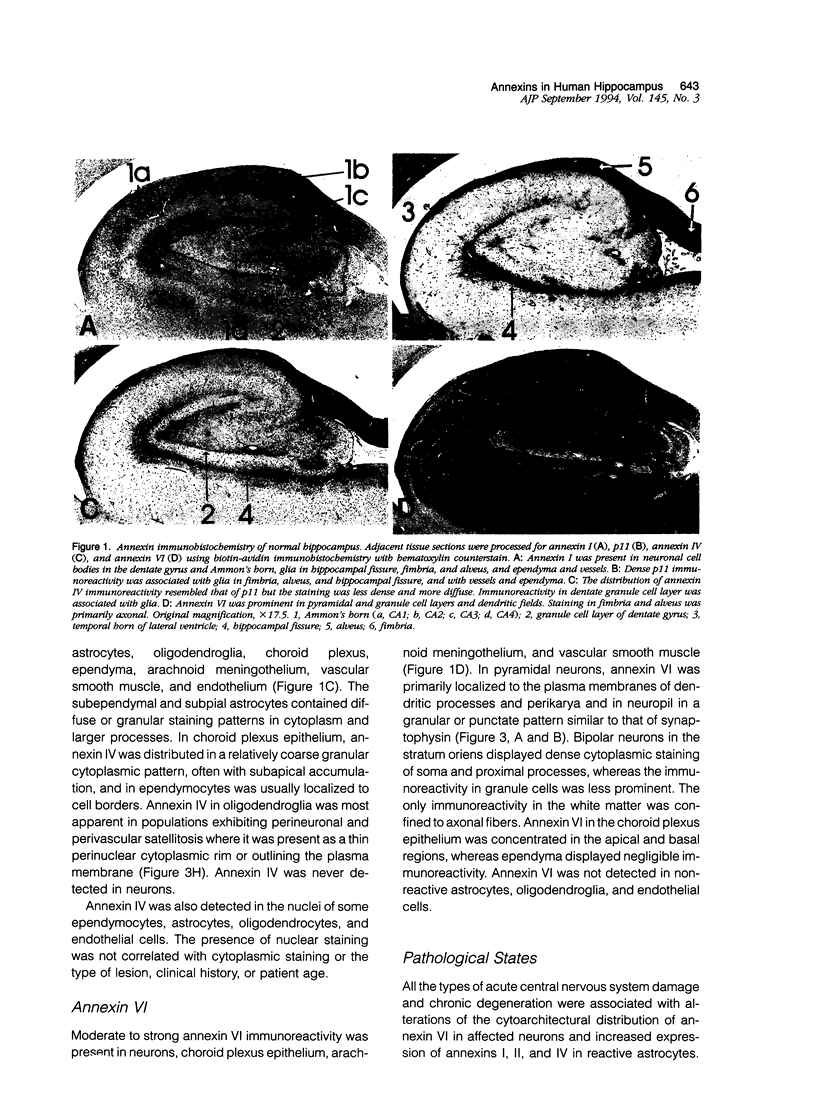
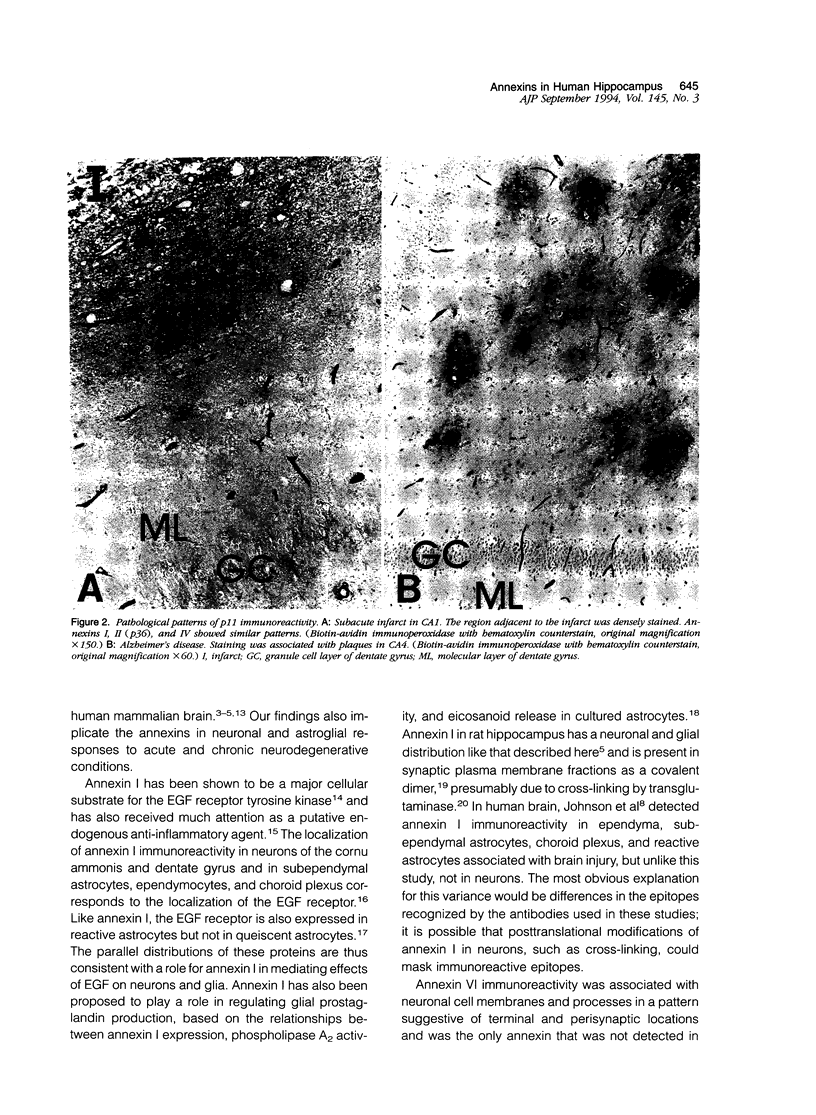
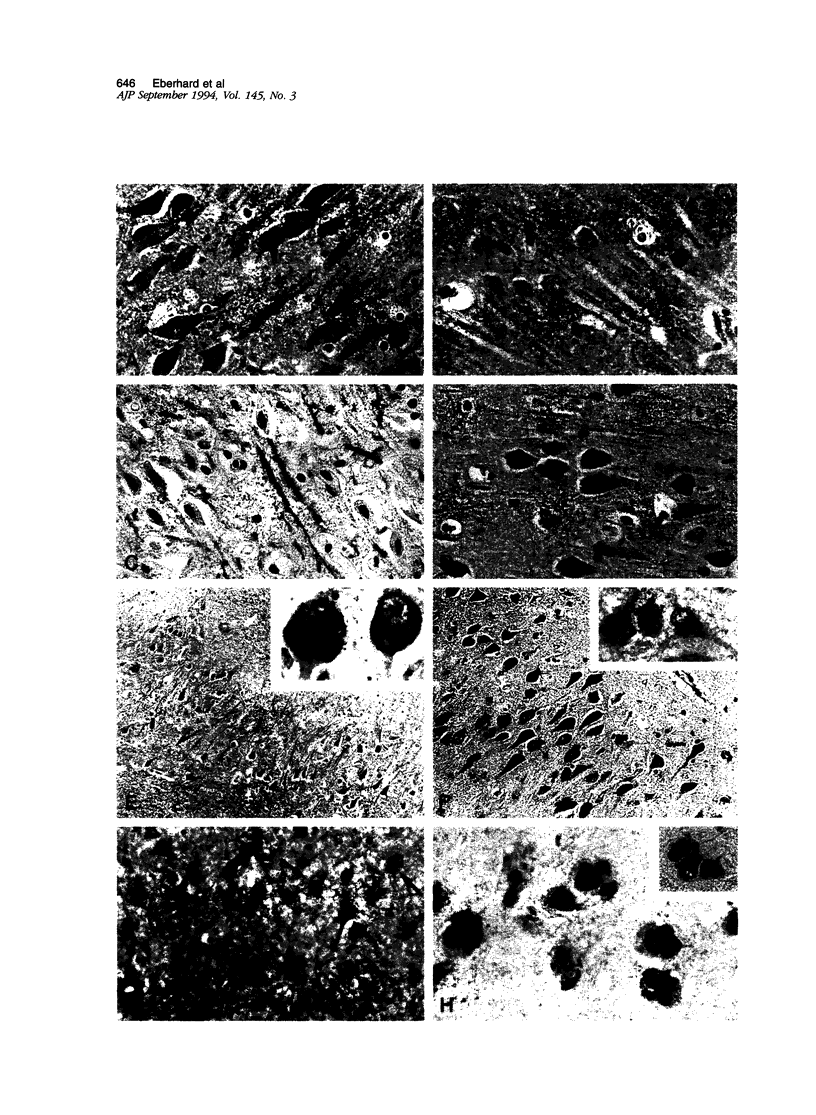
Abstract
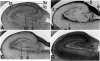
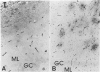
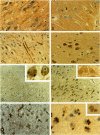
Annexins are Ca(2+)-dependent membrane-binding proteins that are potentially important in Ca(2+)-induced neurotoxicity or neuroprotection. To address the possible involvement of annexins in cellular reactions to brain injury and neurodegenerative disease, we studied the immunohistochemical localization of annexins I, II (p36 and p11), IV, and VI in the adult human hippocampus. Formalin-fixed, paraffin-embedded tissue from autopsy cases representing hypoxic-ischemic injury, seizure disorders, Alzheimer's disease, and age-related controls were examined. Neurons showed cytoplasmic immunoreactivity for annexin I, whereas annexin VI was distributed in patterns suggesting plasma membrane and perisynaptic locations. The cytoarchitectural distribution of annexin VI within neurons was altered in pathological states and annexin VI was strongly associated with neuronal granulovacuolar bodies in Alzheimer's disease. Reactive astrocytes expressed annexins I, II (p36 and p11), and IV, whereas quiescent astrocytes were minimally immunoreactive. Significant annexin immunoreactivity was also detected in oligodendrocytes (annexin IV), ependymocytes (I, II, and IV), choroid plexus (I, IV, and VI), meningothelium (I, II, IV, and VI), and vascular endothelium (II and IV) and smooth muscle (I, IV, and VI). This is the first comparative study of immunoreactivities for multiple annexins in human brain. Neurons and glia display selective and different profiles of annexin protein expression and show immunohistochemical changes in pathological conditions, which suggest involvement of annexins in neuronal and glial reactions to injury.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alafuzoff I., Adolfsson R., Grundke-Iqbal I., Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol. 1987;73(2):160–166. doi: 10.1007/BF00693782. [DOI] [PubMed] [Google Scholar]
