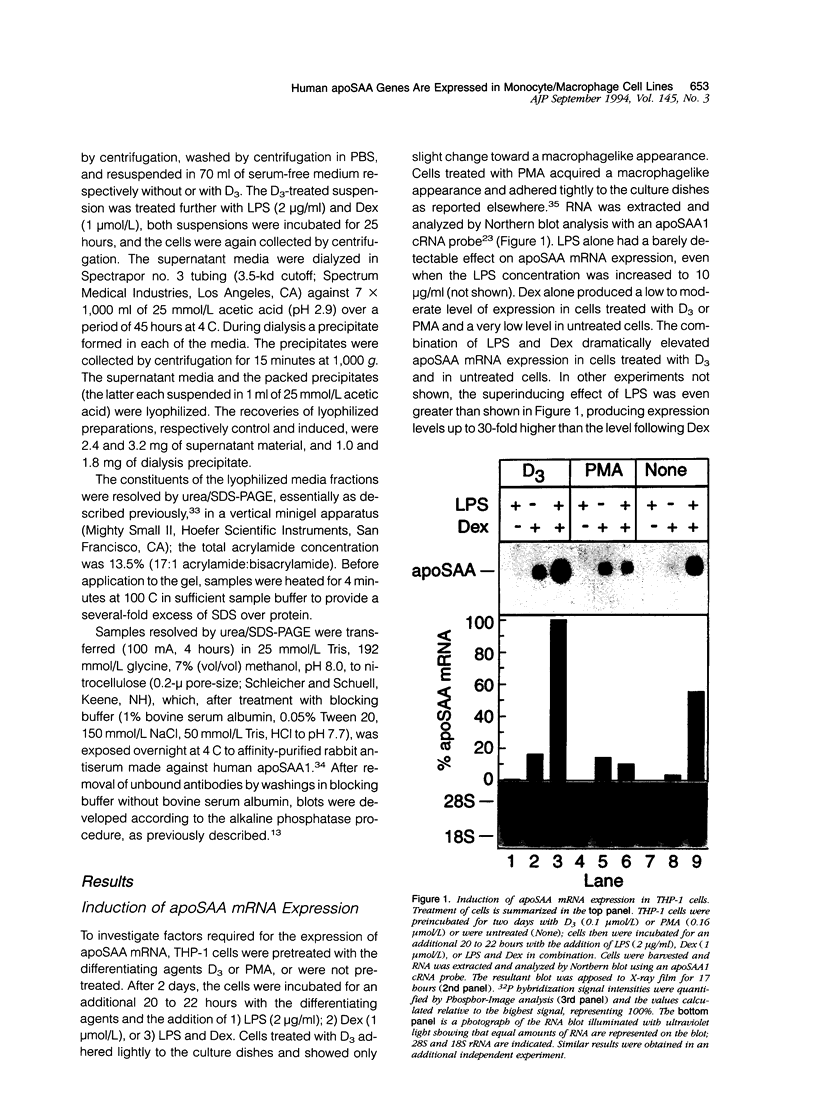
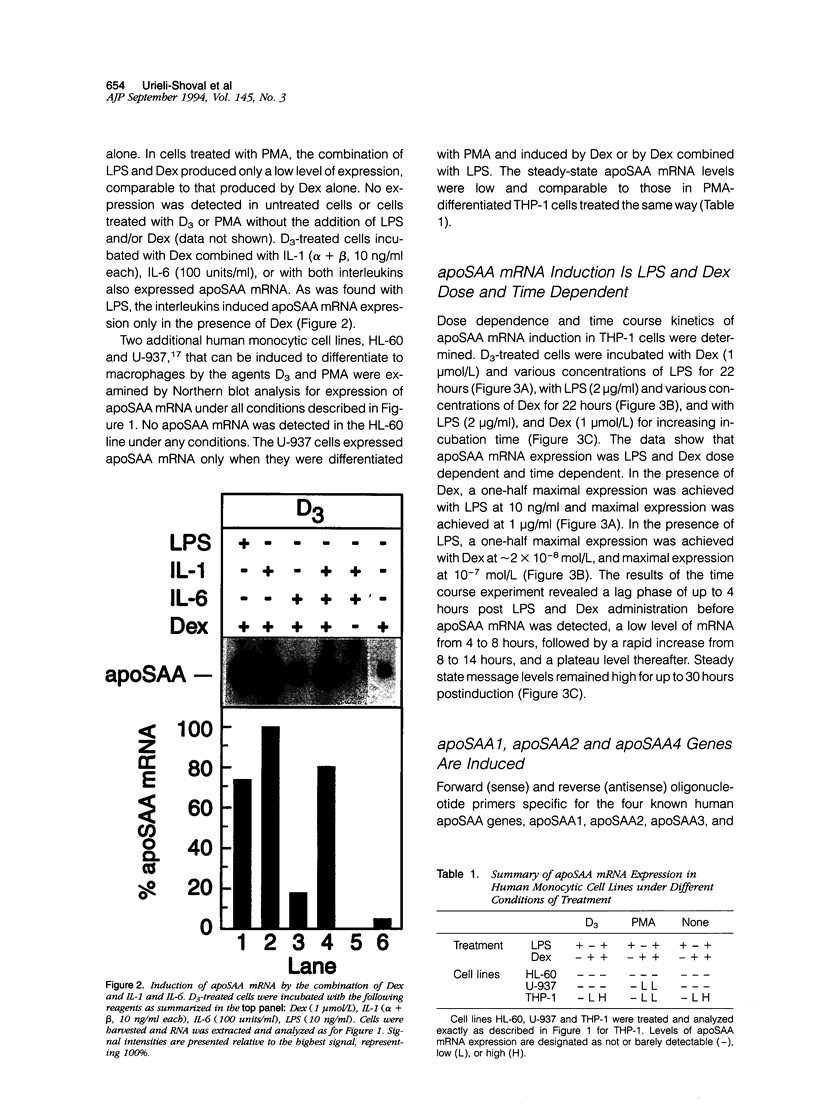
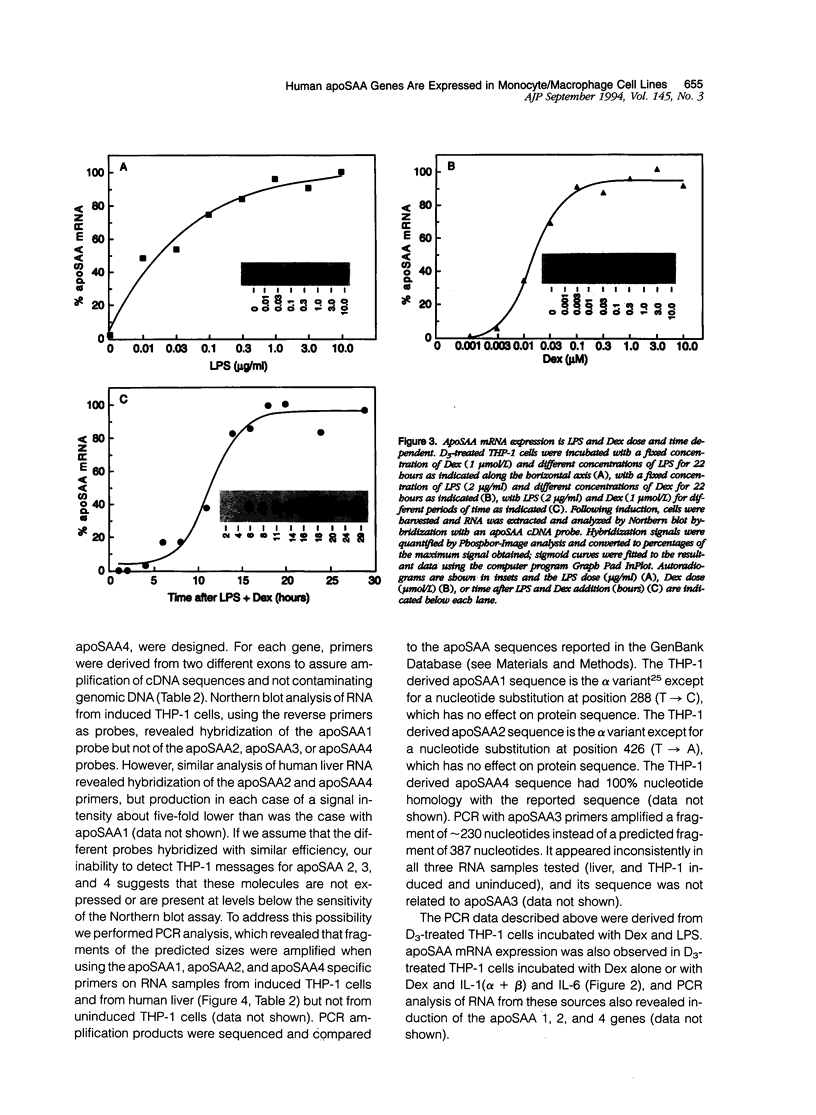
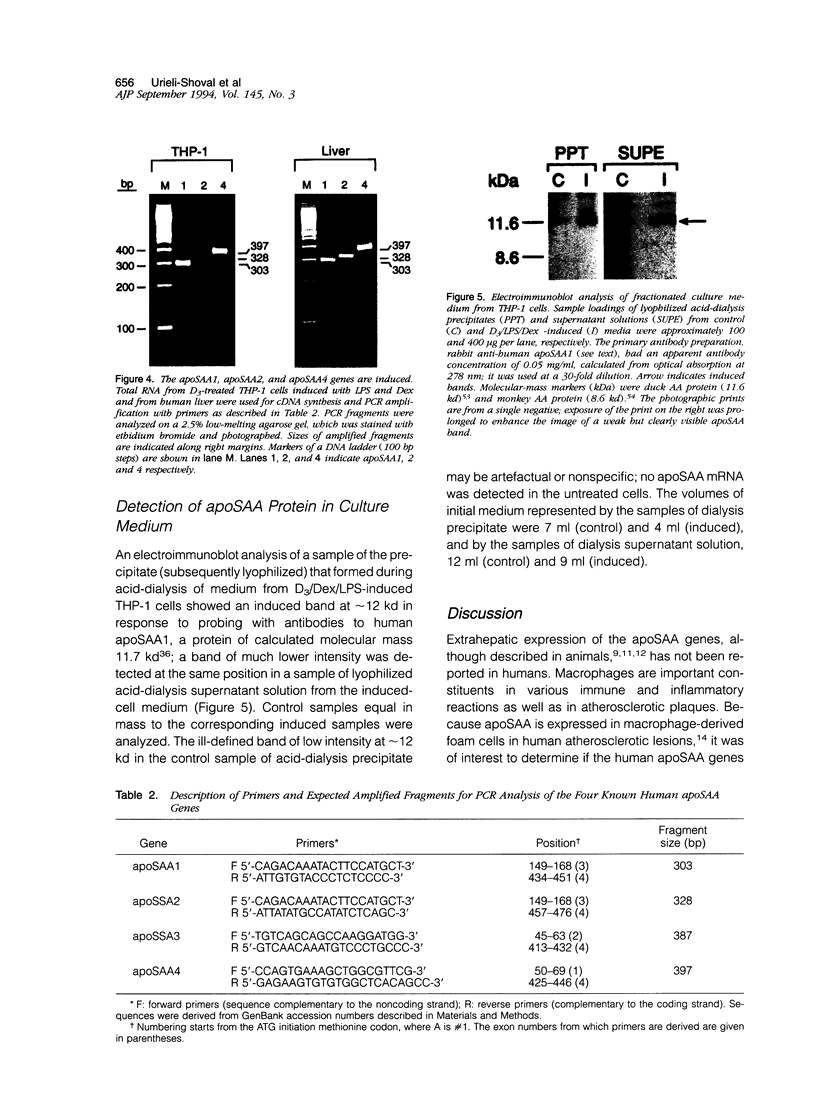
Abstract
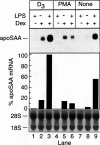
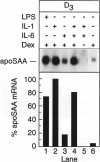
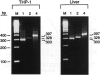
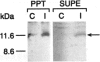
Serum amyloid A (apoSAA) is a family of proteins found, mainly associated with high density lipoproteins, in the blood plasma of mammals and at least one avian species, the Pekin duck. These proteins are present in small amounts under normal circumstances, but their concentration is capable of rising 100- to 1,000-fold in situations involving tissue injury or infection. Like classic acute phase proteins they are produced in the liver; however, expression of one of the apoSAA genes is known to occur in activated macrophages of mice. We examined three human macrophage precursor cell lines (THP-1, U-937, and HL-60), before and after differentiation with phorbol 12-myristate 13-acetate or 1 alpha,25-dihydroxy-vitamin D3, for apoSAA messenger (m)-RNA expression and found that: 1) induction of steady-state apoSAA mRNA by lipopolysaccharide, interleukin-1, or interleukin-6 required the presence of the synthetic glucocorticoid dexamethasone; 2) the three known active genes, apoSAA1, apoSAA2, and apoSAA4, were induced in THP-1 cells, whereas the pseudogene apoSAA3 was not; 3) differentiated and undifferentiated THP-1 cells expressed apoSAA mRNA, but U-937 cells expressed apoSAA mRNA (low levels) only after phorbol 12-myristate 13-acetate differentiation and HL-60 cells did not express apoSAA mRNA whether differentiated or not; 4) apoSAA protein was detectable immunologically at a low level in lyophilized medium from induced THP-1 cells. Our findings are compatible with the hypotheses that 1) apoSAA gene expression in human monocytes/macrophages in vivo is differentiation dependent; 2) activated macrophages provide a local source of apoSAA at sites of tissue injury or inflammation; 3) apoSAA is induced in tissue macrophages by local stimuli, under conditions that may not evoke the systemic acute phase response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahoshi T., Oppenheim J. J., Matsushima K. Induction of high-affinity interleukin 1 receptor on human peripheral blood lymphocytes by glucocorticoid hormones. J Exp Med. 1988 Mar 1;167(3):924–936. doi: 10.1084/jem.167.