Abstract
Icilin, a cooling compound, produces vigorous wet-dog shakes in rats. We have reported previously that icilin-induced wet-dog shakes are blocked by the kappa opioid receptor agonists, nalfurafine and U50,488H, and that icilin evokes a dose- and time-dependent increase in glutamate within the dorsal striatum. Since activation of kappa opioid receptors inhibits glutamate release intrastriatally, we targeted glutamate release within the dorsal striatum using nalfurafine and examined the role of the dorsal striatum in icilin-induced wet-dog shakes, more specifically, the effect that icilin-evoked intrastriatal glutamate release has on the overt stimulant behavior. We report that nalfurafine (0.04 mg/kg) inhibits icilin (0.50 mg/kg)-induced wet-dog shakes and that this inhibition is reversed by intrastriatal perfusion of the kappa opioid receptor antagonist, norbinaltorphimine (100 nM). Furthermore, we antagonized icilin-evoked glutamate release with nalfurafine (0.04 mg/kg), and reversed inhibition of glutamate release with intrastriatal norbinaltorphimine (100 nM). These findings support a central component in the behavioral response to icilin and suggest that activation of kappa opioid receptors antagonizes icilin-induced wet-dog shakes in rats by inhibiting glutamate release within the dorsal striatum.
Keywords: icilin , dorsal striatum, microdialysis, wet-dog shakes, glutamate, kappa opioid receptors
1. Introduction
Icilin is a cold-inducing agent that activates two transient receptor potential channels, TRPM8 and TRPA1, in the dorsal root ganglia and trigeminal neurons of the periphery (McKemy et al., 2002; Peier et al., 2002; Reid et al., 2002; Story et al., 2003; Bandell et al., 2004; Jordt et al., 2004; Liu et al., 2006). Upon application to the skin or on the tongue, icilin produces “mild, pleasant sensations of coolness, similar to menthol but discrete and non-irritating” and is 400-600 times more potent than menthol (Wei and Seid, 1983; Behrendt et al.,2004; Wei, 2005).Icilin may potentially be used in the treatment of pruritus, hemorrhoids, canker sores, arthritis and pain (Wei and Seid, 1983; Wei, 2005).
Current research has targeted icilin as a reference compound in extending the molecular pharmacology of TRPM8 and TRPA1 receptors in the periphery in the hope of providing further insight into cold transduction. TRPM8 is activated by cool temperatures (<25°C), menthol and icilin (McKemy et al., 2002; Peier et al., 2002); whereas TRPA1 is activated by noxious cold (<17°C), icilin, pungent natural compounds (mustard oil, wintergreen oil, clove oil, cinnamon oil, ginger oil) (Bandell et al., 2004), and raw garlic (Bandell et al., 2004; Bautista et al., 2005; Macpherson et al., 2005), but not by menthol (Story et al., 2003; Jordt et al., 2004). Activation of these channels by their respective agonists results in calcium ion influx and desensitization (McKemy et al., 2002; Peier et al., 2002; Story et al., 2003; Jordt et al., 2004; Liu et al., 2006). Investigation of the downstream effect of channel activation remains unclear, supporting the need to investigate the pharmacology of cold-inducing compounds in vivo.
Icilin, the super cold-inducing compound, was first categorized as a member of a class of biologically active non-opiates that precipitate a quasi-morphine withdrawal syndrome when given acutely to rats (Wei, 1976). I.p. icilin precipitates excessive grooming, rapid forepaw movement, abdominal writhing and wet-dog shakes, defined as “rapid twisting of the head and trunk with the forepaws leaving the ground”, in rats (Burford and Chappel, 1972; Wei, 1976; Cowan and Watson, 1978; Wei, 1981). Icilin-induced shaking begins within 2-5 min following administration and the dose of the compound mentioned in the present paper (0.5 mg/kg) produces approximately 70 wet-dog shakes within a 30-min observation period (Werkheiser et al., 2006).