- Ando Y., Imamura S., Owada M. K., Kannagi R. Calcium-induced intracellular cross-linking of lipocortin I by tissue transglutaminase in A431 cells. Augmentation by membrane phospholipids. J Biol Chem. 1991 Jan 15;266(2):1101–1108. [PubMed] [Google Scholar]
- Birecree E., Whetsell W. O., Jr, Stoscheck C., King L. E., Jr, Nanney L. B. Immunoreactive epidermal growth factor receptors in neuritic plaques from patients with Alzheimer's disease. J Neuropathol Exp Neurol. 1988 Sep;47(5):549–560. doi: 10.1097/00005072-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Black M. D., Carey F., Crossman A. R., Relton J. K., Rothwell N. J. Lipocortin-1 inhibits NMDA receptor-mediated neuronal damage in the striatum of the rat. Brain Res. 1992 Jul 10;585(1-2):135–140. doi: 10.1016/0006-8993(92)91198-n. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Wischik C. M., Novak M., Roth M. Sequestration of tau by granulovacuolar degeneration in Alzheimer's disease. Am J Pathol. 1991 Sep;139(3):641–647. [PMC free article] [PubMed] [Google Scholar]
- Braslau D. L., Ringo D. L., Rocha V. Synthesis of novel calcium-dependent proteins associated with mammary epithelial cell migration and differentiation. Exp Cell Res. 1984 Nov;155(1):213–221. doi: 10.1016/0014-4827(84)90782-1. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cambray-Deakin M. A., Norman K. M. Developmental regulation of tyrosine kinase substrate p36 (calpactin heavy chain) in rat cerebellum. J Mol Neurosci. 1989;1(1):47–54. doi: 10.1007/BF02896856. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Christmas P., Callaway J., Fallon J., Jones J., Haigler H. T. