Abstract
It is suggested that mechanoreceptors in muscle play an important role in the exercise pressor reflex. However, it has not been verified whether isolated stimulation of the mechanoreceptors can induce responses in muscle sympathetic nerve activity (MSNA) in young healthy individuals. We tested the hypothesis that passive stretch of muscle can evoke an increase in MSNA in healthy individuals. In 12 young subjects, leg calf muscles were passively stretched, or actively contracted for 5 s followed by a 15–25 s (random length) relaxation period. Stretch and contraction were each repeated 25 times. MSNA, heart rate and blood pressure were analysed, and averaged according to the onset of the force on a beat-by-beat basis. At the 1st to the 3rd heart beat from the onset of stretch, MSNA (199 ± 30%, P < 0.05) as well as heart rate (102.5 ± 0.7%, P < 0.05) increased transiently but significantly from the prior stretch baseline (100%), followed (from 3rd to 7th beat from the onset of stretch) by a transient increase in mean blood pressure (101.9 ± 0.3%, P < 0.05) from the baseline. Similar response patterns were observed during active muscle contractions. The present data show that MSNA responses to isolated stimulation of mechanoreceptors are measurable. Because of baroreflex engagement, the magnitude of the response is small and transient, and the haemodynamic consequences using this protocol may be limited.
Exercise evokes sympathetic nervous system activation and increases heart rate, cardiac output, peripheral vasoconstriction and blood pressure (Sinoway et al. 1989; Rowell, 1993), which is called the exercise pressor reflex. In addition to central mechanisms (central command) (Vissing et al. 1991), inputs from mechanically and chemically sensitive afferents in the exercising muscle play an important role in evoking sympathetic activation (Mark et al. 1985; Victor et al. 1988; Saito et al. 1989; Seals, 1989; Sinoway et al. 1989).
Group III and IV afferent fibres in muscle are suggested to be involved in the exercise pressor reflex (McCloskey & Mitchell, 1972). These afferents are sensitive to mechanical and chemical stimulation (Kaufman & Hayes, 2002). A number of animal studies have shown that mechanoreceptors in cats activate muscle (Hill et al. 1996), and renal (Victor et al. 1989; Hayes & Kaufman, 2002) sympathetic efferents, and evoke an exercise pressor reflex (Hayes & Kaufman, 2001; Li et al. 2004). However, the role played by mechanoreceptors in evoking the exercise pressor reflex in human subjects remains controversial. Direct pressure on the leg muscles (Williamson et al. 1994) increased blood pressure, which was considered as a result of stimulation of mechanoreceptors. However, pressure on arm muscles had no similar effects (McClain et al. 1994). Visual inspection of muscle sympathetic nerve activity (MSNA) revealed that the activity did not increase until 30–60 s after humans started to perform static exercise (Mark et al. 1985). On the other hand, MSNA can be dramatically activated by the metaboreceptor stimulation during post-exercise ischaemia (Mark et al. 1985). Thus, early human studies (Mark et al. 1985; Victor et al. 1988; Saito et al. 1989) suggested that the MSNA responses to exercise were due to stimulation of metaboreceptors, and mechanoreceptors were thought to play little or no role in evoking the reflex. However, handgrip increases blood pressure, which engage baroreflexes. Eventually the baroreflexes are reset to higher blood pressure levels (Ebert, 1986; Cui et al. 2001). But before resetting, the afferent activities from mechanoreceptors may induce transient sympathetic responses, which could be overwhelmed by the higher blood pressure induced activation of baroreflexes. Thus, the visual inspection of mean MSNA burst rate may not be sufficiently sensitive to reveal transient MSNA response due to the stimulation of muscle mechanoreceptors.
To examine the role played by mechanoreceptors in humans, Herr et al. (1999) used signal averaging methods and examined the MSNA responses during repetitive contractions. The results showed that MSNA increased with an onset latency of 4–6 s, and the findings were suggestive of a role for the mechanoreceptors. Nevertheless, in this study stimulation of mechanoreceptors was not isolated from central command and/or metaboreflex engagement. On the other hand, a recent study reported that passive arm exercise via flexing wrist did not evoke significant increase in mean MSNA in healthy subjects, although an increase in MSNA during the stretch was observed in heart failure patients (Middlekauff et al. 2004). Therefore, the role of isolated stimulation of mechanoreceptors in the muscle reflex in healthy individuals is still controversial.
