Abstract
Endothelial β2-adrenoceptor (β2AR) stimulation increases nitric oxide (NO) generation, but the underlying cellular mechanisms are unclear. We examined the role of l-arginine transport and of phosphorylation of NO synthase 3 (NOS-3) in β2AR-mediated NO biosynthesis by human umbilical vein endothelial cells (HUVEC). To this end, we assessed l-arginine uptake, NOS activity (from l-arginine to l-citrulline conversion), membrane potential (using [3H]tetraphenylphosphonium), as well as serine phosphorylation of NOS-3 (by Western blotting and mass spectrometry), in HUVEC treated with βAR agonists or cyclic AMP-elevating agents. β2AR stimulation increased l-arginine transport, as did cyclic AMP elevation with either forskolin or dibutyryl cyclic AMP, and this increase was inhibitable by N-ethylmaleimide. Blockade of l-arginine uptake by l-lysine inhibited NOS activity and, conversely, blockade of NOS using Nω-nitro-l-arginine methyl ester (l-NAME) inhibited l-arginine transport. β2AR stimulation also caused a membrane hyperpolarization inhibitable by l-NAME, suggesting that the increase in l-arginine uptake occurred in response to NO-mediated hyperpolarization. β2AR activation also increased NOS activity and phosphorylation of NOS-3 on serine-1177, and these increases were attenuated by inhibition of protein kinase A (PKA), phosphatidylinositol 3-kinase (PI3K) or Akt, and abolished by coinhibition of PKA and Akt. These findings suggest that β2AR-mediated NOS-3 activation in HUVEC is mediated through phosphorylation of NOS-3 on serine-1177 through both the PKA and the PI3K/Akt systems, and is sustained by an increase in l-arginine uptake resulting from NO-mediated membrane hyperpolarization.
Nitric oxide (NO) is an important endothelium-derived mediator which contributes to vasorelaxation, and is released in response to a number of mechanical and neurohormonal stimuli (Furchgott & Zawadzki, 1980; Katusic et al. 1984; Rubanyi et al. 1986; Vanhoutte & Miller, 1989; Yang et al. 1989). Following its release, NO diffuses to the subjacent vascular smooth muscle, where it elicits vasorelaxation through activation of soluble guanylyl cyclase, which catalyses the formation of cyclic guanosine-3′,5′-monophosphate (cGMP) and hence activation of cGMP-dependent protein kinase (Ignarro et al. 1987; Palmer et al. 1987). Classically, NO is released from endothelial cells following activation of the endothelial or type 3 isoform of NO synthase (NOS-3), which is a Ca2+- and calmodulin-dependent enzyme, and hence many endothelium-dependent vasodilators cause NO release via an increase in intracellular Ca2+.
Vascular endothelial cells express β-adrenoceptors (βAR), which contribute to vasorelaxation through stimulation of endothelial NO biosynthesis, and in at least some vessel types βAR-mediated NO production may greatly outweigh any direct vasorelaxant effect of βAR located on vascular smooth muscle (Ferro et al. 1999; Xu et al. 2000). In the only study to date examining the effect of βAR stimulation on NO production in endothelial cells derived from humans, we previously demonstrated that β2AR, but not β1AR, stimulate NOS activity in human umbilical vein endothelial cells (HUVEC), and that they do so in a Ca2+-independent manner (Ferro et al. 1999). The mechanism by which this occurs is not known, but may involve protein kinase modifications of NOS-3, since serine phosphorylation of NOS-3 by both protein kinase A (PKA) and Akt activates NOS-3 in a Ca2+-independent manner through increasing its sensitivity to Ca2+-calmodulin (Dimmeler et al. 1999; Butt et al. 2000; Fisslthaler et al. 2000; Boo et al. 2002), and serine phosphorylation of NOS-3 occurs with β2AR stimulation of rat aortic rings in vitro (Ferro et al. 2004). Furthermore, cellular uptake of l-arginine (the substrate for NOS) was shown to be increased following β2AR stimulation in HUVEC (Ferro et al. 1999), and this may also be responsible, at least in part, for the observed β2AR-mediated increase in NOS activity. We hypothesized that β2AR-mediated NOS activation in HUVEC may occur partly through PKA- and/or Akt-induced serine phosphorylation of NOS-3, and partly by augmentation of l-arginine uptake. The present study was designed therefore to investigate the respective roles of protein kinase modification of NOS-3 and of l-arginine uptake in mediating β2-adrenergic NOS activation in these cells.
Methods
Materials
CGP 20712A was kindly provided by Novartis International AG (Basel, Switzerland), and ICI 118551 by Zeneca Pharmaceuticals (Macclesfield, UK). Radiochemicals were from Amersham International PLC (Little Chalfont, UK). Medium 199, antibiotics, antimycotics, trypsin-EDTA, Dulbecco's PBS and fetal bovine serum were from Gibco BRL (Paisley, UK). Akt inhibitor and mouse monoclonal anti-phosphoserine IgG were from Calbiochem-Novabiochem Ltd (Nottingham, UK). Mouse monoclonal anti-NOS-3 antibody was from BD Biosciences Pharmingen (San Diego, USA). Rabbit polyclonal anti-phospho-NOS-3 (serine-1177-specific) was from New England Biolabs Ltd (Hitchin, UK). All other chemicals were from Sigma-Aldrich Company Ltd (Poole, UK).