We have reported previously that s.c. pretreatment with nalfurafine (0.02, 0.04 mg/kg) or U50,488H (5 mg/kg), the kappa opioid receptor agonists, inhibit icilin-induced wet-dog shakes, excessive grooming and abdominal writhing, whereas ICI 204,448 (1, 5, 10 mg/kg), the peripherally directed kappa agonist, does not (Werkheiser et al., 2006). Furthermore, icilin (0.25, 0.50, 0.75 mg/kg) evokes a dose- and time-dependent increase in glutamate within the dorsal striatum (Werkheiser et al., 2006) a region of the brain that controls the planning and execution of motor behavior (Blandini et al., 2000; reviewed by Lovinger et al., 2003). Since the glutamate increase in the dorsal striatum did not coincide with wet-dog shaking behavior observed in rats following icilin administration, we conclude that the dorsal striatal glutamate release is most likely an effect of icilin itself and not a consequence of the behavior induced by the compound.
Toth and Lajtha (1989) reported that intrastriatal administration of either NMDA or kainate precipitates wet-dog shakes in rats by increasing glutamate release within the striatum. As a result of this finding, we wished to examine the role of intrastriatal glutamate release in the mediation of icilin-induced wet-dog shakes. However, unlike intrastriatal NMDA and kainate, intrastriatal icilin does not produce wet-dog shakes (unpublished observation) and we believe that the compound acts directly on TRPM8 and TRPA1 in the periphery and elicits unknown downstream signaling to induce wet-dog shakes in rats. Finally, Moghaddam (1993) has previously shown that intense movement increases striatal glutamate levels.
In the present study, we wished to target glutamate release within the dorsal striatum. Since kappa opioid receptors are located presynaptically on dopaminergic (Spanagel et al., 1993) and glutamatergic (Meshul and McGinty, 2000) terminals within the striatum and activation of these receptors decreases neurotransmitter levels (Hill and Brotchie, 1995, Rawls and McGinty, 1998; Gray et al., 1999; Hill and Brotchie, 1999; Rawls et al., 1999; Sbrenna et al., 1999), we investigated whether an effective dose of nalfurafine (0.04 mg/kg) blocks icilin (0.50 mg/kg)-induced wet-dog shakes by inhibiting glutamate release intrastriatally. In doing so, we can confirm involvement of the dorsal striatum following icilin administration and suggest a possible mechanism for kappa opioid mediated inhibition of icilin-induced wet-dog shakes.
2. Methods
2.1. Animals and surgery
Male Sprague Dawley rats (150-175 g, Ace Laboratories, Boyertown, PA) were housed in groups of 4 at 23 ± 1°C with food and water provided ad libitum, according to Temple University Institutional Animal Care and Use Committee regulations. A standard light-dark cycle was maintained with a timer-regulated light period from 0700 to 1900 h.
Rats were anesthetized with ketamine and acepromazine (150 mg/kg + 2.5 mg/kg, i.p.) and secured into a stereotaxic frame (Harvard Apparatus, Holliston, MA) with the incisor bar positioned at –3.3 mm. A microdialysis cannula (CMA/12; CMA/Microdialysis, Chelmsford, MA) was implanted into the dorsal striatum using the following stereotaxic coordinates from bregma, AP +0.8 mm, ML +3.0 mm and DV-4.0 mm (Paxinos and Watson, 1997). Following implantation, the cannula was affixed with the dental acrylic, Durelon™ carboxylate (CMA/Microdialysis). After solidification of the dental cement, each rat was placed singly in a cage and returned to the animal facility for 2 days to recover.
2.2. In vivo microdialysis perfusion
On the day of the experiment, each rat was weighed and briefly anesthetized with isoflurane. The dummy probe was removed from the cannula and replaced with a 2 mm CMA/12 microdialysis probe connected to FEP tubing. The rat was placed into an individual chamber of a Plexiglas observation box.
Upon regaining consciousness, the rat acclimated in its chamber for 30 min and artificial CSF [aCSF; NaCl (147 mM), CaCl2 (1.2 mM), KCl (2.7 mM), MgCl2 (0.85 mM)] flowed through the probe at a rate of 2 μL/min, controlled by a syringe pump. Steady-state equilibrium of dialysate was achieved with a 90-min washout period prior to four consecutive baseline collections. Dialysate samples were collected in 15-min intervals and rat behavior was observed for the duration of the experiment.
In experiment 1, following baseline collections, rats were pretreated with s.c. nalfurafine (0.04 mg/kg) or vehicle (saline) 30 min before i.p. icilin (0.50 mg/kg) or vehicle (1% Tween 80). These agents were administered in a volume of 1 mL/kg. Dialysate samples were collected for 120 min following icilin or vehicle treatment and stored at -80°C for neurochemical analysis. Dialysates from rats with proper probe placements were analyzed using HPLC.