MSNA is closely correlated with changes in blood pressure; it is also influenced by other mechanisms (e.g. central mechanisms, breathing, etc.) (Wallin & Fagius, 1988; Eckberg & Sleight, 1992). Thus, to separate one factor (e.g. stretch) from these non-specific factors, the stimulation paradigm needs to be repeated and the obtained signals need to be averaged. In the aforementioned study (Middlekauff et al. 2004), the stimulation was not repetitive, and the dynamic MSNA response pattern during stretch was not reported. In this report, we hypothesize that the isolated stimulation of mechanoreceptors by passive muscle stretch would evoke responses in MSNA, heart rate and blood pressure in healthy individuals. We used a repetitive stimulus, and observed the averaged dynamic responses of the measured variables.
Methods
Subjects
Twelve subjects (6 male, 6 female) from the Hershey, PA, area and surrounding communities participated in the study. The average age was 30 ± 2 (s.e.m.) year and all were of normal height (175 ± 3 cm) and weight (78 ± 5 kg). All subjects were normotensive (supine blood pressures < 140/90 mmHg), were not taking medications, and were in good health. Subjects refrained from caffeine, alcohol, and exercise 24 h prior to the study. Each subject had the purposes and risks of the protocol explained to them before written informed consent was obtained. The experimental protocol was approved by the Institutional Review Board of the Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki.
Measurements
Blood pressure was recorded on a beat-by-beat basis from a finger via a Finapres device (Finapres, Ohmeda, Madison, WI, USA). Resting blood pressures obtained from the Finapres were verified during the experiment by an automated sphygmomanometer (Dinamap, Critikon, Tampa, FL, USA). A standard electrocardiogram was used to monitor heart rate. Respiratory excursions were monitored with pneumography. Multifibre recordings of MSNA were obtained with a tungsten microelectrode inserted in the peroneal nerve of the non-exercising leg. A reference electrode was placed subcutaneously 2–3 cm from the recording electrode. The recording electrode was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (Vallbo et al. 1979). The nerve signal was amplified, a band-pass filtered with a bandwidth of 500–5000 Hz, and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA, USA). The nerve signal was also routed to a loudspeaker and a computer for monitoring throughout the study. The forces of passive stretch or active contraction were measured with force transducers.
Protocols
All parameters were recorded with the subject in the supine condition. The ambient temperature of the laboratory was controlled at ∼25°C. After the 10 min rest baseline data collection, a foot of the subject was flexed (dorsiflexion) by an investigator for 5 s followed by a period of relaxation with random length (15–25 s, see Fig. 1). The ‘stretch and relaxation’ cycle was repeated 25 times (∼10 min). A computer program-generated sound signal was used to indicate the time for stretching and relaxing, which could be heard only by the investigator during the stretch protocol. The strength of the stretch was as high as possible without evoking pain. After a rest period, subjects voluntarily performed muscle contraction of the leg by pushing a pedal with the foot at 30% maximal voluntary contraction for 5 s and followed by 15–25 s (random length) of relaxation according to the sound signal. The ‘contraction and relaxation’ cycle was repeated 25 times (∼10 min). To avoid any influence from changes in breathing during stretch or contraction, subjects were asked to avoid breath holding during the study. Moreover, the stretch and contraction protocols were performed in both spontaneous and controlled breathing conditions. For the controlled breathing, subjects were asked to follow the rhythm of a visual sign on a computer screen to control the respiratory rate at 0.25 Hz. The strength of breathing was not controlled, although subjects were asked to avoid hyperventilation. The four protocols of stretch with spontaneous breathing, stretch with controlled breathing, contraction with spontaneous breathing, and contraction with controlled breathing, were performed in a random order. The intervals between the protocols allowed the measured haemodynamic variables to return towards baseline.
