Abstract
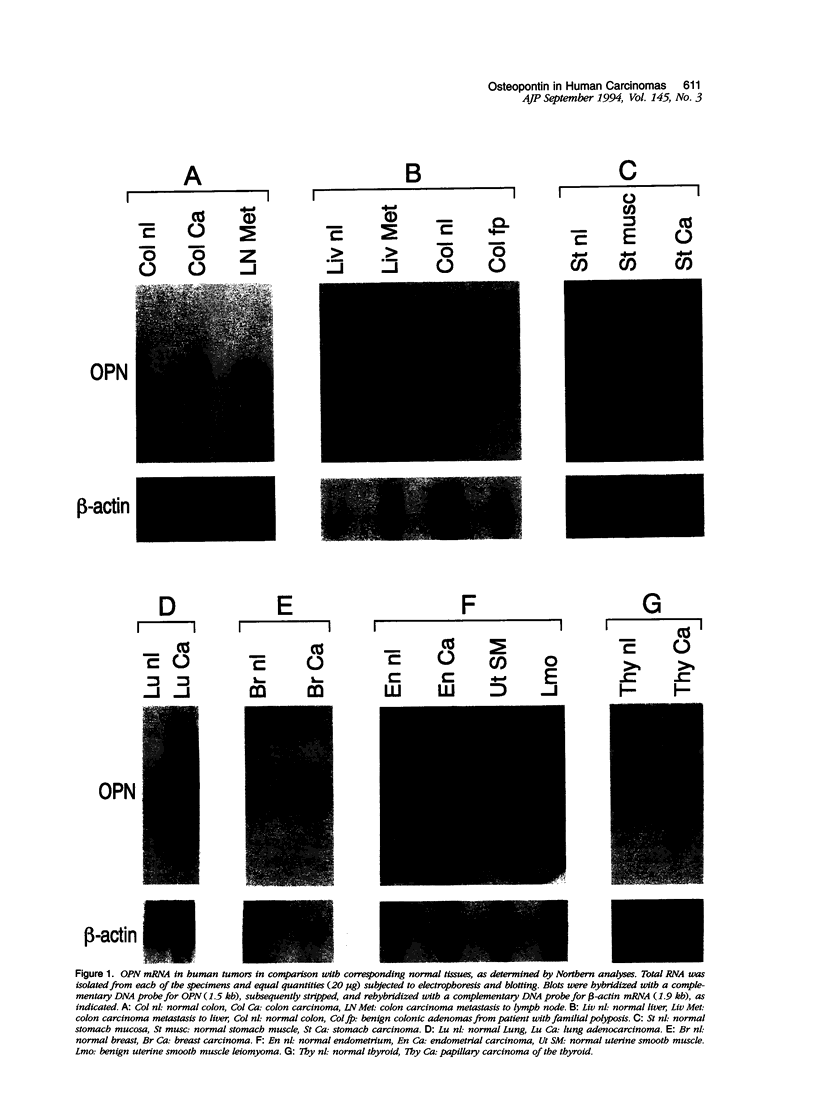
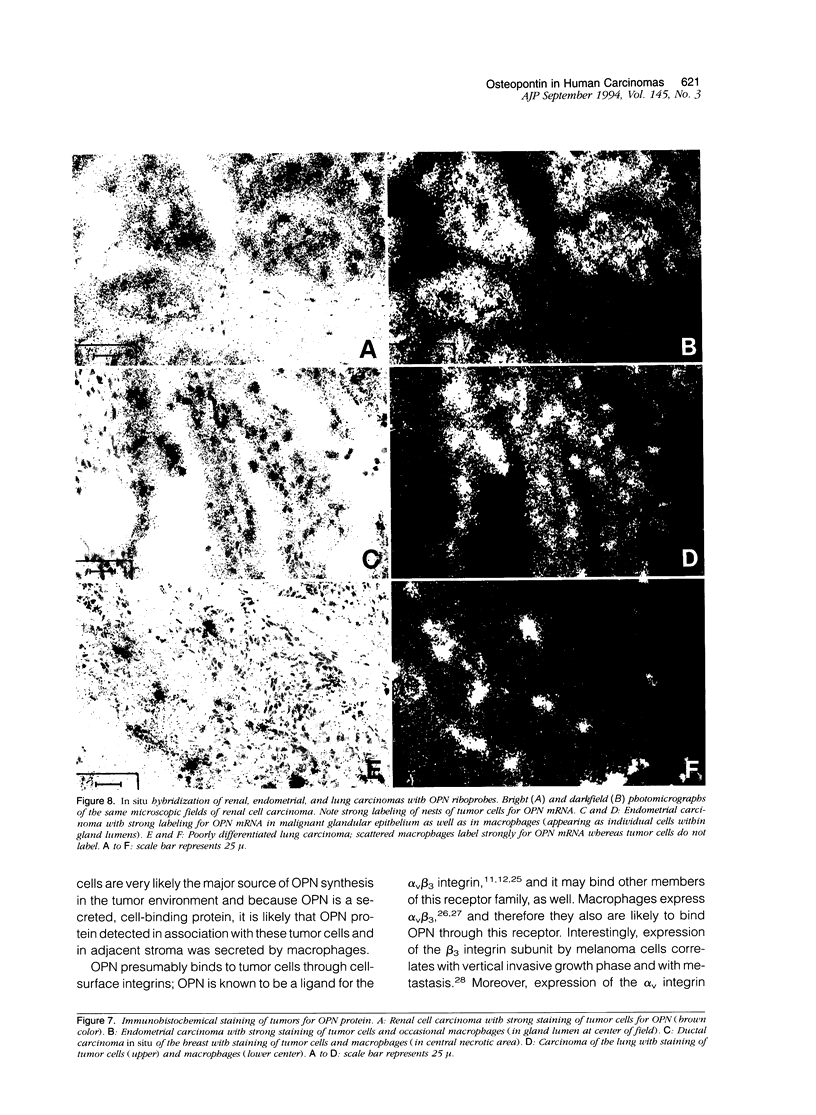
Osteopontin (OPN), a secreted adhesive glycoprotein, is significantly overexpressed in a variety of experimental models of malignancy. Moreover, increased levels of OPN have been detected in the blood of patients with metastatic carcinoma. To investigate OPN expression and distribution in human carcinomas directly, we studied a wide variety of common tumors by Northern analysis, in situ hybridization, and immunohistochemistry. All 14 tumors studied by Northern analysis showed very substantial increases in OPN messenger (m)RNA when compared to corresponding normal tissues. Moreover, intense labeling for OPN mRNA was detected in 71 of 76 carcinomas studied by in situ hybridization. In most of the carcinomas studied (colon, stomach, duodenum, pancreas, breast, lung, bladder, prostate, ovary, thyroid, and melanoma), tumor cells did not label detectably for OPN mRNA; however, macrophages intimately associated with tumor cells labeled strongly for the OPN transcript. In carcinomas of the kidney and endometrium, both tumor cells and host macrophages labeled strongly for OPN mRNA. The presence of OPN mRNA in macrophages was particularly pronounced at the edge of tumors (ie, the tumor/stroma interface) and in areas of tumor necrosis. Although in most cases tumor cells did not label detectably for OPN mRNA, both tumor cells and macrophages stained for OPN protein, suggesting that OPN secreted by macrophages may bind to tumor cells, possibly through the glycine-arginine-glycine-aspartate-serine cell binding domain in OPN. Collectively, these data suggest that OPN functions in adhesive interactions at the tumor/host interface and thereby may influence processes such as invasion and metastasis.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Boukerche H., Berthier-Vergnes O., Bailly M., Doré J. F., Leung L. L., McGregor J. L. A monoclonal antibody (LYP18) directed against the blood platelet glycoprotein IIb/IIIa complex inhibits human melanoma growth in vivo. Blood. 