Abstract
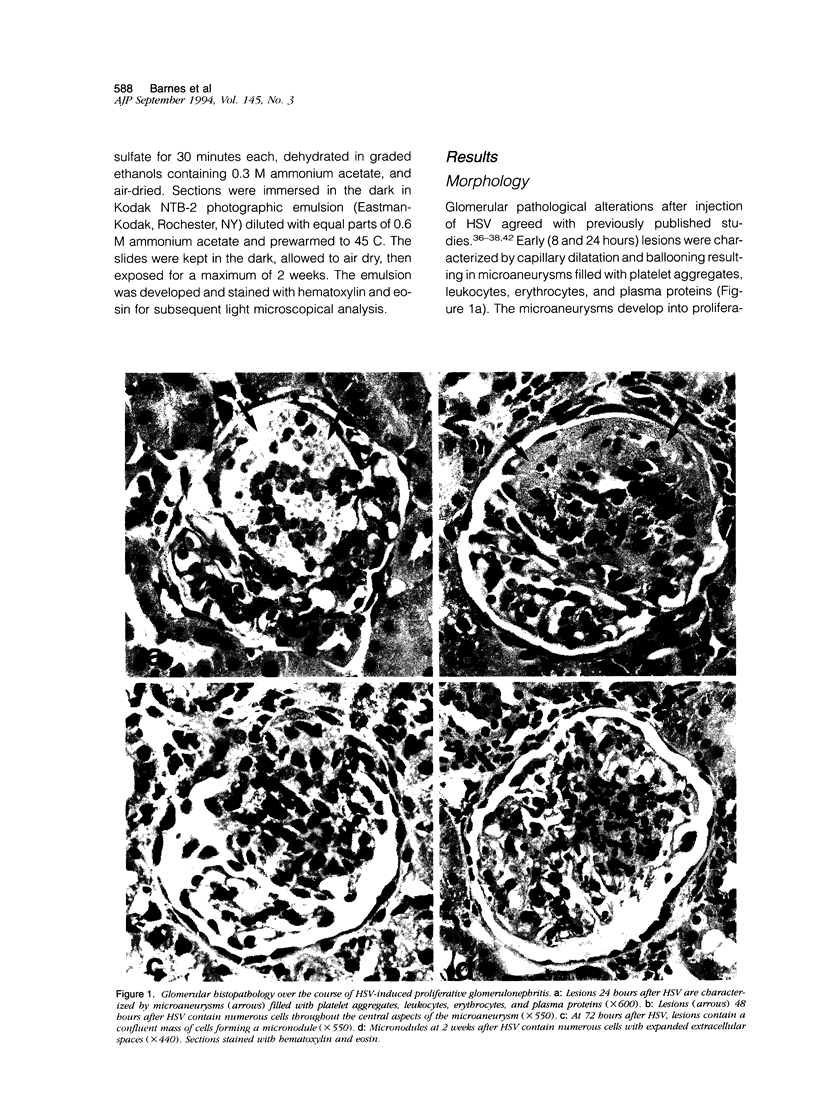
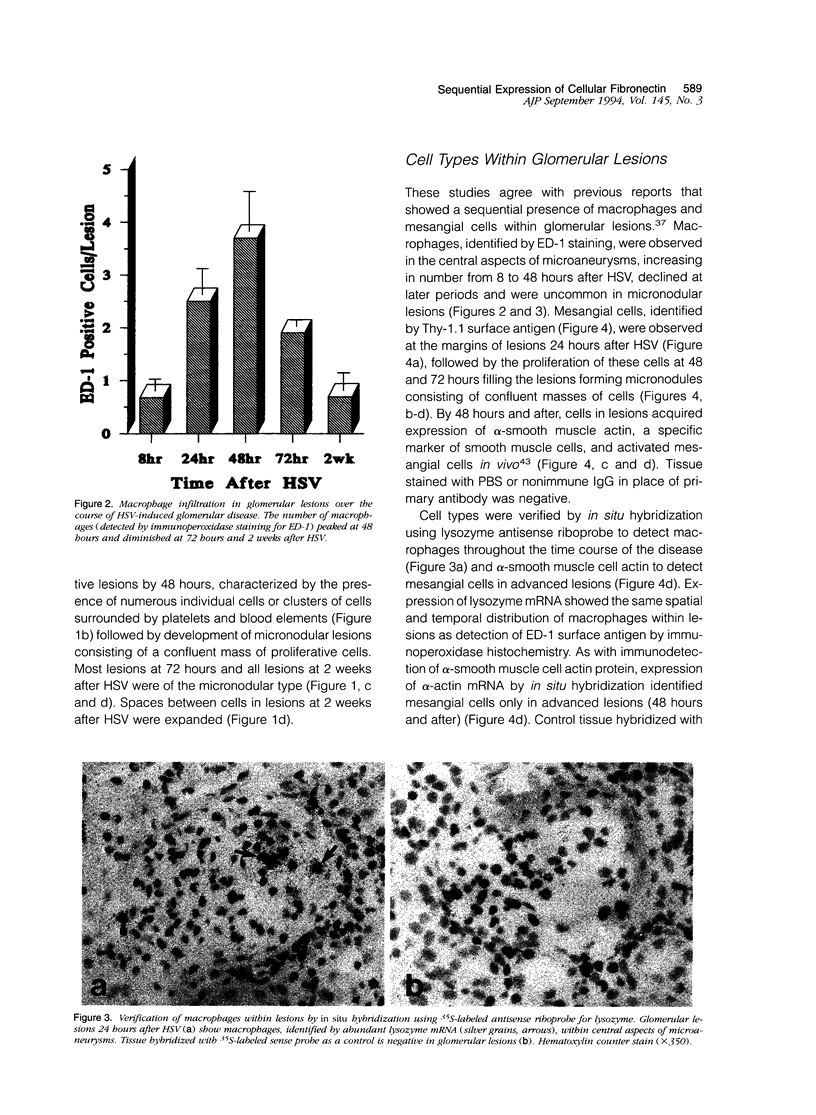
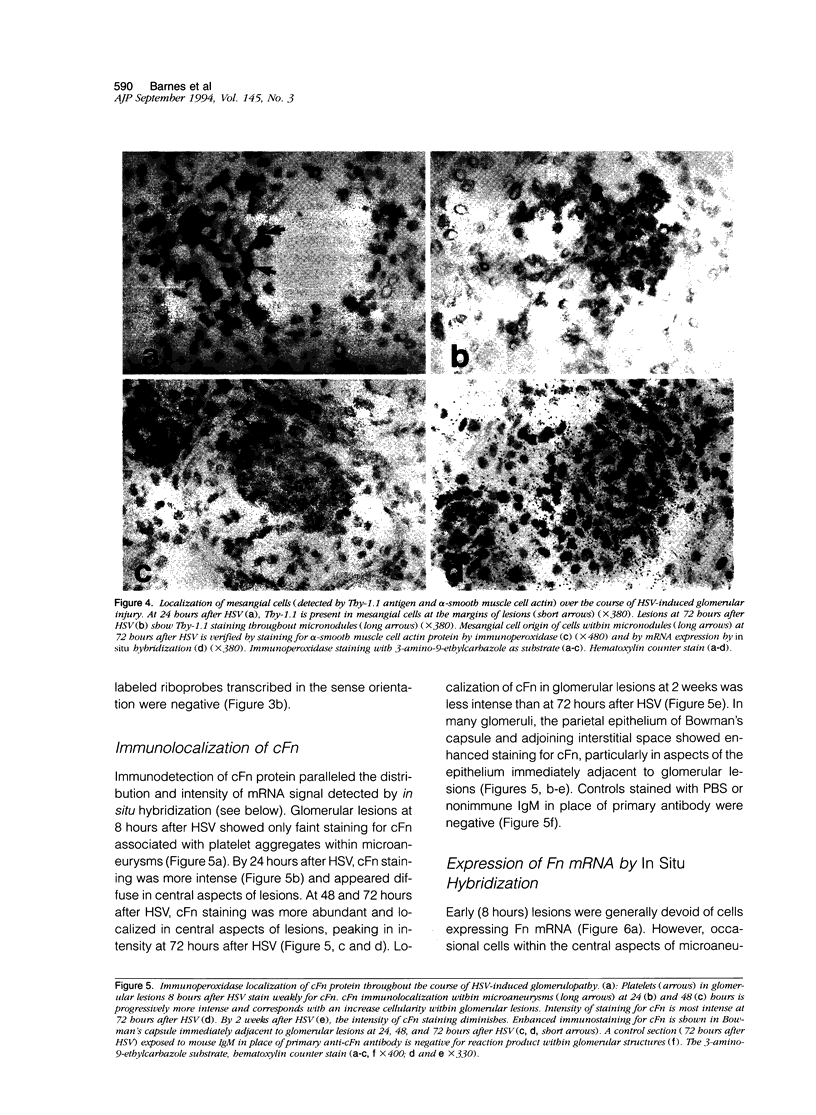
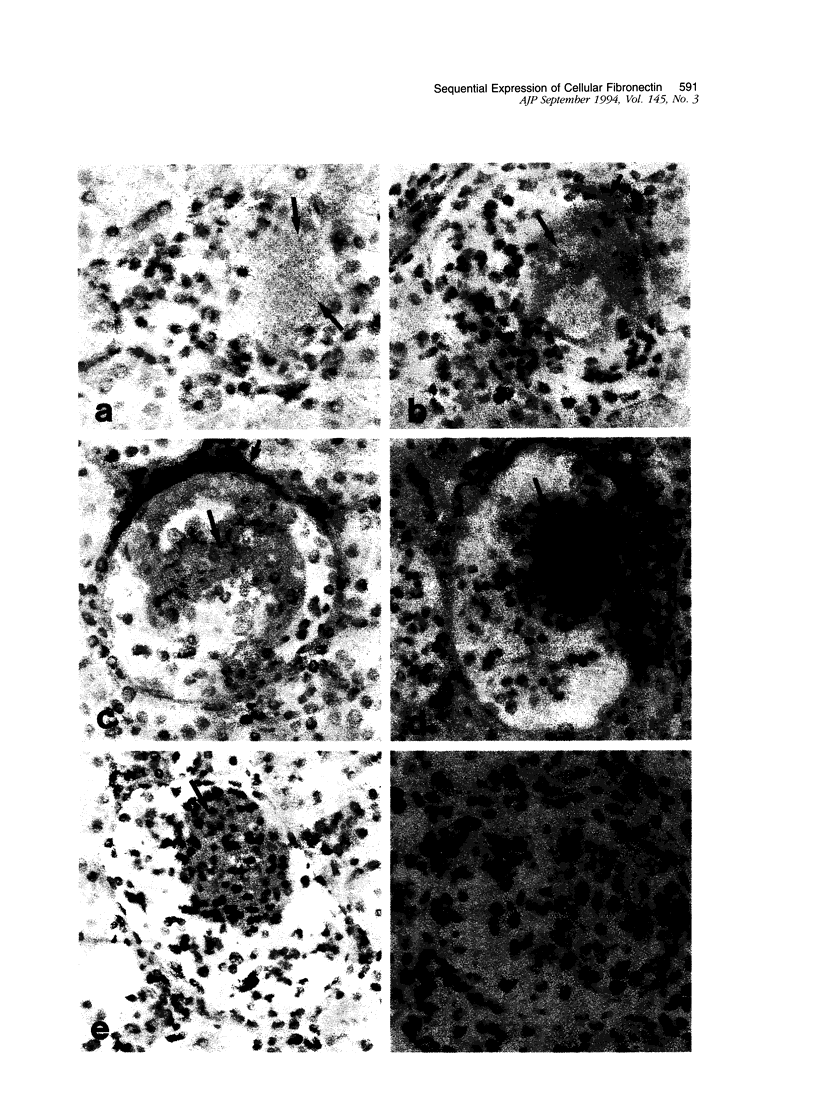
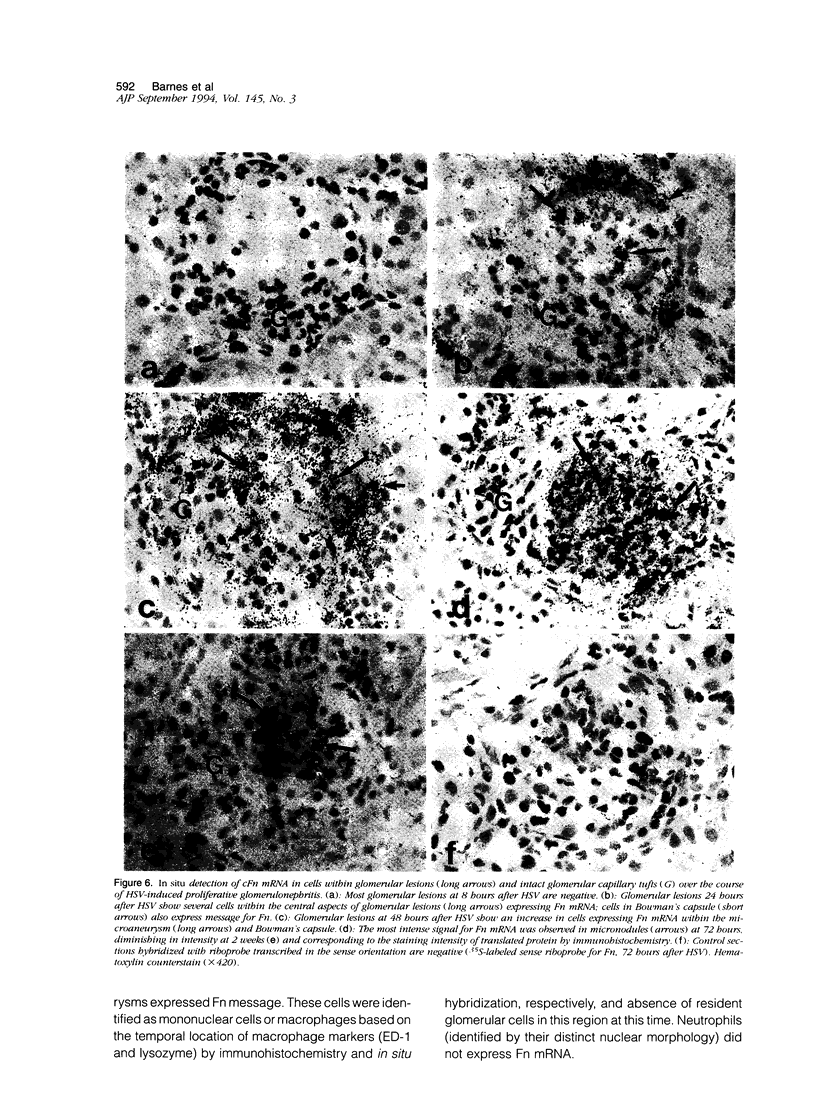
Fibronectin (Fn) regulates cell migration, proliferation, and extracellular matrix formation during embryogenesis, angiogenesis, and wound healing. Fn also promotes mesangial cell migration and proliferation in vitro and contributes to extracellular matrix formation and tissue remodeling during glomerular disease. In this study, we examined, by immunohistochemistry and in situ hybridization, the temporal glomerular localization and cellular sources of Fn in Habu snake venom (HSV)-induced proliferative glomerulonephritis. Early HSV-induced glomerular lesions consisted of microaneurysms devoid of resident glomerular cells and filled with platelets, leukocytes, and erythrocytes. Over the course of the disease, mesangial cells migrated into the lesions, proliferated, and formed a confluent cellular mass. Fn was present in lesions beginning at 8 hours, with highest intensity at 72 hours and diminishing at 2 weeks after HSV. Staining for Fn at 8 and 24 hours after HSV was attributed to platelets and macrophages. In situ hybridization and phenotypic identification of cell types within lesions revealed macrophages as the predominant source of cellular Fn mRNA at these times. At 48 hours after HSV, Fn mRNA was expressed in proliferating mesangial cells in addition to macrophages. Most cells in lesions at 72 hours after HSV were mesangial, at a time when expression of Fn mRNA peaked. Cellular expression for Fn mRNA and translated protein declined at 2 weeks after HSV. These studies support the hypothesis that Fn, derived from platelets and macrophages, provides a provisional matrix involved with mesangial cell migration into glomerular lesions. Fn produced by mesangial cells might contribute to the formation of a stable extracellular matrix.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. Characterization of glomerular epithelial cell matrix receptors. Am J Pathol. 1992 Sep;141(3):571–578. [PMC free article] [PubMed] [Google Scholar]