HUVEC isolation and culture
Fresh umbilical cords were obtained following delivery of healthy babies to healthy normotensive mothers, either by vaginal delivery or by elective Caesarean section. The study conformed to the standards set by the Declaration of Helsinki (last modified 2004). Approval for the study was granted by the Research Ethics Committee, St Thomas' Hospital, London, UK, and all subjects gave written informed consent. HUVEC were isolated from cords and cultured as previously described (Ferro et al. 1999). Confluent cells at passage 3 were used for all experiments.
Determination of L-arginine uptake
HUVEC monolayers in 96-well culture plates were washed three times at 37°C with warmed balanced salt solution (BSS) buffer, of the following composition (mm): NaCl 125, KCl 5.4, NaHCO3 16.2, Hepes 15, NaH2PO4 1, MgSO4 0.8, CaCl2 1.8, glucose 5.5 (pH 7.4). Cells were then incubated with BSS containing unlabelled l-arginine (100 μm) for 15 min, and subsequently with CGP 20712A (300 nm, a selective β1AR antagonist), ICI 118551 (100 nm, a selective β2AR antagonist) or vehicle (Ferro et al. 1999), in the absence or presence of N-ethylmaleimide (0.2 mm) for a further 10 min. Agonists (isoproterenol 1 μm, forskolin 1 μm or dibutyryl cAMP 1 mm) or vehicle were then added and the incubation continued for a further 5 min, for the last 30 s of which 0.4 μCi l-[3H]arginine was added. This was left for 30 s before being aspirated; HUVEC were then washed twice with ice-cold stop solution (BSS containing 10 mm unlabelled l-arginine). Formic acid (100 μl) was added to each well, and the plates were left on ice for 10 min to allow HUVEC lysis to occur. The lysate was removed from each well and placed into a scintillation vial. Lysates from 12 extra vehicle-treated wells from each plate were reserved for protein determination, using the method of Bradford (1976). Ultima Gold Liquid Scintillation Cocktail (5 ml) was added to all samples, mixed well and the radioactivity (counts min−1 (cpm)) in each tube measured on a Wallac Beta liquid scintillation counter. Data were corrected for protein and expressed as pmol (μg protein)−1 min−1.
Determination of membrane potential changes
Influx of 46 nm[3H]tetraphenylphosphonium ([3H]TPP+, 0.5 μCi ml−1), a membrane potential-sensitive probe (Sobrevia et al. 1995; Casanello & Sobrevia, 2002; Flores et al. 2003), was measured over 15–240 s in HUVEC in 96-well plates equilibrated for 15 min with BSS containing 100 μml-arginine, in the absence or presence of Nω-nitro-l-arginine methyl ester (l-NAME, 0.1 mm) and/or isoproterenol (1 μm, + CGP 20712A, 300 nm).
NOS activity measurement and cGMP assay
Conversion of radiolabelled l-arginine to l-citrulline was determined as previously described (Ferro et al. 1999). Following incubation of HUVEC for 20 min with 1 μCi ml−1l-[3H]arginine (57 Ci mmol−1) at 37°C, in an atmosphere of 95% air and 5% CO2, l-NAME (0.1 mm), l-lysine (0.1 mm), H-89 (a selective PKA inhibitor, 100 nm), wortmannin (a selective inhibitor of phosphatidylinositol 3-kinase (PI3K), 500 nm), Akt inhibitor (1l-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate, 10 μm, a selective inhibitor of Akt) or vehicle was added for a further 10 min. Agonists (isoproterenol 1 μm, albuterol 1 μm, forskolin 1 μm, or dibutyryl cAMP 1 mm) or vehicle were then added, and the incubation continued for another 10 min. l-citrulline formation was corrected for protein concentration.
For cGMP measurement, HUVEC treated as above were placed on ice and incubated for 60 min with 0.1 m HCl, which was then stored at −20°C for radioimmunoassay of cGMP following acetylation, as previously described (Sobrevia et al. 1995).
Determination of serine phosphorylation of NOS-3
Confluent HUVEC were equilibrated for 15 min in BSS containing one or more of the following: l-NAME (100 μm), H-89 (100 nm), wortmannin (500 nm), Akt inhibitor (10 μm), the Rp-isomer of 8-bromo-2′-O-monobutyryladenosine-3′,5′-cyclic monophosphorothioate (Rp-8-Br-MB-cAMPS, an alternative inhibitor of PKA; 30 μm), LY 294002 (an alternative inhibitor of PI3K; 10 μm), SH5 (an alternative inhibitor of Akt; 1 μm), pertussis toxin (100 ng ml−1), or corresponding vehicle. Following this period, the selective β2AR agonist albuterol (1 μm) or corresponding vehicle were added for a further 10 min. Reactions were terminated, cells were lysed and NOS-3 was immunoprecipitated. NOS-3 expression as well as serine phosphorylation of NOS-3 (both total serine phosphorylation and serine-1177-specific phosphorylation) were analysed in lysates and immunoprecipitates by Western blotting using anti-NOS-3, anti-phosphoserine and anti-phospho-NOS-3 (serine-1177-specific) primary antibodies, as previously described (Xu et al. 2003). In other experiments, NOS-3 immunoprecipitated from HUVEC following albuterol or vehicle treatment was run on a 10% SDS-PAGE gel and, following Coomassie Blue staining, the identity of the band at 135 kDa was confirmed as NOS-3, and the precise sites of phosphorylation were determined, by liquid chromatography/mass spectrometry/mass spectrometry (LC-MS/MS; Proteome Sciences plc, London, UK).
