Abstract
Immunohistochemical and biochemical studies have demonstrated several different proteins in amyloid deposits that are not intrinsic components of the fibril itself but may play a role in their deposition and fibril formation. We compared the distribution of several amyloid-associated proteins, ie, amyloid P component, apolipoprotein-E, apolipoprotein-J, and vitronectin, in the deposits of several different amyloids, in particular light chain amyloid, with those in the deposits of nonamyloid monoclonal immunoglobulin, which may be considered a form of preamyloid disease. Although 100% of amyloid specimens (7 amyloid A, 15 immunoglobulin light chain, and 1 transthyretin) had amyloid P component and 100% had apolipoprotein-E (2 amyloid A, 10 immunoglobulin light chain, and 1 transthyretin) co-localized with the primary amyloid protein, none of the monoclonal nonamyloid cases (14 light chain deposition disease and 6 light and heavy chain deposition disease) had amyloid P component and only 1 of 11 had apolipoprotein-E. On the other hand, staining for apolipoprotein-J and vitronectin was positive in 100% of cases of amyloid and nonamyloid monoclonal deposits. The association between the presence of apolipoprotein-E and amyloid P component in the fibrillar form of monoclonal light chain deposits and their absence in the nonfibrillar form of deposits suggest a role for these proteins in the process of fibrillogenesis. This lends support for the previously proposed concept that apolipoprotein-E functions as a pathological chaperone by altering the conformation of amyloidogenic proteins.
Full text
PDF

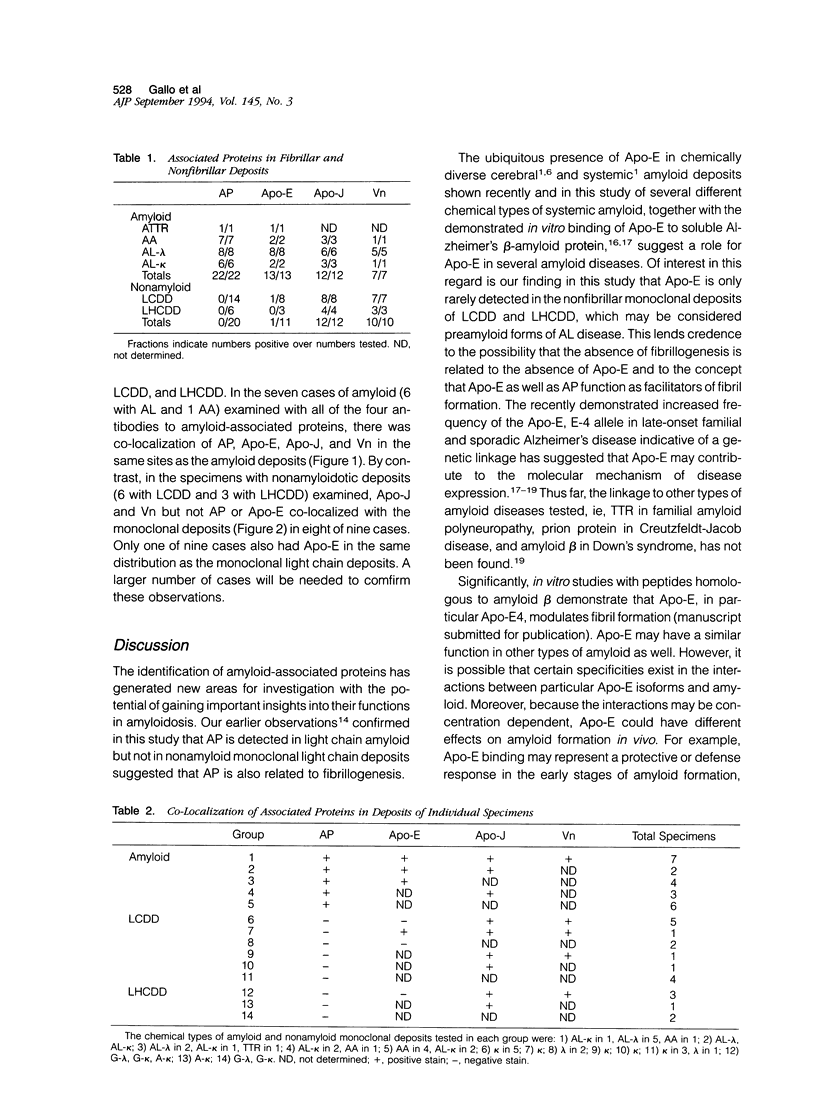


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Choi-Miura N. H., Ihara Y., Fukuchi K., Takeda M., Nakano Y., Tobe T., Tomita M. SP-40,40 is a constituent of Alzheimer's amyloid. Acta Neuropathol. 1992;83(3):260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Mazda T., Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989 Sep;26(9):835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Tobe T., Hara K., Yoshida H., Tomita M. Sandwich ELISA assay for quantitative measurement of SP-40,40 in seminal plasma and serum. J Immunol Methods. 1990 Aug 7;131(2):159–163. doi: 10.1016/0022-1759(90)90186-y. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Podack E., Dalmasso A. P., Jennette J. C. Localization of S protein and its relationship to the membrane attack complex of complement in renal tissue. Am J Pathol. 1987 Apr;127(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- French L. E., Tschopp J., Schifferli J. A. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992 Jun;88(3):389–393. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Picken M., Buxbaum J., Frangione B. The spectrum of monoclonal immunoglobulin deposition disease associated with immunocytic dyscrasias. Semin Hematol. 1989 Jul;26(3):234–245. [PubMed] [Google Scholar]