- Adler S., Eng B. Integrin receptors and function on cultured glomerular endothelial cells. Kidney Int. 1993 Aug;44(2):278–284. doi: 10.1038/ki.1993.242. [DOI] [PubMed] [Google Scholar]
- Alitalo K., Hovi T., Vaheri A. Fibronectin is produced by human macrophages. J Exp Med. 1980 Mar 1;151(3):602–613. doi: 10.1084/jem.151.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins R. C., Holdsworth S. R., Hancock W. W., Thomson N. M., Glasgow E. F. Cellular immune mechanisms in human glomerulonephritis: the role of mononuclear leucocytes. Springer Semin Immunopathol. 1982;5(3):269–296. doi: 10.1007/BF01892089. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Abboud H. E. Temporal expression of autocrine growth factors corresponds to morphological features of mesangial proliferation in Habu snake venom-induced glomerulonephritis. Am J Pathol. 1993 Nov;143(5):1366–1376. [PMC free article] [PubMed] [Google Scholar]
- Barnes J. L. Glomerular localization of platelet secretory proteins in mesangial proliferative lesions induced by habu snake venom. J Histochem Cytochem. 1989 Jul;37(7):1075–1082. doi: 10.1177/37.7.2659663. [DOI] [PubMed] [Google Scholar]
- Barnes J. L., Hevey K. A. Glomerular mesangial cell migration in response to platelet-derived growth factor. Lab Invest. 1990 Mar;62(3):379–382. [PubMed] [Google Scholar]
- Barnes J. L., Hevey K. A. Glomerular mesangial cell migration. Response to platelet secretory products. Am J Pathol. 1991 Apr;138(4):859–866. [PMC free article] [PubMed] [Google Scholar]
- Barnes J. L., Hevey K. A., Hastings R. R., Bocanegra R. A. Mesangial cell migration precedes proliferation in Habu snake venom-induced glomerular injury. Lab Invest. 1994 Apr;70(4):460–467. [PubMed] [Google Scholar]