Data analysis
Each experiment was performed with cells obtained from a separate donor. All data are presented as mean ±s.e.m. Statistical comparisons were made using ANOVA with or without repeated measures as appropriate, with post hoc analyses using Dunnett's test (GraphPad Prism version 4). P < 0.05 (two-tailed) was taken as statistically significant.
Results
β2AR stimulation or cAMP elevation increase L-arginine transport in HUVEC
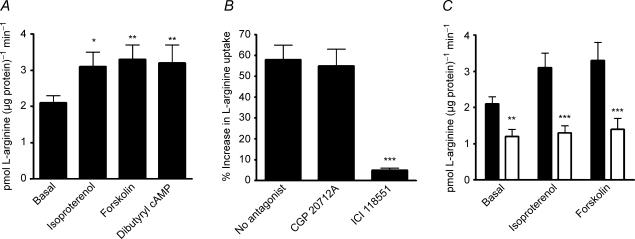
Isoproterenol, forskolin and dibutyryl cAMP each increased l-arginine uptake by HUVEC (Fig. 1A). The response to isoproterenol was inhibited by ICI 118551 but not by CGP 20712A, suggesting that the effect of isoproterenol was mediated solely through β2AR and not β1AR (Fig. 1B). Neither CGP 20712A nor ICI 118551 had any effect on basal l-arginine uptake; moreover, ICI 118551 had no effect on forskolin-induced l-arginine uptake (data not shown).
Figure 1. Effect of βAR stimulation or cAMP elevation on l-arginine uptake in HUVEC.
A, effect of isoproterenol 1 μm, forskolin 1 μm or dibutyryl cAMP 1 mm on l-arginine uptake as compared with basal values (vehicle treatment), by HUVEC. *, **: P < 0.05 and < 0.01, respectively, versus basal. B, effect of isoproterenol on percentage increase in l-arginine uptake above basal, in HUVEC, in the presence of the selective β1AR antagonist CGP 20712A 300 nm, the selective β2AR antagonist ICI 118551 100 nm, or neither antagonist. ***P < 0.001 as compared with no antagonist. C, effect of isoproterenol 1 μm or forskolin 1 μm, as compared with basal values (vehicle treatment), on l-arginine uptake by HUVEC, in the absence (filled bars) or presence (open bars) of 0.2 mmN-ethylmaleimide. **, ***: P < 0.01 and < 0.001 as compared with absence of N-ethylmaleimide. Data are mean ±s.e.m. of 8–10 experiments in different cell cultures.
Transport of l-arginine appears to occur principally through the cationic amino acid transporter-1 (CAT-1), in HUVEC (Mann et al. 2003). The CAT system of amino acid transporters can be specifically inhibited by the sulphydryl reagent N-ethylmaleimide (Devés et al. 1992; Devés & Boyd, 1998; Palacín et al. 1998). We found that basal l-arginine transport was inhibited by N-ethylmaleimide, and that this compound also abolished the increase in l-arginine transport in response to isoproterenol or forskolin (Fig. 1C).
Inhibition of l-arginine transport prevents NO production, and vice versa, in HUVEC
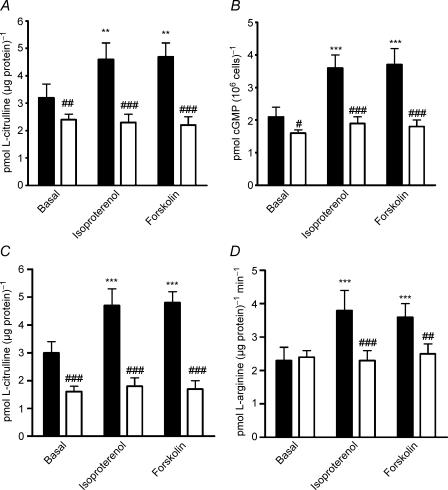
Uptake of l-arginine into HUVEC is inhibited by other cationic amino acids. We therefore examined the effect of coincubation with l-lysine on NOS activity, as measured by l-[3H]arginine to l-[3H]citrulline conversion. l-lysine inhibited basal NOS activity, and also prevented the increase in NOS activity to either isoproterenol or forskolin (Fig. 2A). To confirm that l-lysine indeed prevented NO generation, we also measured intracellular cGMP accumulation in HUVEC treated with isoproterenol or forskolin, in the absence or presence of l-lysine. Basal cGMP was reduced, and the increase in cGMP in response to isoproterenol or forskolin was also abolished, when l-lysine was present (Fig. 2B).
Figure 2. Interdependency of l-arginine uptake and NOS activity on βAR- and cAMP-mediated NO biosynthesis in HUVEC.
A, effect of isoproterenol 1 μm or forskolin 1 μm, as compared with basal values (vehicle treatment), on NOS activity in HUVEC, in the absence (filled bars) or presence (open bars) of 0.1 mml-lysine. **P < 0.01 as compared with basal; ##, ###P < 0.01 and < 0.001 as compared with absence of l-lysine. B, effect of isoproterenol 1 μm or forskolin 1 μm, as compared with basal values (vehicle treatment), on cGMP accumulation in HUVEC, in the absence (filled bars) or presence (open bars) of 0.1 mml-lysine. ***P < 0.001 as compared with basal; #, ###: P < 0.05 and < 0.001 as compared with absence of l-lysine. C, effect of isoproterenol 1 μm or forskolin 1 μm, as compared with basal values (vehicle treatment), on NOS activity in HUVEC, in the absence (filled bars) or presence (open bars) of 0.1 mml-NAME. ***P < 0.001 as compared with basal; ###P < 0.001 as compared with absence of l-NAME. D, effect of isoproterenol 1 μm or forskolin 1 μm, as compared with basal values (vehicle treatment), on l-arginine uptake by HUVEC, in the absence (filled bars) or presence (open bars) of 0.1 mml-NAME. ***P < 0.001 as compared with basal; ##, ###: P < 0.01 and < 0.001 as compared with absence of l-NAME. Data are mean ±s.e.m. of 8–12 experiments in different cell cultures.