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldo-Benson M. A., Benson M. D. SAA suppression of immune response in vitro: evidence for an effect on T cell-macrophage interaction. J Immunol. 1982 Jun;128(6):2390–2392. [PubMed] [Google Scholar]
- Amasino R. M. Acceleration of nucleic acid hybridization rate by polyethylene glycol. Anal Biochem. 1986 Feb 1;152(2):304–307. doi: 10.1016/0003-2697(86)90413-6. [DOI] [PubMed] [Google Scholar]
- Auwerx J. The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia. 1991 Jan 15;47(1):22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Banka C. L., Black A. S., Dyer C. A., Curtiss L. K. THP-1 cells form foam cells in response to coculture with lipoproteins but not platelets. J Lipid Res. 1991 Jan;32(1):35–43. [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Morella K. K. Interaction of cytokine- and glucocorticoid-response elements of acute-phase plasma protein genes. Importance of glucocorticoid receptor level and cell type for regulation of the elements from rat alpha 1-acid glycoprotein and beta-fibrinogen genes. J Biol Chem. 1990 Dec 25;265(36):22275–22281. [PubMed] [Google Scholar]
- Betts J. C., Edbrooke M. R., Thakker R. V., Woo P. The human acute-phase serum amyloid A gene family: structure, evolution and expression in hepatoma cells. Scand J Immunol. 1991 Oct;34(4):471–482. doi: 10.1111/j.1365-3083.1991.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Mitchell T. I., Karmilowicz M. J., Kluve-Beckerman B., Benson M. D. Autocrine induction of collagenase by serum amyloid A-like and beta 2-microglobulin-like proteins. Science. 1989 Feb 3;243(4891):655–657. doi: 10.1126/science.2536953. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Hirano T., Kishimoto T., Heinrich P. C. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett. 1988 May 23;232(2):347–350. doi: 10.1016/0014-5793(88)80766-x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett. 1987 Jun 22;218(1):11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Serum amyloid A (ApoSAA) and lipoproteins. Methods Enzymol. 1986;128:311–320. doi: 10.1016/0076-6879(86)28076-3. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., Rzewnicki D., Samols D., Jiang S. L., Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991 Aug 15;147(4):1261–1265. [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Hass R. Retrodifferentiation--an alternative biological pathway in human leukemia cells. Eur J Cell Biol. 1992 Jun;58(1):1–11. [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R., Subrahmanyan L. Serum amyloid A changes high density lipoprotein's cellular affinity. A clue to serum amyloid A's principal function. Lab Invest. 1992 Jun;66(6):778–785. [PubMed] [Google Scholar]
- Kluve-Beckerman B., Drumm M. L., Benson M. D. Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 1991 Nov;10(9):651–661. doi: 10.1089/dna.1991.10.651. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke R. P., Bock V., Valet G., Rothe G. Inhibition of the oxidative burst response of N-formyl peptide-stimulated neutrophils by serum amyloid-A protein. Biochem Biophys Res Commun. 1991 May 15;176(3):1100–1105. doi: 10.1016/0006-291x(91)90397-p. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Malle E., Steinmetz A., Raynes J. G. Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis. 1993 Sep;102(2):131–146. doi: 10.1016/0021-9150(93)90155-n. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Eriksen N., Benditt E. P. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7949–7952. doi: 10.1073/pnas.89.17.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Eriksen N., Benditt E. P. Serum amyloid A in the mouse. Sites of uptake and mRNA expression. Am J Pathol. 1989 Aug;135(2):411–419. [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Urieli-Shoval S., Benditt E. P. Expression of apolipoprotein serum amyloid A mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid A function. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3186–3190. doi: 10.1073/pnas.91.8.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menju M., Tajima S., Yamamoto A. Expression of the apolipoprotein E gene in a human macrophage-like cell line, THP-1. J Biochem. 1989 Sep;106(3):505–510. doi: 10.1093/oxfordjournals.jbchem.a122882. [DOI] [PubMed] [Google Scholar]