In experiment 2, following baseline collections, norbinaltorphimine (NBNI; 100 nM) or aCSF was infused through the microdialysis probe for the duration of the experiment. Sixty min following the start of this infusion, rats were given s.c. nalfurafine (0.04 mg/kg) or saline. Icilin or vehicle was given 30 min after nalfurafine. Dialysate was collected for 120 min following icilin administration and stored at -80°C for neurochemical analysis. Samples from rats with proper probe placements were analyzed using HPLC.
2.3. Behavioral experiment
Similar to the microdialysis experiment, rats were cannulated intrastriatally with a CMA/12 microdialysis probe and recovered for 2 days prior to experimentation. On the day of the experiment, rats were allowed to acclimate for 2.5 h (correlating with the microdialysis time). Norbinaltorphimine (100 nM) or aCSF was infused through the microdialysis probe for the duration of the experiment. Sixty min after the start of infusion, rats were pretreated with s.c. nalfurafine (0.04 mg/kg) or saline for 30 min, followed by icilin (0.50 mg/kg). Icilin-induced wet-dog shakes were counted in 5-min increments for 30 min noting onset of action and the presence of side effects (e.g. abdominal writhing). Because of the intensity of icilin-induced wet-dog shakes, we were unable to monitor shaking behavior while making sample collections simultaneously. Similar to the previous microdialysis experiment, rats were euthanized and brains removed to check for probe placement and damage.
2.4. Histology
Rats were euthanized with carbon dioxide gas and decapitated following microdialysis or behavioral experiments. Brains were extracted and placed in 4% formaldehyde in phosphate buffered saline (pH 7.0). Sections were sectioned with a cryostat and stained with 0.5% cresyl violet. Probe placement and histological damage were determined by microscopic visualization.
2.5. Amino acid analysis
Samples were usually run 5-7 days following experimentation. Probe placement and histology were confirmed prior to analysis. For glutamate derivatization, 5 μL of dialysate or amino acid standard was mixed with 5 μL sodium borate (8 mM, pH 9.5), followed by addition of 5 μL KCN (12 mM). The resulting solution was mixed with 4 μL of naphthalene dicarboxylate acid (NDA) and derivatized for 5 min. Reaction of glutamate in the samples with NDA, in the presence of cyanide ions in borate buffer, produces a stable, electrochemically and UV-detectable 1-cyano-(f)-isoindole derivative.
After derivatization, 15 μL of the mixture was injected on to a 5-μm C-18 reverse phase column (150 × 4.6 mm) (Phenomenex Inc. Torrance, CA) with a LKB 2150 HPLC pump (Pharmacia Biotech, Uppsala, Sweden) and eluted with 20 mM sodium citrate buffer (pH 7.5) containing 50% methanol, using a linear gradient with 100% methanol. Varying the buffer and the gradient conditions affected glutamate retention times but did not influence quantitative analysis. The flow rate was 0.60 mL/min. Glutamate was detected with a model HP 1050 diode array detector (Hewlett Packard Company, Atlanta, GA). Glutamate was identified by overlaying absorption spectra at 420 and 440 nm and quantifying at 420 nm using HP Chemstation software (Hewlett Packard Company) based on peak area by comparison with an external standard calibration curve ranging from 0.50 to 5 μM. The detection limit was 500 nM, based on signal to noise ratio.
2.7. Materials
Icilin (AG-3-5), a gift from Delmar Chemicals Ltd. (Montreal, Canada), was suspended in 1% Tween 80/distilled water. NBNI was purchased from Tocris Chemicals (Ellisville, MO) and dissolved in aCSF. Nalfurafine, a gift from Adolor Company (Exton, PA), was dissolved in saline. Amino acids were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO) and dissolved in double distilled water.
2.6. Data analysis
Glutamate levels were obtained prior to icilin or vehicle (1% Tween 80) administration. Each post-baseline sample was expressed as the percentage of respective baseline values ± SEM of results obtained from 5-11 rats. Results were analyzed using two-way analysis of variance (ANOVA) for treatment x time with the repeated measures design followed by the Bonferroni post-hoc test (Prism, GraphPad Software, San Diego, CA). Results were considered significant at p<0.05.