l-NAME is a neutral analogue of l-arginine, which specifically inhibits NOS, with no direct effect on l-arginine transport via system y+/CAT-1 (Bogle et al. 1992). Indeed, in our system, l-NAME did not affect basal l-arginine uptake (2.3 ± 0.3 versus 2.4 ± 0.3 pmol (μg protein)−1 min−1 in the absence and presence of l-NAME, respectively; n = 8), whereas, by contrast, the alternative l-arginine analogue Nω-monomethyl-l-arginine (l-NMMA, at the same concentration, 0.1 mm) reduced basal l-arginine uptake from 2.2 ± 0.3 to 1.2 ± 0.2 pmol (μg protein)−1 min−1 (n = 4; P < 0.05). As expected, l-NAME inhibited basal as well as isoproterenol- and forskolin-stimulated NOS activity (Fig. 2C); additionally, although l-NAME did not affect basal l-arginine uptake, it abolished both isoproterenol- and forskolin-stimulated l-arginine uptake (Fig. 2D).
β2AR-mediated NO generation causes membrane hyperpolarization in HUVEC
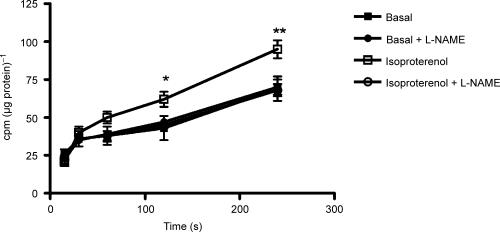
We have previously shown that adenosine-stimulated NO release causes membrane hyperpolarization in HUVEC, resulting in an increased rate of l-arginine influx (Wyatt et al. 2002). In the present experiments, isoproterenol in the presence of CGP 20712A increased the uptake of the membrane potential-sensitive probe [3H]TPP+, and this increase was inhibited by l-NAME (Fig. 3), confirming that β2AR activation causes a membrane hyperpolarization as a result of NO generation.
Figure 3. NO dependency of β2AR-mediated membrane hyperpolarization in HUVEC.
Effect of isoproterenol 1 μm on the time course of uptake of the membrane potential-sensitive probe [3H]TPP+ by HUVEC in the presence of the selective β1AR antagonist CGP 20712A and the absence or presence of 0.1 mml-NAME. *, **: P < 0.05 and < 0.01 as compared with basal. Data are expressed as counts min−1 (cpm) corrected for cellular protein, and are mean ±s.e.m. of eight experiments in different cell cultures.
Activation of NOS by β2AR is dependent on both PKA and Akt, in HUVEC
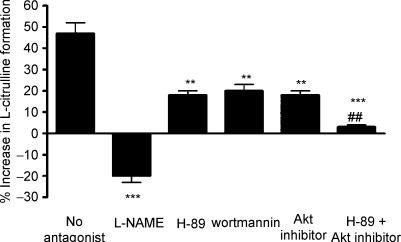
NOS stimulation by β2AR agonists occurs with no observable change in intracellular Ca2+ (Ferro et al. 1999). Since Ca2+-independent NOS-3 activation can occur through serine phosphorylation of NOS-3 by PKA or Akt (especially on serine residue 1177, but also serine-116 and serine-633), we determined the effect of selective inhibition of PKA, PI3K (the upstream activator of Akt) or Akt on NOS activity, in HUVEC treated with albuterol or vehicle. As expected, NOS activity increased in response to albuterol, and this increase was inhibited by coincubation with l-NAME. H-89, wortmannin or Akt inhibitor alone each partially inhibited the albuterol-induced increase in NOS activity, while the combination of H-89 and Akt inhibitor completely abolished the albuterol response (Fig. 4). H-89, wortmannin and Akt inhibitor had no effect on basal NOS activity (data not shown).
Figure 4. Role of the PKA and PI3K/Akt pathways in β2AR-mediated NOS activation in HUVEC.
Percentage increase above basal (vehicle treatment) in NOS activity in HUVEC treated with albuterol 1 μm, in the absence or presence of: l-NAME 0.1 mm; the selective PKA inhibitor H-89 100 nm; the selective PI3K inhibitor wortmannin 500 nm; Akt inhibitor 10 μm; or the combination of H-89 100 nm and Akt inhibitor 10 μm. **, ***: P < 0.01 and < 0.01 as compared with no antagonist; ##P < 0.01 as compared with H-89 alone. Data are mean ±s.e.m. of six experiments in different cell cultures.