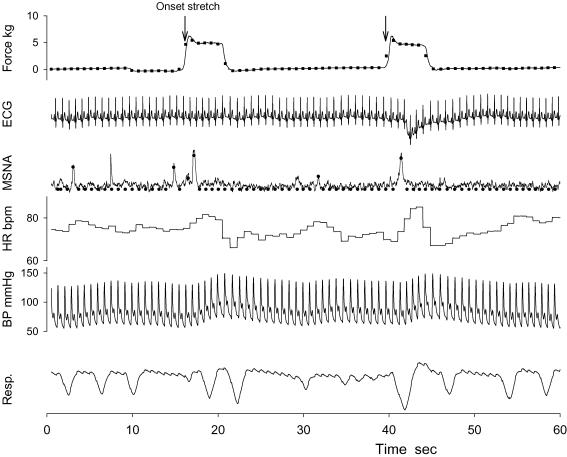
Figure 1. Representative tracing of stretch force, ECG, muscle sympathetic nerve activity (MSNA), heart rate (HR), arterial blood pressure (BP), and respiratory excursion (Resp.) during two passive stretch bouts.
The stretch bouts were repeated 25 times in each trial, and the intervals between 2 bouts were of random length (15–25 s). The filled circles and squares represent the processed beat-by-beat data of MSNA and force, respectively. The arrows indicate the beat of the onset stretch, and were used to align the data segments of the beat-by-beat data in signal averaging.
To separate the effects of arousal stimulation that may be associated with the stretch and contraction protocol, a control study was performed in four subjects during the second visit. All parameters were recorded in the same conditions as previous protocols. After the baseline data collection, low pressure (∼17 mmHg) was applied on an ankle of the subjects for 5 s followed by an off period with random length (15–25 s). This intervention was used as an arousal stimulus. This stimulus did not cause changes in foot position, and the level of pressure selected was insufficient to evoke circulatory arrest. All subjects reported that they could clearly feel the on/off of the pressure. The pressure on/off cycle was repeated 25 times (∼10 min). After an interval, this protocol was repeated when subjects controlled their respiration rate at 0.25 Hz.
Data analysis
Data were sampled at 200 Hz via a data acquisition system (MacLab, ADInstruments, Castle Hill, Australia). MSNA bursts were first identified in real time by visual inspection, coupled with the burst sound from the audio amplifier. These bursts were further evaluated via a computer software program that identified bursts based upon fixed criteria, including an appropriate latency following the R-wave of the electrocardiogram (Cui et al. 2004). Integrated MSNA was normalized by assigning a value of 100 to the mean amplitude of the large sympathetic bursts during the 10 min rest baseline period (Halliwill, 2000). Normalization of the MSNA signal was performed to reduce variability between subjects attributed to factors including needle placement, signal amplification, etc. Total MSNA was identified from burst area of the integrated neurogram, and was measured on a beat-by-beat basis. If no MSNA burst was detected for a particular cardiac cycle, a zero value was assigned for this cardiac cycle (see Fig. 1). This can eliminate the influences of the artificial factors and noises. Beat-by-beat systolic and diastolic pressure, mean blood pressure and transient heart rate were calculated simultaneously. The mean force during each cardiac cycle was also calculated (Fig. 1).
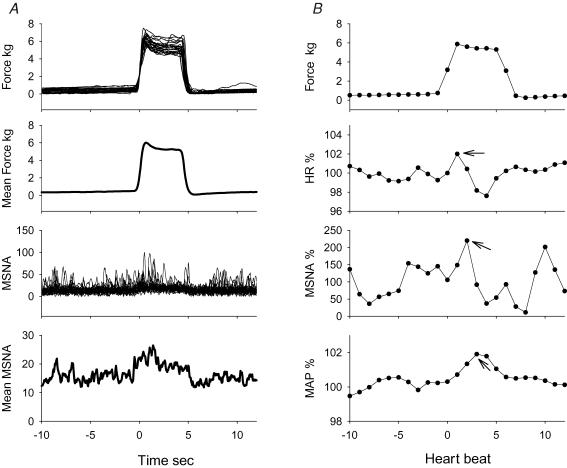
As previously described (Herr et al. 1999), the signal-averaging process uses signal summation to progressively increase signal events correlated with the stimulus, while decreasing the amplitude of uncorrelated ‘stray’ events (blood pressure variation, breathing, etc.). The improved signal-to-noise ratio due to signal averaging permits the detection of small, correlated MSNA bursts which occur at definite periods during the course of the response, while these activities can be overlooked by calculating mean burst rate or total activity. In the present study, MSNA signal averaging was performed on a beat-by-beat basis (Halliwill, 2000). This has the following advantages over direct averaging of the neurogram. First, MSNA is modulated by baroreflexes in a beat-by-beat fashion (Eckberg & Sleight, 1992). MSNA bursts are cardiac cycle synchronized, and occur in a defined time window within the cardiac cycle. MSNA activity will not occur outside this defined time window. Thus, averaging MSNA on a beat-by-beat basis yields clearer signal summation than is noted with direct averaging of the neurograms. Second, there are always some artificial factors and background noise in the neurogram tracings. These artificial factors cannot be eliminated with ‘direct’ signal averaging, and this can lead to erroneous results. In Fig. 2, data from one subject is used to demonstrate the two respective methods of signal averaging the MSNA responses evoked by muscle stretch.