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J Biol Chem. 1991 Feb 5;266(4):2499–2507. [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz M., Hamilton S. L., Kaetzel M. A., Hazarika P., Dedman J. R. Modulation of Ca2+ release channel activity from sarcoplasmic reticulum by annexin VI (67-kDa calcimedin). J Biol Chem. 1990 Sep 15;265(26):15894–15899. [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Flower R. J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988 Aug;94(4):987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. T., Prentice D. A., Hughes J. P. Increases in p11 and annexin II proteins correlate with differentiation in the PC12 pheochromocytoma. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1188–1193. doi: 10.1016/0006-291x(91)90666-u. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter P. J., Schobert A., Dieter P., Honegger P., Hertting G. Regulation and glucocorticoid-independent induction of lipocortin I in cultured astrocytes. J Neurochem. 1991 Jul;57(1):175–183. doi: 10.1111/j.1471-4159.1991.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Iida H., Hatae T., Shibata Y. Immunocytochemical localization of 67 KD Ca2+ binding protein (p67) in ventricular, skeletal, and smooth muscle cells. J Histochem Cytochem. 1992 Dec;40(12):1899–1907. doi: 10.1177/40.12.1453007. [DOI] [PubMed] [Google Scholar]
- Jackson A. P. Endocytosis in the brain: the role of clathrin light-chains. Biochem Soc Trans. 1992 Aug;20(3):653–655. doi: 10.1042/bst0200653. [DOI] [PubMed] [Google Scholar]
- Jindal H. K., Chaney W. G., Anderson C. W., Davis R. G., Vishwanatha J. K. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase alpha. J Biol Chem. 1991 Mar 15;266(8):5169–5176. [PubMed] [Google Scholar]
- Johnson M. D., Kamso-Pratt J. M., Whetsell W. O., Jr, Pepinsky R. B. Lipocortin-1 immunoreactivity in the normal human central nervous system and lesions with astrocytosis. Am J Clin Pathol. 1989 Oct;92(4):424–429. doi: 10.1093/ajcp/92.4.424. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Südhof T. C., Anderson R. G. Annexin VI is required for budding of clathrin-coated pits. Cell. 1992 Jul 24;70(2):283–291. doi: 10.1016/0092-8674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- McKanna J. A., Chuncharunee A., Munger K. A., Breyer J. A., Cohen S., Harris R. C. Localization of p35 (annexin I, lipocortin I) in normal adult rat kidney and during recovery from ischemia. J Cell Physiol. 1992 Dec;153(3):467–476. doi: 10.1002/jcp.1041530305. [DOI] [PubMed] [Google Scholar]