β2AR activation increases serine-1177 phosphorylation of NOS-3 in HUVEC, through both PKA and Akt
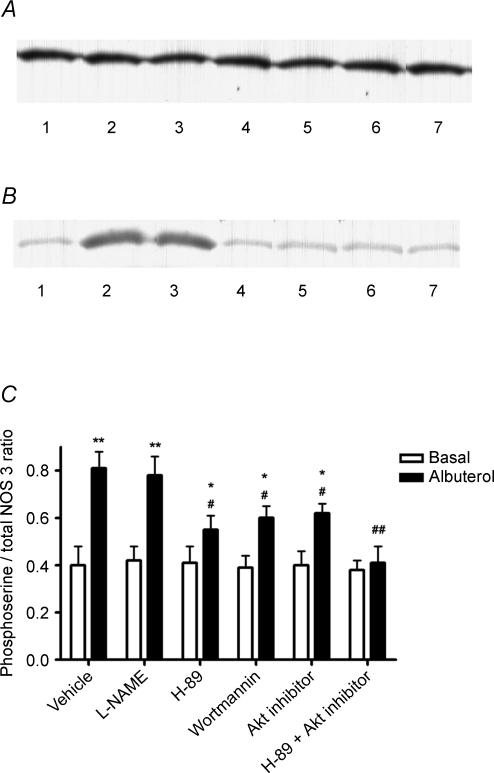
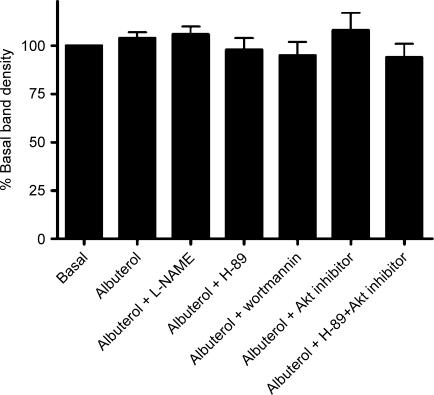
To confirm that β2AR agonism induced an increase in serine phosphorylation of NOS-3, HUVEC were treated with albuterol or vehicle, NOS-3 was immunoprecipitated, and the immunoprecipitates subjected to Western blotting for both NOS-3 and phosphoserine. Using the anti-NOS-3 antibody, a band was detected at 135 kDa, the known molecular mass of NOS-3 (Fig. 5A); and using the anti-phosphoserine IgG a band was detected at this same position, thus confirming that NOS-3 undergoes serine phosphorylation in HUVEC (Fig. 5B). Albuterol increased NOS-3 serine phosphorylation, and this increase was not affected by NOS inhibition with l-NAME; the albuterol-induced increase in NOS-3 serine phosphorylation was partially inhibited by H-89, wortmannin or Akt inhibitor, and was completely abolished by the combination of H-89 and Akt inhibitor (Fig. 5C). None of the inhibitors (H-89, wortmannin or Akt inhibitor) had any effect on basal NOS-3 phosphorylation (Fig. 5C). By contrast, Western blotting of HUVEC lysates using the anti-NOS-3 antibody showed no change in NOS-3 expression, following each of these treatments (Fig. 6). Additionally, Western blotting of HUVEC lysates using an anti-NOS-2 monoclonal antibody (Santa Cruz Biotechnology, USA) revealed no bands corresponding to NOS-2 (data not shown).
Figure 5. Role of the PKA and PI3K/Akt pathways in β2AR-mediated NOS-3 serine phosphorylation in HUVEC.
A, Western blot depicting the presence of a 135 kDa band (the known molecular mass of NOS-3) in NOS-3 immunoprecipitates prepared from HUVEC lysates, probed with anti-NOS-3 antibody. B, Western blot depicting the presence of a 135 kDa band (the known molecular mass of NOS-3) in NOS-3 immunoprecipitates prepared from HUVEC lysates, probed with anti-phosphoserine IgG. Lanes: 1 = basal (vehicle treatment); 2 = albuterol 1 μm; 3 = albuterol +l-NAME 0.1 mm; 4 = albuterol + H-89 100 nm; 5 = albuterol + wortmannin 500 nm; 6 = albuterol + Akt inhibitor 10 μm; 7 = albuterol + H-89 + Akt inhibitor. C, densitometric ratio of 135 kDa phosphoserine/NOS-3 bands, in HUVEC treated as shown, expressed as mean ±s.e.m. of six experiments. *, **: P < 0.05 and < 0.01 as compared with basal; #, ##: P < 0.05 and < 0.01 as compared with albuterol alone.
Figure 6. Effect of β2AR stimulation, and of concomitant inhibition of the PKA and PI3K/Akt pathways, on NOS-3 expression in HUVEC.
Density of 135 kDa band detected by Western blotting for NOS-3 in HUVEC lysates, expressed as a percentage of basal (vehicle-treated HUVEC). Basal density is set at 100%, in order to allow interblot normalization of densities. Results are shown for HUVEC following treatment with albuterol 1 μm, in the absence or presence of: l-NAME 0.1 mm; H-89 100 nm; wortmannin 500 nm; Akt inhibitor 10 μm; and the combination of H-89 with Akt inhibitor. Data are mean ±s.e.m. of six experiments.