To reflect overall changes in glutamate levels, area under the curve (AUC) values were calculated from 15-60 min following icilin treatment, using KaleidaGraph (Synergy Software, Reading, PA) and expressed in histograms. One-way analysis of variance was applied to each of the AUC values and compared using Newman-Keuls (GraphPad Prism). Results were considered significant at p<0.05.
In behavioral experiments, group data are expressed as mean wet-dog shakes ± SEM of the results obtained from 6-8 rats. Comparisons were performed using one-way analysis of variance followed by the Newman-Keuls post-hoc test (GraphPad Prism). Results were considered significant at p<0.05.
3. Results
3.1. Histology
Eighty-one of 94 rats were confirmed to have proper probe placement in both behavioral and microdialysis experiments. The histology and placement depicted in Fig. 1 represent probe placement and damage in our standard microdialysis experiments. Rats not confirmed as having proper probe placement were excluded from our studies.
Fig.1.
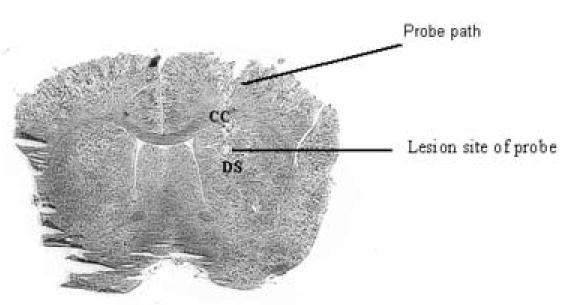
A representative cresyl violet (0.5%) stained brain section that depicts probe placement and histological damage resulting from insertion of microdialysis probe and subsequent microdialysis experiment. The anatomical placement within the dorsal striatum of all dialysis probes used in the study (AP +0.8 mm, ML +3.0 mm DV-4.0 mm) was based on Paxinos and Watson (1997). CC=Corpus callosum; DS= Dorsal striatum.
3.2. Nalfurafine on icilin-evoked glutamate stimulation within the dorsal striatum
Two-way ANOVA with repeated measures design showed time and treatment significance [F (df 3,17)=2.231; p<0.05]. Icilin (0.50 mg/kg) significantly (p<0.01) increased glutamate 15-min post administration, and this was significant (p<0.01) when compared to those icilin-treated rats pretreated with nalfurafine or rats given nalfurafine/vehicle and vehicle/vehicle. Although glutamate levels appeared slightly elevated 30-90 min following i.p. icilin, given the variability at these time points, this elevation was not significant (Fig. 2A).
Fig. 2.
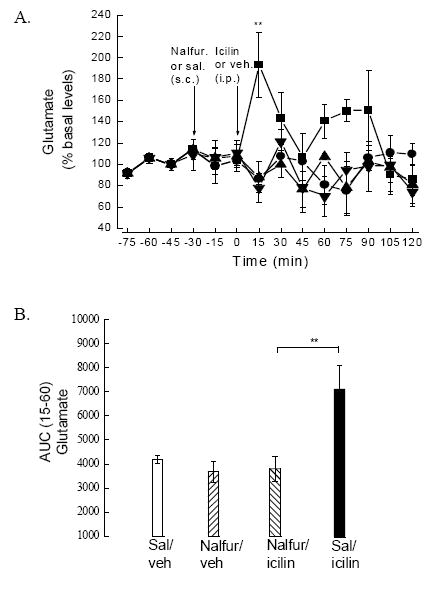
A. Rats were injected with either icilin or vehicle after the 0 min collection. At 15 min, 0.50 mg/kg icilin (■) increased glutamate levels significantly compared to icilin rats pretreated with nalfurafine (▲) or controls [saline (sal)/vehicle (veh) (●) or nalfurafine (nalfur)/veh (▼)] as well as pretreatment levels (**p<0.01; two-way ANOVA followed by Bonferroni post hoc test). B. Area under the curve (AUC) was analyzed for the first 60 min (15-60 min) following icilin and confirmed inhibition of icilin-evoked glutamate release by nalfurafine (**p< 0.01; one way ANOVA followed by Newman Keuls post-hoc test). Nalfurafine had no effect on glutamate levels compared to pretreatment glutamate levels or saline/vehicle.