- Mizutani A., Usuda N., Tokumitsu H., Minami H., Yasui K., Kobayashi R., Hidaka H. CAP-50, a newly identified annexin, localizes in nuclei of cultured fibroblast 3Y1 cells. J Biol Chem. 1992 Jul 5;267(19):13498–13504. [PubMed] [Google Scholar]
- Pradel L. A., Rendon A. Annexin 1 is present in different molecular forms in rat cerebral cortex. FEBS Lett. 1993 Jul 19;327(1):41–44. doi: 10.1016/0014-5793(93)81035-x. [DOI] [PubMed] [Google Scholar]
- Reeves S. A., Chavez-Kappel C., Davis R., Rosenblum M., Israel M. A. Developmental regulation of annexin II (Lipocortin 2) in human brain and expression in high grade glioma. Cancer Res. 1992 Dec 15;52(24):6871–6876. [PubMed] [Google Scholar]
- Regnouf F., Rendon A., Pradel L. A. Biochemical characterization of annexins I and II isolated from pig nervous tissue. J Neurochem. 1991 Jun;56(6):1985–1996. doi: 10.1111/j.1471-4159.1991.tb03457.x. [DOI] [PubMed] [Google Scholar]
- Relton J. K., Strijbos P. J., O'Shaughnessy C. T., Carey F., Forder R. A., Tilders F. J., Rothwell N. J. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J Exp Med. 1991 Aug 1;174(2):305–310. doi: 10.1084/jem.174.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römisch J., Schüler E., Bastian B., Bürger T., Dunkel F. G., Schwinn A., Hartmann A. A., Pâques E. P. Annexins I to VI: quantitative determination in different human cell types and in plasma after myocardial infarction. Blood Coagul Fibrinolysis. 1992 Feb;3(1):11–17. [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto S., Tashiro T., Komiya Y. Two 68-kDa proteins in slow axonal transport belong to the 70-kDa heat shock protein family and the annexin family. J Neurochem. 1991 May;56(5):1774–1782. doi: 10.1111/j.1471-4159.1991.