Figure 2. Example of signal averaging in one representative subject.
A, raw tracing and the averaged signals of force and neurogram during stretch in one subject. The averaging was performed directly with recorded signals. It should note that averaged neurogram tracing was not low in the later portion of the stretch, while MSNA was clearly suppressed in the period. B, averaged force and responses of heart rate (HR), MSNA, mean blood pressure (MAP) by stretch with the processed beat-by-beat data in the same subject. The responses were expressed as a percentage of the respective mean values of 10-beats data prior to the stretch. There were transient increases in heart rate, MSNA and blood pressures after onset of the stretch. The values of the peak responses (indicated with arrows) and the delay were selected from each subject, and the mean peak responses and the delays are shown in Fig. 4 and Table 2, respectively.
For the 25 stretch, contraction bouts or pressure on ankle in each trial, the beat-by-beat total MSNA, blood pressures, heart rate and force data were divided into 25 segments, aligned according to the onset beat of force (see Fig. 1), and then the mean response courses were calculated on beat-by-beat bases, respectively. Because the individual differences in baseline MSNA, blood pressures and heart rate were lager than the responses to stimulation, all parameters were normalized by assigning a value of 100 to the mean value of the parameters of the 10 beats data prior the stretch or contraction, respectively. Thus, the final dynamic response courses were expressed as a percentage of the respective data value noted before stimulation.
Statistics
Differences in the mean values of haemodynamic parameters between the resting baselines, passive stretch, and voluntary contraction trials during spontaneous or controlled breathing conditions were evaluated via post hoc analysis after repeated-measures two-way ANOVA. The differences between the stretch/contraction induced peak responses (see Fig. 2) in haemodynamic parameters from the prior stretch/contraction baseline were evaluated using Student's t test for paired data. Differences in the peak responses between stretch and contraction during spontaneous and controlled breathing trials were evaluated via post hoc analyses after repeated-measures two-way ANOVA. All values are reported as means ±s.e.m.P-values of < 0.05 were considered statistically significant.
Results
The mean haemodynamic parameters over the rest baseline, and whole period of the stretch or contraction protocols are reported in Table 1. Neither stretch nor contraction evoked significant changes in the mean haemodynamic parameters as compared to the corresponding baseline values (all P > 0.05). There was no significant difference in the haemodynamic parameters between the spontaneous and controlled breathing condition.
Table 1.
Hemodynamic responses to passive stretch and active contraction
| Spontaneous breathing | Controlled breathing | |||||
|---|---|---|---|---|---|---|
| Baseline | Stretch | Contraction | Baseline | Stretch | Contraction | |
| Heart rate (bpm) | 64.1 ± 3.4 | 62.6 ± 3.6 | 64.4 ± 3.1 | 64.6 ± 3.4 | 64.4 ± 3.5 | 66.4 ± 3.0 |
| MSNA (bursts min−1) | 13.2 ± 1.8 | 14.1 ± 1.8 | 13.7 ± 2.0 | 12.4 ± 1.9 | 13.7 ± 1.7 | 14.6 ± 2.2 |
| MSNA (bi) | 22.2 ± 3.7 | 24.0 ± 3.6 | 21.8 ± 3.1 | 20.7 ± 3.7 | 22.4 ± 3.4 | 22.7 ± 3.2 |
| MSNA (units min−1) | 227 ± 36 | 250 ± 33 | 252 ± 44 | 205 ± 32 | 240 ± 37 | 297 ± 47 |
| SBP (mmHg) | 121.3 ± 4.6 | 123.1 ± 4.4 | 127.3 ± 4.4 | 123.2 ± 4.8 | 123.0 ± 4.7 | 126.2 ± 3.9 |
| DBP (mmHg) | 68.6 ± 2.0 | 69.3 ± 2.7 | 72.1 ± 2.1 | 68.7 ± 1.9 | 69.5 ± 2.0 | 71.0 ± 2.4 |
| MAP (mmHg) | 86.2 ± 2.6 | 87.2 ± 2.9 | 90.5 ± 2.6 | 86.9 ± 2.5 | 87.3 ± 2.6 | 89.4 ± 2.6 |
| Resp. (cycles min−1) | 15.7 ± 0.7 | 15.2 ± 0.6 | 14.4 ± 0.7 | 15.0 ± 0.1 | 14.9 ± 0.1 | 14.7 ± 0.1 |
| Force (kg) | — | 6.8 ± 0.6 | 16.8 ± 1.5* | — | 6.7 ± 0.6 | 17.7 ± 1.5* |
| Subject number | 12 | 12 | 11 | 12 | 12 | 10 |
Values are means ±s.e.m. Mean blood pressure (MAP) was calculated as one-third systolic blood pressure (SBP) pulse, two-thirds diastolic blood pressure (DBP), which was measured by auscultation of brachial artery. MSNA, muscle sympathetic nerve activity, bi: bursts per 100 heart beats. The forces were the mean values of the forces only during the stretch or contraction.