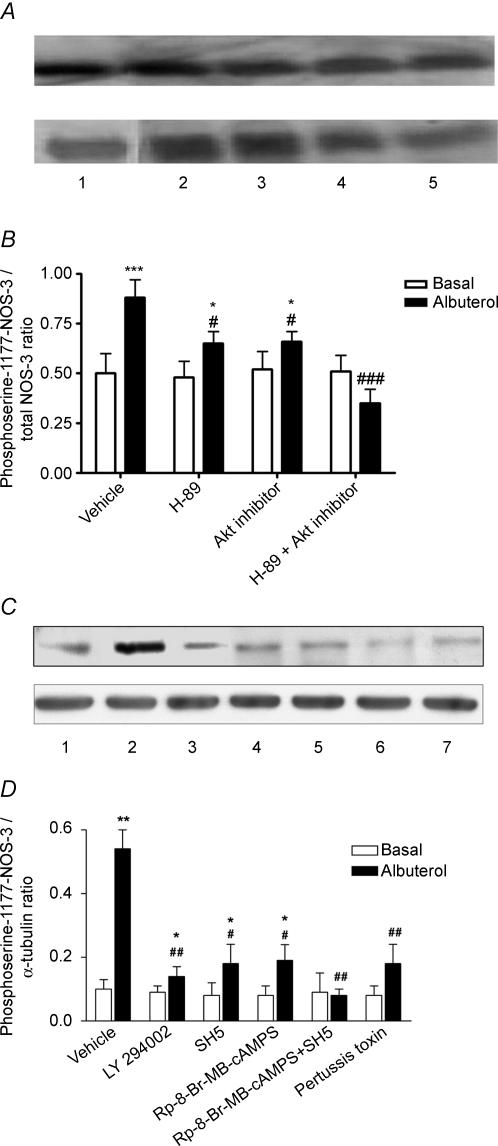
In further experiments, HUVEC were treated with albuterol or vehicle, and lysates were subjected to Western blotting for phosphoserine-1177-modified NOS-3. Albuterol increased NOS-3 serine phosphorylation at position 1177, which was partially inhibited by H-89 or Akt inhibitor, and completely abolished by the combination of H-89 and Akt inhibitor (Fig. 7A and B). Using different inhibitors of H-89, PI3K and Akt, namely Rp-8-Br-MB-cAMPS, LY 294002 and SH5, respectively, it was found that each of these also partially inhibited the albuterol-induced increase in NOS-3 phosphorylation at serine-1177, and the combination of Rp-8-Br-MB-cAMPS and SH5 completely abolished it (Fig. 7C and D). Once again, no effect was seen of the different inhibitors on basal phosphorylation. Moreover, whereas pertussis toxin did not affect basal NOS-3 serine phosphorylation, it largely inhibited the increase in this phosphorylation to albuterol (Fig. 7C and D).
Figure 7. Role of the PKA and PI3K/Akt pathways, and of Gi protein, in β2AR-mediated phosphorylation of NOS-3 specifically on serine-1177 in HUVEC.
A, Western blot depicting the presence of a 135 kDa band (the known molecular mass of NOS-3) in NOS-3 immunoprecipitates prepared from HUVEC lysates, probed with anti-NOS-3 antibody (upper blot) and anti-phospho-NOS-3 (serine-1177-specific) antibody (lower blot). Lanes: 1 = basal (vehicle treatment); 2 = albuterol 1 μm; 3 = albuterol + H-89 100 nm; 4 = albuterol + Akt inhibitor 10 μm; 5 = albuterol + H-89 + Akt inhibitor. B, densitometric ratio of phosphoserine-1177-NOS-3/total NOS-3 bands, in HUVEC treated as shown, expressed as mean ±s.e.m. of six experiments. C, Western blot depicting the presence of a 135 kDa band in HUVEC lysates, probed with anti-phospho-NOS-3 (serine-1177-specific) antibody (upper blot) and anti-α-tubulin antibody (lower blot). Lanes: 1 = basal (vehicle treatment); 2 = albuterol 1 μm; 3 = albuterol + LY 294002 10 μm; 4 = albuterol + Rp-8-Br-MB-cAMPS 30 μm; 5 = albuterol + SH5 1 μm; 6 = albuterol + SH5 + Rp-8-Br-8-MB-cAMPS; 7 = albuterol + pertussis toxin 100 ng ml−1D, densitometric ratio of phosphoserine-1177-NOS-3/tubulin bands, in HUVEC treated as shown, expressed as mean ±s.e.m. of three experiments. *, **, ***: P < 0.05, < 0.01 and < 0.001 as compared with basal; #, ##, ##: P < 0.05, < 0.01 and < 0.001 as compared with albuterol alone.
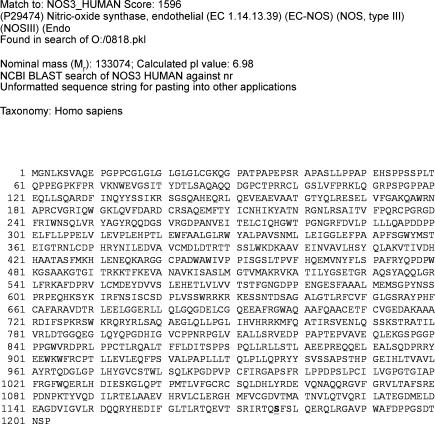
To confirm that NOS-3 serine phosphorylation occurred at position 1177, NOS-3 immunoprecipitates from HUVEC treated with either albuterol or vehicle were run on a 10% SDS-polyacrylamide gel and, following Coomassie Blue staining, the 135 kDa band thus visualized was excised and trypsin digests were analysed by LC-MS/MS. In both albuterol- and vehicle-treated samples, the only phosphorylation site detected was serine-1177 (Fig. 8).
Figure 8. Phosphorylation sites of NOS-3 in HUVEC.
LC-MS/MS result for trypsin digest of 135 kDa band seen on Coomassie Blue staining of gel following SDS-PAGE of NOS-3 immunoprecipitate from HUVEC. Phosphorylation sites are shown underscored.
Discussion
The present study sheds light on the mechanisms by which β2AR-mediated NO biosynthesis occurs. HUVEC take up extracellular l-arginine predominantly through the system y+ (CAT-1 transporter), and this is the predominant source of l-arginine used as substrate for NOS-3, rather than intracellular l-arginine (Hardy & May, 2002). It seems likely therefore that both the uptake and the subsequent intracellular conversion by NOS-3 of l-arginine would be closely linked or coregulated. Indeed, our experiments show that β2AR stimulation or cAMP elevation by other means simultaneously activates l-arginine uptake and NOS activity. Furthermore, inhibition of l-arginine uptake prevents NO biosynthesis and, conversely, inhibition of NOS prevents l-arginine uptake, in response to β2AR stimulation or cAMP elevation in HUVEC.