One-way ANOVA [F (df 3,20)=5.784] following AUC analysis showed that nalfurafine pretreatment significantly; (p<0.01) reduced icilin-stimulated increase in glutamate levels within the striatum compared to icilin-treated rats (Fig. 2B). Icilin evoked a significant (p<0.01) increase in glutamate levels when compared to nalfurafine/vehicle and vehicle/vehicle.
3.3. Intrastriatal infusion of NBNI reverses nalfurafine inhibition of icilin-induced wet-dog shakes
One-way ANOVA [F (df 3,21)=6.774] showed that nalfurafine inhibition of icilin-induced wet-dog shakes was significantly (p<0.05) reversed by intrastriatal infusion of NBNI. NBNI infusion did not influence the incidence of icilin-induced shaking behavior (Fig. 3).
Fig. 3.
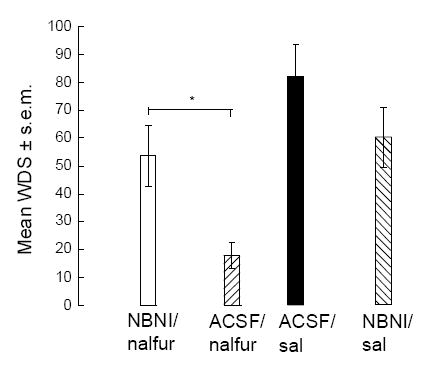
Perfusion of norbinaltorphimine (NBNI) directly into the dorsal striatum significantly reversed nalfurafine inhibition of icilin (0.5 mg/kg)-induced wet-dog shakes (WDS) over the 30 min test period (* p< 0.05; one-way ANOVA followed by Newman Keuls post-hoc test).
3.4. Intrastriatal perfusion of NBNI reverses the inhibition of icilin-evoked glutamate changes by nalfurafine
Analysis of AUC values using one-way ANOVA [F (df 5,29)=5.078)] followed by Newman-Keuls post-hoc test showed that NBNI significantly (p<0.01) reversed nalfurafine inhibition of icilin-evoked glutamate stimulation (Fig. 4) and was significant (p<0.05) compared to controls (aCSF/nalfurafine; NBNI/saline; NBNI/nalfurafine; aCSF/saline).
Fig. 4.
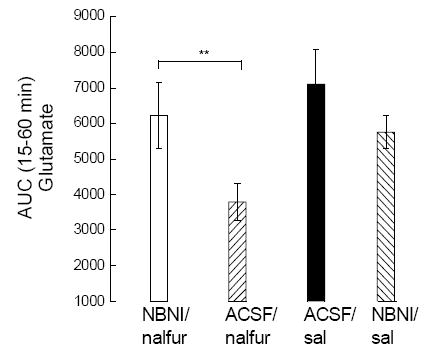
AUC analysis for the first 60 min following icilin showed that NBNI significantly (** p<0.01) reversed nalfurafine inhibition of icilin (0.05 mg/kg) evoked glutamate increase (one-way ANOVA followed by Newman-Keuls post-hoc test).
4. Discussion
Nalfurafine (0.04 mg/kg, s.c.) inhibits icilin-induced wet-dog shakes (Werkheiser et al., 2006). Here, we report that nalfurafine also inhibits icilin-evoked glutamate release within the dorsal striatum, suggesting that kappa receptor agonists, such as nalfurafine, may inhibit icilin-induced wet-dog shakes through this mechanism. To confirm this, we successfully reversed nalfurafine antagonism of icilin-evoked wet-dog shakes by unilateral infusion of the kappa antagonist, NBNI, into the dorsal striatum. Likewise, we have also shown that intrastriatal NBNI can reverse nalfurafine inhibition of icilin-evoked glutamate release which implicates the dorsal striatum in its behavioral action in rats. From our findings, we implicate the central nervous system and, more specifically, the dorsal striatum in icilin-induced shaking behavior.
