Smith ME, Smith J, Simpson ER. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3′,5′-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology. 1986;118:968–973. doi: 10.1210/endo-118-3-968. [DOI] [PubMed] [Google Scholar]
- [34].Bouchard MF, Taniguchi H, Viger RS. Protein kinase A-dependent synergism between GATA factors and the nuclear receptor, liver receptor homolog-1, regulates human aromatase (CYP19) PII promoter activity in breast cancer cells. Endocrinology. 2005;146:4905–4916. doi: 10.1210/en.2005-0187. [DOI] [PubMed] [Google Scholar]
- [35].Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, Moriya T, Simpson ER, Sasano H, Clyne CD. Interactions between prostaglandin E2, liver receptor homologue-1, and aromatase in breast cancer. Cancer Res. 2005;65:657–663. [PubMed] [Google Scholar]
- [36].Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K. Synergy between LRH-1 and β-catenin induces G1 cyclin-mediated cell proliferation. Mol. Cell. 2004;15:499–509. doi: 10.1016/j.molcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [37].Yang C, Zhou D, Chen S. Modulation of aromatase expression in the breast tissue by ERRα-1 orphan receptor. Cancer Res. 1998;58:5695–5700. [PubMed] [Google Scholar]
- [38].Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H. Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–4676. doi: 10.1158/0008-5472.CAN-04-0250. [DOI] [PubMed] [Google Scholar]
- [39].Ariazi EA, Clark GM, Mertz JE. Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res. 2002;62:6510–6518. [PubMed] [Google Scholar]
- [40].Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- [41].Benoit V, Relic B, Leval X, Chariot XA, Merville MP, Bours V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004;23:1631–1635. doi: 10.1038/sj.onc.1207295. [DOI] [PubMed] [Google Scholar]
- [42].Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 2001;10:1213–1217. [PubMed] [Google Scholar]
- [43].Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J. Clin. Endocrinol. Metab. 2005;90:2563–2570. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- [44].Baldwin ASJ. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- [45].Cao Y, Karin M. NF-κB in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia. 2003;8:215–223. doi: 10.1023/a:1025905008934. [DOI] [PubMed] [Google Scholar]
- [46].Biswas DK, Dai SC, Cruz A, Weiser B, Graner E, Pardee AB. The nuclear factor κB (NF-κB): A potential therapeutic target for estrogen receptor negative breast cancers. Proc. Natl. Acad. Sci. U S A. 2001;98:10386–10391. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhou Y, Eppenberger-Castori S, Marx C, Yau C, Scott GK, Eppenberger U, Benz CC. Activation of nuclear factor-κB (NF-κB) identifies a high-risk subset of hormone-dependent breast cancers. Int. J. Biochem. Cell Biol. 2005;37:1130–1144. doi: 10.1016/j.biocel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- [48].Demicco EG, Kavanagh KT, Romieu-Mourez R, Wang X, Shin SR, Landesman-Bollag E, Seldin DC, Sonenshein GE. RelB/p52 NF-κB complexes rescue an early delay in mammary gland development in transgenic mice with targeted superrepressor IκB-α expression and promote carcinogenesis of the mammary gland. Mol. Cell. Biol. 2005;25:10136–10147. doi: 10.1128/MCB.25.22.10136-10147.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc. Natl. Acad. Sci. U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O′Malley BW. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol. Cell. 2004;15:937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- [51].Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- [52].Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, Tsai MJ, O′Malley BW. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by IκB kinase. Mol. Cell. Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Osborne CK, Yochmowitz MG, Knight WA, III, McGuire WL. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980;46:2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- [54].Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J. Clin. Oncol. 1992;10:1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- [55].Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- [56].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [57].Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J. Clin. Oncol. 1989;7:1120–1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- [58].Lebeau A, Unholzer A, Amann G, Kronawitter M, Bauerfeind I, Sendelhofert A, Iff A, Lohrs U. EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 2003;79:187–198. doi: 10.1023/a:1023958324448. [DOI] [PubMed] [Google Scholar]
- [59].Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, Elledge RM. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J. Natl. Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- [60].Horwitz KB, Francis MD, Wei LL. Hormone-dependent covalent modification and processing of human progesterone receptors in the nucleus. DNA. 1985;4:451–460. doi: 10.1089/dna.1985.4.451. [DOI] [PubMed] [Google Scholar]