Our experiments further demonstrate that β2AR stimulation in HUVEC gives rise to membrane hyperpolarization, which is prevented by NOS inhibition with l-NAME, consistent with β2AR activation causing hyperpolarization through NO release. Indeed, we have previously shown that NO released by HUVEC following acute treatment with adenosine can similarly cause membrane hyperpolarization accompanied by increased arginine influx (Wyatt et al. 2002). Furthermore, other studies have shown that membrane potential has an important effect on CAT-1 activity, with hyperpolarization increasing l-arginine transport (Mann et al. 2003). Several workers have demonstrated that [3H]TPP+ is a robust, sensitive and specific probe which can be utilized to show changes in membrane potential (Kuroki et al. 1982; Friedman et al. 1985; Hofer & Kunemund, 1985; Arcangeli & Olivotto, 1986; Sobrevia et al. 1995; Casanello & Sobrevia, 2002; Flores et al. 2003). Our data suggest therefore that β2AR stimulate NOS, eliciting an increase in NO biosynthesis, which is then sustained through increased l-arginine influx via NO-induced membrane hyperpolarization. We therefore further examined the mechanisms by which β2AR activate NOS.
Phosphorylation of NOS-3, the principal isoform of NOS found in endothelial cells, by PKA or Akt can elicit Ca2+-independent activation (Dimmeler et al. 1999; Butt et al. 2000; Fisslthaler et al. 2000; Boo et al. 2002). Here, we have confirmed that β2AR stimulation increases the degree of serine phosphorylation of NOS-3, specifically on serine-1177, which can explain β2AR-mediated Ca2+-independent NOS activation in HUVEC. Blockade either of PKA or of the PI3K-Akt pathway partially inhibits both NOS activation and NOS-3 serine phosphorylation in response to β2AR stimulation, and combined PKA and Akt inhibition abolishes both of these. Serine-1177 phosphorylation was identified in both vehicle- and albuterol-treated HUVEC by LC-MS/MS, but no other phosphorylation of NOS-3 was detected; and the degree of this phosphorylation was increased by albuterol, as determined by Western blotting. Although serine-1177 on NOS-3 is well characterized as undergoing phosphorylation by Akt, recent evidence has shown that PKA can also phosphorylate this residue, thereby causing Ca2+-independent NOS-3 activation, in endothelial cells (Bae et al. 2003).
βAR couple, via the stimulatory G-protein Gs, to adenylyl cyclase, which catalyses the conversion of ATP to cAMP; cAMP in turn activates PKA. Recently, it has become apparent that β2AR, but not β1AR, can also activate the inhibitory G-protein Gi (Xiao et al. 1995, 1999), and this may provide a mechanism whereby β2AR can stimulate Akt, since β/γ subunits derived from Gi following its activation can stimulate PI3K, which in turn activates Akt (Brock et al. 2003). In the present experiments, pertussis toxin largely prevented the β2AR-mediated increase in NOS-3 phosphorylation at serine-1177, suggesting that β2AR do indeed mediate NOS-3 phosphorylation at this residue, and hence its activation, at least partially through a Gi-dependent mechanism. Our data provide, for the first time, a mechanistic explanation for β2AR-mediated NOS-3 activation at the subcellular level.
The question arises as to the specificities of H-89, wortmannin and Akt inhibitor used in the present experiments. Based on previously published activity and selectivity data for each of these inhibitors (Chijiwa et al. 1990; Davies et al. 2000; Hu et al. 2000), we were careful to use concentrations that would cause maximal or near-maximal inhibition of the chosen kinase, with little cross-reactivity with other pathways. Nevertheless, both H-89 and wortmannin have been previously demonstrated to inhibit certain other kinase pathways at concentrations close to those used here (Davies et al. 2000). We therefore confirmed the findings with these inhibitors by also using Rp-8-Br-MB-cAMPS (an alternative PKA inhibitor), LY 294002 (an alternative PI3K inhibitor) and SH5 (an alternative Akt inhibitor). We are confident therefore that our data truly reflect selective kinase inhibition as stated.
In conclusion, there is now abundant evidence that endothelial β2AR play an important role in mediating β-adrenergic vasorelaxation in a variety of blood vessel types through stimulation of NO production. The data presented here provide a mechanism by which this occurs. Our results suggest that, in HUVEC, β2AR stimulate both the PKA and PI3K-Akt pathways, both of which can give rise to phosphorylation on serine-1177 – and hence Ca2+-independent activation – of NOS-3. This increase in NOS-3 activity augments NO biosynthesis, which in turn hyperpolarizes the plasmalemma, increasing CAT-1 activity and l-arginine uptake, thereby sustaining an increase in NO production. Our study provides important novel information about the physiological mechanisms underlying β-adrenergic regulation of vascular tone.
Acknowledgments
This work was supported by a Guy's and St Thomas' Charitable Foundation Project Grant to A. Ferro, a Wellcome Trust Travelling Research Fellowship to Y. Ji, and a Wellcome Trust International Research Development Award to B. Xu. The authors are grateful to Drs Malcolm Ward and Kit-Yi Leung (Proteome Sciences plc, London, UK) for performing the protein LC/MS/MS analyses.