- Brown L. F., Dubin D., Lavigne L., Logan B., Dvorak H. F., Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993 Mar;142(3):793–801. [PMC free article] [PubMed] [Google Scholar]
- Cattell V., Bradfield J. W. Focal mesangial proliferative glomerulonephritis in the rat caused by habu snake venom. A morphologic study. Am J Pathol. 1977 Jun;87(3):511–524. [PMC free article] [PubMed] [Google Scholar]
- Cosio F. G., Sedmak D. D., Nahman N. S., Jr Cellular receptors for matrix proteins in normal human kidney and human mesangial cells. Kidney Int. 1990 Nov;38(5):886–895. doi: 10.1038/ki.1990.287. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Kanwar Y. S., Hynes R. O., Farquhar M. G. Fibronectin localization in the rat glomerulus. J Cell Biol. 1980 Dec;87(3 Pt 1):691–696. doi: 10.1083/jcb.87.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Pesek-Diamond I., Diamond J. R. Cholesterol, macrophages, and gene expression of TGF-beta 1 and fibronectin during nephrosis. Am J Physiol. 1993 Apr;264(4 Pt 2):F577–F584. doi: 10.1152/ajprenal.1993.264.4.F577. [DOI] [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Kornblihtt A. R., Thiery J. P. The role of fibronectins in embryonic cell migrations. Trends Genet. 1988 Jul;4(7):198–203. doi: 10.1016/0168-9525(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Alpers C. E., Burns M. W., Pritzl P., Gordon K., Couser W. G., Johnson R. J. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Lab Invest. 1992 Apr;66(4):485–497. [PubMed] [Google Scholar]
- Floege J., Johnson R. J., Gordon K., Yoshimura A., Campbell C., Iruela-Arispe L., Alpers C. E., Couser W. G. Altered glomerular extracellular matrix synthesis in experimental membranous nephropathy. Kidney Int. 1992 Sep;42(3):573–585. doi: 10.1038/ki.1992.321. [DOI] [PubMed] [Google Scholar]