1989 Aug 15;74(3):909–912. [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Behrend E. I., Wilson S. M., Denhardt D. T. Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Res. 1992 Jan-Feb;12(1):43–47. [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Chen J., Singh K., Mukherjee B. B., Sodek J. Developmental expression of osteopontin (OPN) mRNA in rat tissues: evidence for a role for OPN in bone formation and resorption. Matrix. 1993 Mar;13(2):113–123. doi: 10.1016/s0934-8832(11)80070-3. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Structure, function and biological properties of integrin alpha v beta 3 on human melanoma cells. Cancer Metastasis Rev. 1991 May;10(1):3–10. doi: 10.1007/BF00046839. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Bowden G. T., Chambers A. F., Spearman M. A., Greenberg A. H., Wright J. A., McLeod M., Denhardt D. T. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990 Jul 15;46(1):133–137. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Nemir M., Mukherjee B. B., Chambers A. F., Denhardt D. T. Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):166–173. doi: 10.1016/s0006-291x(88)80028-7. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992 Jun;89(6):2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horny H. P., Schaumburg-Lever G., Bolz S., Geerts M. L., Kaiserling E. Use of monoclonal antibody KP1 for identifying normal and neoplastic human mast cells. J Clin Pathol. 1990 Sep;43(9):719–722. doi: 10.1136/jcp.43.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann H. G., Dedhar S. Distribution of integrins on human peripheral blood mononuclear cells. Blood. 1989 Sep;74(4):1348–1354. [PubMed] [Google Scholar]
- Krissansen G. W., Elliott M. J., Lucas C. M., Stomski F. C., Berndt M. C., Cheresh D. A., Lopez A. F., Burns G. F. Identification of a novel integrin beta subunit expressed on cultured monocytes (macrophages). Evidence that one alpha subunit can associate with multiple beta subunits. J Biol Chem. 1990 Jan 15;265(2):823–830. [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Alvarez J., Greenfield E. M., Teti A., Grano M., Colucci S., Zambonin-Zallone A., Ross F. P., Teitelbaum S. L., Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991 Oct 25;266(30):20369–20374. [PubMed] [Google Scholar]