- Mitchell T. I., Coon C. I., Brinckerhoff C. E. Serum amyloid A (SAA3) produced by rabbit synovial fibroblasts treated with phorbol esters or interleukin 1 induces synthesis of collagenase and is neutralized with specific antiserum. J Clin Invest. 1991 Apr;87(4):1177–1185. doi: 10.1172/JCI115116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Colten H. R. Expression and regulation of the murine serum amyloid A (SAA) gene in extrahepatic sites. J Immunol. 1985 Dec;135(6):3645–3647. [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr, Talbot C. C., Jr The human serum amyloid A locus SAA4 is a pseudogene. Biochem Biophys Res Commun. 1992 Mar 16;183(2):362–366. doi: 10.1016/0006-291x(92)90489-8. [DOI] [PubMed] [Google Scholar]
- Schooltink H., Schmitz-Van de Leur H., Heinrich P. C., Rose-John S. Up-regulation of the interleukin-6-signal transducing protein (gp130) by interleukin-6 and dexamethasone in HepG2 cells. FEBS Lett. 1992 Feb 10;297(3):263–265. doi: 10.1016/0014-5793(92)80552-r. [DOI] [PubMed] [Google Scholar]
- Sellar G. C., Whitehead A. S. Localization of four human serum amyloid A (SAA) protein superfamily genes to chromosome 11p: characterization of a fifth SAA-related gene sequence. Genomics. 1993 Jun;16(3):774–776. doi: 10.1006/geno.1993.1265. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Colten H. R., Goldberger G., Edge M. D., Tack B. F., Cohen A. S., Whitehead A. S. Human serum amyloid A (SAA): biosynthesis and postsynthetic processing of preSAA and structural variants defined by complementary DNA. Biochemistry. 1985 Jun 4;24(12):2931–2936. doi: 10.1021/bi00333a018. [DOI] [PubMed] [Google Scholar]