References
- Arcangeli A, Olivotto M. Plasma membrane potential of murine erythroleukemia cells: approach to measurement and evidence for cell-density dependence. J Cell Physiol. 1986;127:17–27. doi: 10.1002/jcp.1041270104. [DOI] [PubMed] [Google Scholar]
- Bae SW, Kim HS, Cha YN, Park YS, Jo SA, Jo I. Rapid increase in endothelial nitric oxide production by bradykinin is mediated by protein kinase A signaling pathway. Biochem Biophys Res Commun. 2003;306:981–987. doi: 10.1016/s0006-291x(03)01086-6. [DOI] [PubMed] [Google Scholar]
- Bogle RG, Moncada S, Pearson JD, Mann GE. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell 1-arginine transporter. Br J Pharmacol. 1992;105:768–770. doi: 10.1111/j.1476-5381.1992.tb09053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, et al. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt E, Bernhardt M, Smolenski A, Kotsonis P, Frohlich LG, Sickmann A, et al. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- Casanello P, Sobrevia L. Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y+/hCAT-1 and y+/hCAT-2B, and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ Res. 2002;91:127–134. doi: 10.1161/01.res.0000027813.55750.e7. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devés R, Boyd CAR. Transporters for cationic amino acids in animal cells: discovery, structure and function. Physiol Rev. 1998;78:487–545. doi: 10.1152/physrev.1998.78.2.487. [DOI] [PubMed] [Google Scholar]
- Devés R, Chavez P, Boyd CAR. Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol. 1992;454:491–501. doi: 10.1113/jphysiol.1992.sp019275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Ferro A, Coash M, Yamamoto T, Rob J, Ji Y, Queen L. Nitric oxide-dependent β2-adrenergic dilatation of rat aorta is mediated through activation of both protein kinase A and Akt. Br J Pharmacol. 2004;143:397–403. doi: 10.1038/sj.bjp.0705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A, Queen LR, Priest RM, Xu B, Ritter JM, Poston L, et al. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br J Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- Flores C, Rojas S, Aguayo C, Parodi J, Mann G, Pearson JD, et al. Rapid stimulation of l-arginine transport by d-glucose involves p42/44 (MAPK) and nitric oxide in human umbilical vein endothelium. Circ Res. 2003;92:64–72. doi: 10.1161/01.res.0000048197.78764.d6. [DOI] [PubMed] [Google Scholar]
- Friedman JE, Lelkes PI, Lavie E, Rosenheck K, Schneeweiss F, Schneider AS. Membrane potential and catecholamine secretion by bovine adrenal chromaffin cells: use of tetraphenylphosphonium distribution and carbocyanine dye fluorescence. J Neurochem. 1985;44:1391–1402. doi: 10.1111/j.1471-4159.1985.tb08775.x. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Hardy TA, May JM. Coordinate regulation of l-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic Biol Med. 2002;32:122–131. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- Hofer M, Kunemund A. Tetraphenylphosphonium ion is a true indicator of negative plasma-membrane potential in the yeast Rhodotorula glutinis. Experiments under osmotic stress and at low external pH values. Biochem J. 1985;225:815–819. doi: 10.1042/bj2250815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, et al. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem. 2000;43:3045–3051. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic ZS, Shepherd JT, Vanhoutte PM. Vasopressin causes endothelium-dependent relaxations of the canine basilar artery. Circ Res. 1984;55:575–579. doi: 10.1161/01.res.55.5.575. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Kamo N, Kobatake Y, Okimasu E, Utsumi K. Measurement of membrane potential in polymorphonuclear leukocytes and its changes during surface stimulation. Biochim Biophys Acta. 1982;693:326–334. doi: 10.1016/0005-2736(82)90439-4. [DOI] [PubMed] [Google Scholar]
- Mann GE, Yudilevich DL, Sobrevia L. Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev. 2003;83:183–252. doi: 10.1152/physrev.00022.2002. [DOI] [PubMed] [Google Scholar]
- Palacín M, Estévez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced increase of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Sobrevia L, Cesare P, Yudilevich DL, Mann GE. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells association with membrane hyperpolarization. J Physiol. 1995;489:183–192. doi: 10.1113/jphysiol.1995.sp021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Miller VM. α2-Adrenoceptors and endothelium-derived relaxing factor. Am J Med. 1989;87(Suppl. 3C):1S–5S. doi: 10.1016/0002-9343(89)90496-8. [DOI] [PubMed] [Google Scholar]
- Wyatt AW, Steinert JR, Wheeler-Jones CP, Morgan AJ, Sugden D, Pearson JD, et al. Early activation of the p42/p44 MAPK pathway mediates adenosine-induced nitric oxide production in human endothelial cells: a novel calcium-insensitive mechanism. FASEB J. 2002;16:1584–1594. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, et al. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Ji X, Lakatta EG. Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- Xu B, Chibber R, Ruggiero D, Kohner E, Ritter J, Ferro A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;17:1289–1291. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- Xu B, Li J, Gao L, Ferro A. Nitric oxide-dependent vasodilatation of rabbit femoral artery by β2-adrenergic stimulation or cyclic AMP elevation in vivo. Br J Pharmacol. 2000;129:969–974. doi: 10.1038/sj.bjp.0703155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Diederich D, Schneider K, Siebenmann R, Stulz P, von Segesser L, et al. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the internal mammary artery and saphenous vein. Circulation. 1989;80:1041–1048. doi: 10.1161/01.cir.80.4.1041. [DOI] [PubMed] [Google Scholar]