- Goldstein C. S., Garrick R. E., Polin R. A., Gerdes J. S., Kolski G. B., Neilson E. G., Douglas S. D. Fibronectin and complement secretion by monocytes and peritoneal macrophages in vitro from patients undergoing continuous ambulatory peritoneal dialysis. J Leukoc Biol. 1986 Apr;39(4):457–464. doi: 10.1002/jlb.39.4.457. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Martinez-Lacabe V., Virtanen I., Sahlin K. M., Schwartz M. M. Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest. 1992 Jul;67(1):71–79. [PubMed] [Google Scholar]
- Goyal M., Wiggins R. Fibronectin mRNA and protein accumulation, distribution, and breakdown in rabbit anti-glomerular basement membrane disease. J Am Soc Nephrol. 1991 Jun;1(12):1334–1342. doi: 10.1681/ASN.V1121334. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibronectin and wound healing. J Cell Biochem. 1984;26(2):107–116. doi: 10.1002/jcb.240260206. [DOI] [PubMed] [Google Scholar]
- Houser M. T., Scheinman J. I., Basgen J., Steffes M. W., Michael A. F. Preservation of mesangium and immunohistochemically defined antigens in glomerular basement membrane isolated by detergent extraction. J Clin Invest. 1982 May;69(5):1169–1175. doi: 10.1172/JCI110553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Ikeya M., Nagase M., Honda N. Intraglomerular distribution of fibronectin in primary glomerular diseases. Clin Nephrol. 1985 Aug;24(2):53–59. [PubMed] [Google Scholar]
- Ishimura E., Sterzel R. B., Budde K., Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. Am J Pathol. 1989 Apr;134(4):843–855. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Iida H., Alpers C. E., Majesky M. W., Schwartz S. M., Pritzi P., Gordon K., Gown A. M. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991 Mar;87(3):847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Analysis of alpha-smooth-muscle actin mRNA expression in rat aortic smooth-muscle cells using a specific cDNA probe. Differentiation. 1987;34(3):201–209. doi: 10.1111/j.1432-0436.1987.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Laitinen L., Vartio T., Virtanen I. Cellular fibronectins are differentially expressed in human fetal and adult kidney. Lab Invest. 1991 Apr;64(4):492–498. [PubMed] [Google Scholar]