- Gallo G., Picken M., Frangione B., Buxbaum J. Nonamyloidotic monoclonal immunoglobulin deposits lack amyloid P component. Mod Pathol. 1988 Nov;1(6):453–456. [PubMed] [Google Scholar]
- Ghiso J., Matsubara E., Koudinov A., Choi-Miura N. H., Tomita M., Wisniewski T., Frangione B. The cerebrospinal-fluid soluble form of Alzheimer's amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993 Jul 1;293(Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. C., Finch C. E. Sulfated glycoprotein 2: new relationships of this multifunctional protein to neurodegeneration. Trends Neurosci. 1992 Oct;15(10):391–396. doi: 10.1016/0166-2236(92)90190-j. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Davies D. J., Morrow W., d'Apice A. J. Localization of terminal complement components S-protein and SP-40,40 in renal biopsies. Pathology. 1989 Oct;21(4):275–278. doi: 10.3109/00313028909061073. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Tomonaga M., Kawasaki H., Otomo E., Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991 Feb 8;541(1):163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- Picken M. M., Larrondo-Lillo M., Coria F., Gallo G. R., Shelanski M. L., Frangione B. Distribution of the protease inhibitor alpha 1-antichymotrypsin in cerebral and systemic amyloid. J Neuropathol Exp Neurol. 1990 Jan;49(1):41–48. doi: 10.1097/00005072-199001000-00005. [DOI] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Schmader K., Breitner J. C., Benson M. D., Brown W. T., Goldfarb L., Goldgaber D., Manwaring M. G., Szymanski M. H., McCown N. Apolipoprotein E epsilon 4 allele distributions in late-onset Alzheimer's disease and in other amyloid-forming diseases. Lancet. 1993 Sep 18;342(8873):710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- Skinner M., Cohen A. S., Shirahama T., Cathcart E. S. P-component (pentagonal unit) of amyloid: isolation, characterization, and sequence analysis. J Lab Clin Med. 1974 Oct;84(4):604–614. [PubMed] [Google Scholar]
- Snow A. D., Willmer J., Kisilevsky R. Sulfated glycosaminoglycans: a common constituent of all amyloids? Lab Invest. 1987 Jan;56(1):120–123. [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Duvic C. R., Wetterau J. R., Ray M. J., Ferguson D. G., Albers H. W., Smith W. R., Harmony J. A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990 Aug 5;265(22):13240–13247. [PubMed] [Google Scholar]