- [61].Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol. Endocrinol. 1990;4:1833–1840. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- [62].Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O′Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 1993;7:1244–1255. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- [63].Chalbos D, Galtier F. Differential effect of forms A and B of human progesterone receptor on estradiol-dependent transcription. J. Biol. Chem. 1994;269:23007–23012. [PubMed] [Google Scholar]
- [64].Clarke RB, Anderson E, Howell A. Steroid receptors in human breast cancer. Trends Endocrinol. Metab. 2004;15:316–323. doi: 10.1016/j.tem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- [65].Mote PA, Bartow S, Tran N, Clarke CL. Loss of coordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res. Treat. 2002;72:163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- [66].Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr., Shyamala G, Conneely OM, O′Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- [67].Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- [68].Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. U S A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Condon JC, Hardy DB, Kovaric K, Mendelson CR. Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- [70].Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol. Endocrinol. 1996;10:1379–1387. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- [71].Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J. Steroid Biochem. Mol. Biol. 1997;62:287–297. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- [72].Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol. Endocrinol. 2006 doi: 10.1210/me.2006-0112. in press. [DOI] [PubMed] [Google Scholar]
- [73].Loudon JA, Elliott CL, Hills F, Bennett PR. Progesterone represses interleukin-8 and cyclo-oxygenase-2 in human lower segment fibroblast cells and amnion epithelial cells. Biol. Reprod. 2003;69:331–337. doi: 10.1095/biolreprod.102.013698. [DOI] [PubMed] [Google Scholar]
- [74].Hardy DB, MacDonald E, Smith M, Mendelson CR. Developmental regulation of the eicosanoid pathway mediates the induction of surfactant protein-A (SP-A) expression in the fetal lung. J. Soc. Gynecol. Invest. 2006;13(Supplement2):242A. [Google Scholar]
- [75].Tazawa R, Xu XM, Wu KK, Wang LH. Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem. Biophys. Res. Commun. 1994;203:190–199. doi: 10.1006/bbrc.1994.2167. [DOI] [PubMed] [Google Scholar]
- [76].Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-κB in the transcriptional control of COX-2 gene expression by IL-1β. Biochem. Biophys. Res. Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- [77].Soloff MS, Cook DL, Jr., Jeng YJ, Anderson GD. In situ analysis of interleukin-1-induced transcription of COX-2 and IL-8 in cultured human myometrial cells. Endocrinology. 2004;145:1248–1254. doi: 10.1210/en.2003-1310. [DOI] [PubMed] [Google Scholar]
- [78].Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-κB and the progesterone receptor. J. Biol. Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- [79].Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ′functional progesterone withdrawal′. Mol. Hum. Reprod. 2001;7:581–586. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- [80].Dumont A, Hehner SP, Schmitz ML, Gustafsson JA, Liden J, Okret S, van der Saag PT, Wissink S, van der Burg B, Herrlich P, Haegeman G, De Bosscher K, Fiers W. Cross-talk between steroids and NF-κB: what language? Trends Biochem. Sci. 1998;23:233–235. doi: 10.1016/s0968-0004(98)01212-2. [DOI] [PubMed] [Google Scholar]
- [81].Wissink S, van Heerde EC, Schmitz ML, Kalkhoven E, van der Burg B, Baeuerle PA, van der Saag PT. Distinct domains of the RelA NF-κB subunit are required for negative cross-talk and direct interaction with the glucocorticoid receptor. J. Biol. Chem. 1997;272:22278–22284. doi: 10.1074/jbc.272.35.22278. [DOI] [PubMed] [Google Scholar]
- [82].McKay LI, Cidlowski JA. CBP (CREB binding protein) integrates NF-κB (nuclear factor-κB) and glucocorticoid receptor physical interactions and antagonism. Mol. Endocrinol. 2000;14:1222–1234. doi: 10.1210/mend.14.8.0506. [DOI] [PubMed] [Google Scholar]
- [83].Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-κB signaling by CREB-binding protein and steroid receptor coactivator-1. J. Biol. Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- [84].Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- [85].Miller L, Hunt JS. Regulation of TNF-α production in activated mouse macrophages by progesterone. J. Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- [86].Deroo BJ, Archer TK. Differential activation of the IκBα and mouse mammary tumor virus promoters by progesterone and glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 2002;81:309–317. doi: 10.1016/s0960-0760(02)00072-9. [DOI] [PubMed] [Google Scholar]