Nalfurafine (Seki et al., 1999; Togashi et al., 2002; Inan and Cowan, 2004) an effective antipruritic in uremic pruritus (Wikström et al., 2005) should act similarly to other kappa opioid receptor agonists, e.g. U50,488H and U69,593, commonly used in microdialysis studies. When given alone, U50,488H (You et al., 1999) and U69,593 (Rawls and McGinty, 1998; Gray et al., 1999), do not affect glutamate levels within the striatum. We are first to confirm, through microdialysis, that the clinically useful kappa receptor agonist, nalfurafine, does not affect glutamate levels in the striatum. In addition, Rawls et al. (1998) reported that NBNI increases glutamate levels within the region when given at a higher concentration; however, the concentration we selected (100 nM) did not affect glutamate levels (data not shown).
It is interesting that local infusion of an antagonist can reverse the activity of a systemically administered agonist, but not uncommon. Rawls and McGinty (2000) perfused naltrindole into the striatum and significantly reduced amphetamine-evoked glutamate levels in this region. Likewise, Gray et al. (1999) perfused NBNI directly into the striatum and reversed U69,593 inhibition of amphetamine-evoked glutamate stimulation within this region. Shippenberg et al. (1993) blocked the development of conditioned place preference induced by systemic morphine by infusing the D1 antagonist, SCH-23390, unilaterally into the ventral striatum. Lastly, perfusion of the NMDA antagonist, (+/-)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid, unilaterally into the striatum, blocked stimulant behavior caused by the systemic administration of the D1 receptor agonist, SKF-38393 (Keefe and Gerfen, 1996).
Since activation of kappa opioid receptors decreases glutamate release intrastriatally (Hill and Brotchie, 1995; Rawls and McGinty, 1998; Gray et al., 1999; Hill and Brotchie, 1999; Rawls et al., 1999) and extrastriatally (Gannon and Terrian, 1992; Maneuf et al., 1995; Sbrenna et al., 1999), we cannot establish how icilin acts to increase glutamate levels in the dorsal striatum or how a kappa agonist, like nalfurafine, reverses the behavioral or neurochemical action of icilin in rats. Although we have shown that icilin increases glutamate release we have not observed overt seizures in rats following intraperitoneal, intrastriatal or intracerebroventricular injection of icilin (data not shown). In future experiments, we must separate glutamatergic stimulation by extrastriatal afferents (e.g. corticostriatal and thalamocortico processes) from that of trans-synaptic glutamate release by targeting components of striatal circuitry (e.g. substantia nigra, globus pallidus, subthalamic nucleus) and better determine the role of the dorsal striatum in icilin-induced wet-dog shakes.
Finally, nalfurafine antagonizes icilin-evoked glutamate release within the dorsal striatum, yet fails to completely abolish the shaking behavior observed with even a low dose of icilin (0.50 mg/kg). Since kappa opioid receptor activation inhibits glutamate release in many extrastriatal brain regions, the failure of nalfurafine to completely antagonize icilin-induced wet-dog shakes along with the intensity of its overt stimulant behavior, supports the involvement of other brain regions [e.g. the hypothalamus (Maldonado et al., 1992), locus coereleus (Hoshi et al., 1987; Liu et al., 1999) hippocampus (Ohno et al., 1987) and ventral tegmental area (Wang et al., 2004)] and neurotransmitters [e.g. serotonin (Yap and Taylor, 1983; Fone et al., 1991), dopamine (David et al., 2005), acetylcholine (Turski et al., 1984)] in icilin-induced wet-dog shakes. In the future, it is imperative to investigate the role of multiple brain regions and neurotransmitters in icilin-evoked shaking behavior.
Nevertheless, we believe that our findings implicate a role for the central nervous system and, more specifically, the dorsal striatum in icilin-induced wet-dog shakes. By doing so, we suggest a possible mechanism for kappa-mediated inhibition of icilin-induced wet-dog shakes and extend the in vivo pharmacological profile of icilin, a therapeutically beneficial cold-inducing agent, beyond its role as an agonist on TRPM8 and TRPA1 receptors within the periphery.