- Limper A. H., Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992 Jun;101(6):1663–1673. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- Linder E., Miettinen A., Törnroth T. Fibronectin as a marker for the glomerular mesangium in immunohistology of kidney biopsies. Lab Invest. 1980 Jan;42(1):70–75. [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Stein H., Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991 Dec;139(6):1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Mosquera J. A. Increase production of fibronectin by glomerular cultures from rats with nephrotoxic nephritis. Macrophages induce fibronectin production in cultured mesangial cells. Lab Invest. 1993 Apr;68(4):406–412. [PubMed] [Google Scholar]
- Oberley T. D., Mosher D. F., Mills M. D. Localization of fibronectin within the renal glomerulus and its production by cultured glomerular cells. Am J Pathol. 1979 Sep;96(3):651–662. [PMC free article] [PubMed] [Google Scholar]
- Oomura A., Nakamura T., Arakawa M., Ooshima A., Isemura M. Alterations in the extracellular matrix components in human glomerular diseases. Virchows Arch A Pathol Anat Histopathol. 1989;415(2):151–159. doi: 10.1007/BF00784353. [DOI] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Plow E. F., Birdwell C., Ginsberg M. H. Identification and quantitation of platelet-associated fibronectin antigen. J Clin Invest. 1979 Mar;63(3):540–543. doi: 10.1172/JCI109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991 Oct;13(10):527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Tararak E. M., Samokhin G. P., Mitkevich O. V., Mazurov A. V., Vinogradov D. V., Vlasik T. N., Kalantarov G. F., Koteliansky V. E. Visualization of apo B, fibrinogen/fibrin, and fibronectin in the intima of normal human aorta and large arteries and during atherosclerosis. Atherosclerosis. 