- Moore M. A., Gotoh Y., Rafidi K., Gerstenfeld L. C. Characterization of a cDNA for chicken osteopontin: expression during bone development, osteoblast differentiation, and tissue distribution. Biochemistry. 1991 Mar 5;30(9):2501–2508. doi: 10.1021/bi00223a029. [DOI] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross F. P., Chappel J., Alvarez J. I., Sander D., Butler W. T., Farach-Carson M. C., Mintz K. A., Robey P. G., Teitelbaum S. L., Cheresh D. A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993 May 5;268(13):9901–9907. [PubMed] [Google Scholar]
- Senger D. R., Asch B. B., Smith B. D., Perruzzi C. A., Dvorak H. F. A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells. Nature. 1983 Apr 21;302(5910):714–715. doi: 10.1038/302714a0. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos-Sergiou A., Van de Water L. Adhesive properties of osteopontin: regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol Biol Cell. 1994 May;5(5):565–574. doi: 10.1091/mbc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A. Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res. 1989 Sep-Oct;9(5):1291–1299. [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Papadopoulos A., Tenen D. G. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989 Jun 13;996(1-2):43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Wirth D. F., Hynes R. O. Transformation-specific secreted phosophoproteins. Nature. 1980 Aug 7;286(5773):619–621. doi: 10.1038/286619a0. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Butler W. T., Foster R. A., Moehring J. M., Sauk J. J. Cell attachment activity of the 44 kilodalton bone phosphoprotein is not restricted to bone cells. Matrix. 1989 Jan;9(1):49–54. doi: 10.1016/s0934-8832(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]