Significantly different from stretch (P < 0.05).
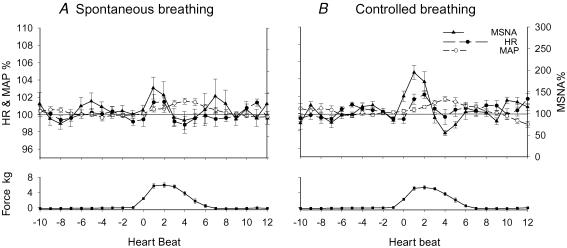
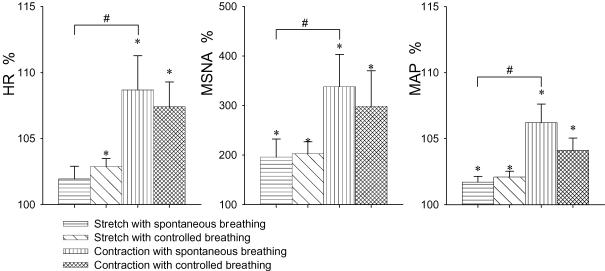
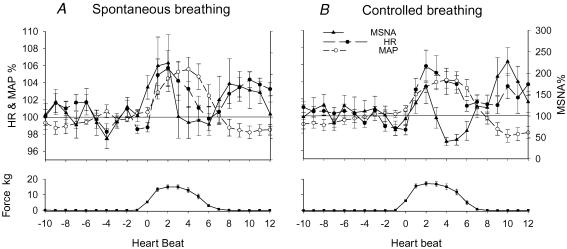
The mean results of the averaged dynamic responses to stretch are shown in Fig. 3. After the onset of stretch, MSNA and heart rate increased transiently from the baseline prior to stretch. Heart rate and MSNA were higher during beats 1–3. This was followed by a transient increase in mean blood pressure during beats 3–7. After the bouts of stretch were completed, blood pressure fell. This response pattern was observed in most trials of stretch with spontaneous or controlled breathing. The peak responses and the delays (beats) of the peak responses from the onset of stretch were estimated from each individual, and the mean values are shown in Fig. 4 and Table 2, respectively. There was no significant difference in the peak responses and delays between the spontaneous and controlled breathing conditions. Thus, the peak responses by the stretch with spontaneous and controlled breathing conditions were combined. The passive stretch of muscles induced transient and significant increases in MSNA (199 ± 30%, P < 0.05), heart rate (102.5 ± 0.7%, P < 0.05) and mean blood pressure (101.9 ± 0.3%, P < 0.05) from the prior stretch baseline (100%). Because the increases in MSNA and heart rate due to stretch were transient, and the delays of the response peaks varied among the individuals, the mean peak responses in Fig. 4 are different from the mean values in Fig. 3.
Figure 3. Mean dynamic responses of heart rate (HR), MSNA and mean blood pressure (MAP) by passive stretch with spontaneous (A) and controlled (B) breathing in all subjects.
The responses were expressed as a percentage of the respective mean values of 10-beats data prior to the stretch. Subject number = 12.
Figure 4. Peak responses of heart rate (HR), MSNA and mean blood pressure (MAP) to passive stretch and active contraction.