- Snyers L., De Wit L., Content J. Glucocorticoid up-regulation of high-affinity interleukin 6 receptors on human epithelial cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2838–2842. doi: 10.1073/pnas.87.7.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel D. M., Rogers J. T., DeBeer M. C., DeBeer F. C., Whitehead A. S. Biosynthesis of human acute-phase serum amyloid A protein (A-SAA) in vitro: the roles of mRNA accumulation, poly(A) tail shortening and translational efficiency. Biochem J. 1993 May 1;291(Pt 3):701–707. doi: 10.1042/bj2910701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel D. M., Sellar G. C., Uhlar C. M., Simon S., DeBeer F. C., Whitehead A. S. A constitutively expressed serum amyloid A protein gene (SAA4) is closely linked to, and shares structural similarities with, an acute-phase serum amyloid A protein gene (SAA2). Genomics. 1993 May;16(2):447–454. doi: 10.1006/geno.1993.1209. [DOI] [PubMed] [Google Scholar]
- Steinkasserer A., Weiss E. H., Schwaeble W., Linke R. P. Heterogeneity of human serum amyloid A protein. Five different variants from one individual demonstrated by cDNA sequence analysis. Biochem J. 1990 May 15;268(1):187–193. doi: 10.1042/bj2680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlar C. M., Burgess C. J., Sharp P. M., Whitehead A. S. Evolution of the serum amyloid A (SAA) protein superfamily. Genomics. 1994 Jan 15;19(2):228–235. doi: 10.1006/geno.1994.1052. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G., Coade S., Woo P. Analysis of the genomic and derived protein structure of a novel human serum amyloid A gene, SAA4. Scand J Immunol. 1992 Nov;36(5):703–712. doi: 10.1111/j.1365-3083.1992.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Whitehead A. S., de Beer M. C., Steel D. M., Rits M., Lelias J. M., Lane W. S., de Beer F. C. Identification of novel members of the serum amyloid A protein superfamily as constitutive apolipoproteins of high density lipoprotein. J Biol Chem. 1992 Feb 25;267(6):3862–3867. [PubMed] [Google Scholar]
- Woo P., Sipe J., Dinarello C. A., Colten H. R. Structure of a human serum amyloid A gene and modulation of its expression in transfected L cells. J Biol Chem. 1987 Nov 15;262(32):15790–15795. [PubMed] [Google Scholar]
- Zimlichman S., Danon A., Nathan I., Mozes G., Shainkin-Kestenbaum R. Serum amyloid A, an acute phase protein, inhibits platelet activation. J Lab Clin Med. 1990 Aug;116(2):180–186. [PubMed] [Google Scholar]