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Solito E., Raugei G., Melli M., Parente L. Dexamethasone induces the expression of the mRNA of lipocortin 1 and 2 and the release of lipocortin 1 and 5 in differentiated, but not undifferentiated U-937 cells. FEBS Lett. 1991 Oct 21;291(2):238–244. doi: 10.1016/0014-5793(91)81293-h. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., Volpe P. Ryanodine receptors: how many, where and why? Trends Pharmacol Sci. 1993 Mar;14(3):98–103. doi: 10.1016/0165-6147(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Spreca A., Rambotti M. G., Giambanco I., Pula G., Bianchi R., Ceccarelli P., Donato R. Immunocytochemical localization of annexin V (CaBP33), a Ca(2+)-dependent phospholipid- and membrane-binding protein, in the rat nervous system and skeletal muscles and in the porcine heart. J Cell Physiol. 1992 Sep;152(3):587–598. doi: 10.1002/jcp.1041520319. [DOI] [PubMed] [Google Scholar]
- Strijbos P. J., Tilders F. J., Carey F., Forder R., Rothwell N. J. Localization of immunoreactive lipocortin-1 in the brain and pituitary gland of the rat. Effects of adrenalectomy, dexamethasone and colchicine treatment. Brain Res. 1991 Jul 12;553(2):249–260. doi: 10.1016/0006-8993(91)90833-h. [DOI] [PubMed] [Google Scholar]
- Walker J. H., Boustead C. M., Brown R., Koster J. J., Middleton C. A. Tissue and subcellular distribution of endonexin, a calcium-dependent phospholipid-binding protein. Biochem Soc Trans. 1990 Dec;18(6):1235–1236. doi: 10.1042/bst0181235. [DOI] [PubMed] [Google Scholar]
- Werner M. H., Nanney L. B., Stoscheck C. M., King L. E. Localization of immunoreactive epidermal growth factor receptors in human nervous system. J Histochem Cytochem. 1988 Jan;36(1):81–86. doi: 10.1177/36.1.3275713. [DOI] [PubMed] [Google Scholar]
- Woolgar J. A., Boustead C. M., Walker J. H. Characterization of annexins in mammalian brain. J Neurochem. 1990 Jan;54(1):62–71. doi: 10.1111/j.1471-4159.1990.tb13283.x. [DOI] [PubMed] [Google Scholar]