The responses were expressed as a percentage of the mean values of 10-beats data prior to the stretch or contraction (100%). Subject numbers for the respective condition are shown in Table 1. There was no significant difference in the peak responses between the spontaneous and controlled breathing conditions. *Significantly different from the prior stretch/contraction baseline (P < 0.05). #Significantly different from the stretch with the same breathing condition (P < 0.05).
Table 2.
Delays (beats) of the response peak of heart rate (HR), MSNA, and mean blood pressure (MAP) from the onset of passive stretch or active contraction
| Spontaneous breathing | Controlled breathing | |||
|---|---|---|---|---|
| Stretch | Contraction | Stretch | Contraction | |
| Delay of HR | 2.0 ± 0.3 | 2.0 ± 0.3 | 1.8 ± 0.3 | 2.3 ± 0.3 |
| Delay of MSNA | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.0 ± 0.3 | 1.6 ± 0.3 |
| Delay of MAP | 3.5 ± 0.4 | 3.1 ± 0.4 | 3.7 ± 0.4 | 3.0 ± 0.5 |
There was no significant difference in the delays between the conditions. Subject numbers for the respective condition are shown in Table 1.
The patterns of responses to active contraction were similar to the response pattern noted with muscle stretch (Fig. 5). The peak responses were estimated from each individual, and the mean values are shown in Fig. 4. The delays of peak responses from the onset of contraction were not significantly different from the respective delays of the peak responses evoked with stretch (Table 2). Compared with stretch, the peak responses of heart rate, MSNA and blood pressures with contraction were significantly greater than those with stretch (Fig. 4). There was no significant difference in the peak responses and delays by the contraction between the spontaneous and controlled breathing conditions. With the combined data, the active contraction induced transient and significant increases in MSNA (318 ± 53%, P < 0.05), heart rate (108.0 ± 2.2%, P < 0.05) and mean blood pressure (105.2 ± 1.2%, P < 0.05) from the prior stretch baseline (100%). Also because the delays of the response peaks varied in the individuals, the mean peak responses by contraction in Fig. 4 are different from the mean values in Fig. 5. The stretch/contraction did not induce a clear and definite respiratory pattern in the present study.
Figure 5. Mean dynamic responses of heart rate (HR), MSNA and mean blood pressure (MAP) by contraction with spontaneous (A) and controlled (B) breathing.
The responses were expressed as a percentage of the respective mean values of 10-beats data prior to the contraction (100%). Subject numbers for the respective conditions are shown in Table 1.
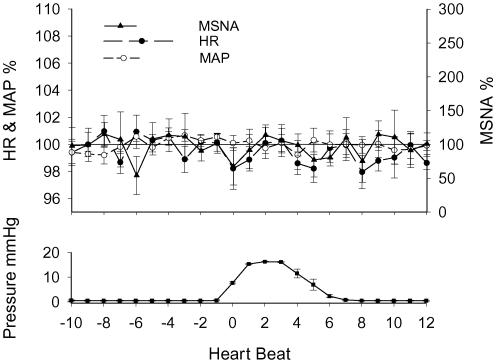
With the combined data of spontaneous and controlled breathing, the mean results of the averaged responses to the low pressure on the ankle are shown in Fig. 6. The arousal stimulation did not induce a clear and definite response in heart rate, MSNA and mean blood pressure as those seen during stretch/contraction.
Figure 6. Mean dynamic responses of heart rate (HR), MSNA and mean blood pressure (MAP) to arousal stimulation by low pressure on an ankle.
The responses were expressed as percentage of the respective mean values of 10-beats data prior to the contraction (100%). Subject number = 4.
Discussion
The primary purpose of this study was to identify the sympathetic responses to the stimulation of mechanoreceptors in healthy individuals. The unique finding of the present study is that passive muscle stretch in young healthy subjects induces transient increases in MSNA, heart rate and blood pressure with short latencies from the onset of stretch. Moreover, active muscle contraction with a similar protocol induces similar response patterns. These data support the concept that mechanoreceptors in muscles have a role in evoking sympathetic responses in healthy humans.