Acknowledgments
This study was supported by DA13429 and T32DA07237 from the National Institute on Drug Abuse. The authors wish to thank Dr. Ronald J. Tallarida for advice on statistical analysis and Dr. Gennady N. Smagin for his helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Burford RG, Chappel CI. Abstracts of the Fifth International Congress of Pharmacology. Vol. 33 IUPHAR; San Francisco: 1972. “Wet dog shake” induction in rats by a novel compound AG-3-5. [Google Scholar]
- Butt NM, Collier HO, Cuthbert NJ, Francis DL, Saeed SA. Mechanism of quasi-morphine withdrawal behaviour induced by methylxanthines. Eur J Pharmacol. 1979;53:375–378. doi: 10.1016/0014-2999(79)90462-x. [DOI] [PubMed] [Google Scholar]
- Collier HO, Cuthbert NJ, Francis DL. Character and meaning of quasi-morphine withdrawal phenomena elicited by methylxanthines. Fed Proc. 1981;40:1513–1518. [PubMed] [Google Scholar]
- Cowan A. Quasi-morphine withdrawal syndrome: recent developments. Introduction. Fed Proc. 1981;40:1489–1490. [PubMed] [Google Scholar]
- Cowan A, Watson T. Lysergic acid diethylamide antagonizes shaking induced in rats by five chemically different compounds. Psychopharmacology. 1978;57:43–46. doi: 10.1007/BF00426956. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of "intact" animals. Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Fone KC, Robinson AJ, Marsden CA. Characterization of the 5-HT receptor subtypes involved in the motor behaviours produced by intrathecal administration of 5-HT agonists in rats. Br J Pharmacol. 1991;103:1547–1555. doi: 10.1111/j.1476-5381.1991.tb09825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon RL, Terrian DM. Kappa opioid agonists inhibit transmitter release from guinea pig hippocampal mossy fiber synaptosomes. Neurochem Res. 1992;17:741–747. doi: 10.1007/BF00969007. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Hill MP, Brotchie JM. Modulation of glutamate release by a kappa-opioid receptor agonist in rodent and primate striatum. Eur J Pharmacol. 1995;281:R1–R2. doi: 10.1016/0014-2999(95)00385-x. [DOI] [PubMed] [Google Scholar]
- Hill MP, Brotchie JM. Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum. Br J Pharmacol. 1999;127:275–283. doi: 10.1038/sj.bjp.0702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi K, Ma T, Oh S, Ho IK. Increased release of excitatory amino acids in rat locus coeruleus in kappa-opioid agonist dependent rats precipitated by nor-binaltorphimine. Brain Res. 1997;753:63–68. doi: 10.1016/s0006-8993(96)01492-8. [DOI] [PubMed] [Google Scholar]
- Inan S, Cowan A. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur J Pharmacol. 2004;502:233–237. doi: 10.1016/j.ejphar.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy D, Zygmont PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–266. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1 dopamine receptor-mediated induction of zif268 and c-fos in the dopamine-depleted striatum: differential regulation and independence from NMDA receptors. J Comp Neurol. 1996;367:165–176. doi: 10.1002/(SICI)1096-9861(19960401)367:2<165::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Liu N, Ho IK, Rockhold RW. Contribution of glutamatergic systems in locus coeruleus to nucleus paragigantocellularis stimulation-evoked behavior. Pharmacol Biochem Behav. 1999;63:555–567. doi: 10.1016/s0091-3057(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lubin ML, Reitz TL, Wang Y, Colburn RW, Flores CM, Qin N. Molecular identification and functional characterization of a temperature-sensitive transient receptor potential channel (TRPM8) from canine. Eur J Pharmacol. 2006;530:23–32. doi: 10.1016/j.ejphar.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC. Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann N Y Acad Sci. 2003;1003:226–240. doi: 10.1196/annals.1300.014. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Stinus L, Gold LH, Koob GF. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- Maneuf YP, Mitchell IJ, Crossman AR, Woodruff GN, Brotchie JM. Functional implications of kappa opioid receptor-mediated modulation of glutamate transmission in the output regions of the basal ganglia in rodent and primate models of Parkinson’s disease. Brain Res. 1995;683:102–108. doi: 10.1016/0006-8993(95)00358-w. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Araki H, Ueki S. The effect of kappa-opioid agonist U50, 488H on wet-dog shaking behavior induced by hippocampal stimulation in rats. Psychopharmacology. 