1990 Jun;82(3):213–226. doi: 10.1016/0021-9150(90)90043-i. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Culp L. A., Dunn M. J. Rat mesangial cell-matrix interactions in culture. Exp Cell Res. 1989 Oct;184(2):484–498. doi: 10.1016/0014-4827(89)90346-7. [DOI] [PubMed] [Google Scholar]
- Sottile J., Mosher D. F., Fullenweider J., George J. N. Human platelets contain mRNA transcripts for platelet factor 4 and actin. Thromb Haemost. 1989 Dec 29;62(4):1100–1102. [PubMed] [Google Scholar]
- Tamaki K., Okuda S., Ando T., Iwamoto T., Nakayama M., Fujishima M. TGF-beta 1 in glomerulosclerosis and interstitial fibrosis of adriamycin nephropathy. Kidney Int. 1994 Feb;45(2):525–536. doi: 10.1038/ki.1994.68. [DOI] [PubMed] [Google Scholar]
- Weiss M. A., Ooi B. S., Ooi Y. M., Engvall E., Ruoslahti E. Immunofluorescent localization of fibronectin in the human kidney. Lab Invest. 1979 Oct;41(4):340–347. [PubMed] [Google Scholar]
- Yamamoto T., Nakamura T., Noble N. A., Ruoslahti E., Border W. A. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Noble N. A., Miller D. E., Border W. A. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int. 1994 Mar;45(3):916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Martinet Y., Crystal R. G. Modulation of fibronectin gene expression in human mononuclear phagocytes. J Clin Invest. 1987 Dec;80(6):1720–1727. doi: 10.1172/JCI113263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama T., Nagase M., Ikeya M., Hishida A., Honda N. Intraglomerular fibronectin in rat experimental glomerulonephritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(3):179–188. doi: 10.1007/BF02899681. [DOI] [PubMed] [Google Scholar]
- van Goor H., van der Horst M. L., Fidler V., Grond J. Glomerular macrophage modulation affects mesangial expansion in the rat after renal ablation. Lab Invest. 1992 May;66(5):564–571. [PubMed] [Google Scholar]