1987;91:189–192. doi: 10.1007/BF00217060. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF. Kappa receptor activation attenuates L-trans-pyrrolidine-2,4-dicarboxylic acid-evoked glutamate levels in the striatum. J Neurochem. 1998;70:626–634. doi: 10.1046/j.1471-4159.1998.70020626.x. [DOI] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF. Delta opioid receptors regulate calcium-dependent, amphetamine-evoked glutamate levels in the rat striatum: an in vivo microdialysis study. Brain Res. 2000;861:296–304. doi: 10.1016/s0006-8993(00)02030-8. [DOI] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF, Terrian DM. Presynaptic kappa-opioid and muscarinic receptors inhibit the calcium-dependent component of evoked glutamate release from striatal synaptosomes. J Neurochem. 1999;73:1058–1065. doi: 10.1046/j.1471-4159.1999.0731058.x. [DOI] [PubMed] [Google Scholar]
- Reid G, Babes A, Pluteanu F. A cold- and menthol-activated current in rat dorsal root ganglion neurons: properties and role in cold transduction. J Physiol. 2002;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrenna S, Marti M, Morari M, Calo G, Guerrini R, Beani L, Bianchi C. L-glutamate and gamma-aminobutyric acid efflux from rat cerebrocortical synaptosomes: modulation by kappa- and mu- but not delta- and opioid receptor like-1 receptors. J Pharmacol Exp Ther. 1999;291:1365–1371. [PubMed] [Google Scholar]
- Seki T, Awamura S, Kimura C, Ide S, Sakano K, Minami M, Nagase H, Satoh M. Pharmacological properties of TRK-820 on cloned mu-, delta- and kappa-opioid receptors and nociceptin receptor. Eur J Pharmacol. 1999;376:159–167. doi: 10.1016/s0014-2999(99)00369-6. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Nat Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Turski WA, Czuczwar SJ, Turski L, Sieklucka-Dziuba M, Kleinrok Z. Studies on the mechanism of wet-dog shakes produced by carbachol in rats. Pharmacology. 1984;28:112–120. doi: 10.1159/000137951. [DOI] [PubMed] [Google Scholar]
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, Tanaka T, Nagase H. Antipruritic activity of the kappa-opioid receptor agonist, TRK-820. Eur J Pharmacol. 2002;435:259–264. doi: 10.1016/s0014-2999(01)01588-6. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A. Motor effects of intracaudate injection of excitatory amino acids. Pharmacol Biochem Behav. 1989;33:175–179. doi: 10.1016/0091-3057(89)90447-4. [DOI] [PubMed] [Google Scholar]
- Wang HL, Zhao Y, Xiang XH, Wang HS, Wu WR. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area attenuates the physical signs of morphine withdrawal in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1079–1087. doi: 10.1016/j.pnpbp.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Wei ET. Chemical stimulants of shaking behaviour. J Pharm Pharmacol. 1976;28:722–724. doi: 10.1111/j.2042-7158.1976.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Wei ET. Pharmacological aspects of shaking behavior produced by TRH, AG-3-5, and morphine withdrawal. Fed Proc. 1981;40:1491–1496. [PubMed] [Google Scholar]
- Wei ET. How best to fight that nasty itch – from new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches (Commentary 3) Exp Dermatol. 2005;14:234–235. doi: 10.1111/j.0906-6705.2005.0321a.x. [DOI] [PubMed] [Google Scholar]
- Wei ET, Seid DA. AG-3-5: a chemical producing sensations of cold. J Pharm Pharmacol. 1983;35:110–112. doi: 10.1111/j.2042-7158.1983.tb04279.x. [DOI] [PubMed] [Google Scholar]
- Werkheiser JL, Rawls SM, Cowan A. Mu and kappa opioid receptor agonists antagonize icilin-induced wet-dog shaking in rats. Eur J Pharmacol. 2006;547:101–105. doi: 10.1016/j.ejphar.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Werkheiser JL, Rawls SM, Cowan A. Icilin evokes a dose-and time-dependent increase in glutamate within the dorsal striatum of rats. Amino Acids. 2006;30:307–309. doi: 10.1007/s00726-005-0306-6. [DOI] [PubMed] [Google Scholar]
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y. Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol. 2005;16:3742–3747. doi: 10.1681/ASN.2005020152. [DOI] [PubMed] [Google Scholar]
- Yap CY, Taylor DA. Involvement of 5-HT2 receptors in the wet-dog shake behaviour induced by 5-hydroxytryptophan in the rat. Neuropharmacology. 1983;22:801–804. doi: 10.1016/0028-3908(83)90123-5. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]