The present study was designed to observe whether isolated stimulation of muscle mechanoreceptors could evoke a dynamic MSNA response. The mean MSNA burst rate and total activity, heart rate and blood pressure over the whole period of the stretch or contraction protocols were not significantly different from the respective resting values. These data suggest that baroreflexes were not reset by the stimulation in the study. The averaged dynamic responses demonstrated that the stretch induced transient increases in heart rate and MSNA during beats 1–3 after the onset of stretch. This was followed by an increase in mean blood pressure, which occurred during beats 3–7 after onset of stretch (see Fig. 3). This increase in blood pressure is likely to be due to MSNA induced vasoconstriction coupled with a rise in cardiac output due to the rise in heart rate. In turn, this higher blood pressure led to suppression of the MSNA and heart rate as baroreflexes were engaged. The present data confirm the observation of Baum et al. (1995) that passive stretch increased blood pressure in the initial period of the stimulation. On the other hand, a recent study by Middlekauff et al. (2004) demonstrated that passive arm flexion did not evoke an increase in mean total MSNA activity in healthy subjects. The different findings might be due to differences in exercise protocols as well as differences in methods of data analysis employed. It should be noted that observations from animal studies support the concept that stimulation of muscle mechanoreceptors can induce sympathetic responses with a short latency. Group III muscle afferents, which were suggested to be involved in the exercise pressor reflex (McCloskey & Mitchell, 1972; Mitchell et al. 1983), respond vigorously at the onset of titanic contraction, with the first impulse often being discharged within 200 ms of the start of the contraction (Kaufman et al. 1983). Moreover, evoked muscle contractions in cats induced increases in signal unit muscle (Hill et al. 1996) and renal (Hayes & Kaufman, 2002) sympathetic discharges with onset latencies in the range of seconds. Therefore, the present results suggest that isolated stimulation of mechanoreceptors can evoke the sympathetic response in healthy humans.
Passive stretch also increased heart rate with a short latency, which is consistent with previous observations (Hollander & Bouman, 1975; Gelsema et al. 1985; Nobrega & Araujo, 1993; Gladwell & Coote, 2002; Fisher et al. 2005). For example, electrically induced contraction of leg muscles increased heart rate within 500 ms of its initiation in human (Hollander & Bouman, 1975) and cats (Gelsema et al. 1985). Moreover, Gladwell & Coote (2002) reported that 1 min of sustained passive triceps surae stretch increased heart rate. These authors concluded that stimulation of mechanoreceptors in muscle inhibits cardiac vagal activity and increases heart rate. Passive rhythmic movement of leg muscles also rapidly increases the heart rate (Nobrega & Araujo, 1993). Thus, the present results and the previous observations suggest that isolated stimulation of mechanoreceptors in human muscles can induce increase in heart rate.
Although subjects were shielded from the sound signal for stretching, arousal stimulation could not be excluded during the passive stretch. However, it is well known that MSNA is not sensitive to certain forms of arousal stimulation, and this stimulation (e.g. sound) is used to discern MSNA from skin sympathetic nerve activity (Vallbo et al. 1979; Wallin & Fagius, 1988). Moreover, electrical skin stimulation used as sensory stimulation has been shown to cause a transient decrease in MSNA in some subjects (Donadio et al. 2002a, b). In the present study, to verify that MSNA increase during passive stretch was not evoked by arousal stimulation, control studies were performed by applying low levels of pressure to the ankle using an on/off cycle identical to that used for stretch. The data were analysed with the same method. The results show that the arousal stimulation did not cause any clear increase in MSNA, heart rate or blood pressure such as those seen during passive stretch (see Fig. 6). Therefore, the responses during passive stretch were evoked by the stimulation of mechanoreceptors.
In the present study, active muscle contraction induced a similar response pattern to that noted with muscle stretch. Because the contraction period of each bout in the present study was short (5 s), and the interval time between the bouts was relative long (15–25 s), the metaboreceptor engagement should not play an important role in evoking MSNA responses to this exercise protocol. Thus, the responses to contraction are likely to be due to engagement of central commands and mechanoreceptors. The present results are consistent with the previous observations of Herr et al. (1999), which suggested that active muscle contractions evoked MSNA responses with an onset latency of ∼4–6 s. Therefore, the present results of passive stretch and active contraction indicate that both central commands and mechanoreceptors in humans can contribute to the exercise pressor reflex through both the vascular (MSNA) and cardiac components (heart rate) of the reflex arc.
Although the response patterns for contraction and stretch were similar in the present report, the response amplitudes evoked by contraction were greater than those seen with stretch. Possible explanations for this difference include the following. First, the force generated by contraction was much greater than that generated by muscle stretch. Thus, afferent activation of muscle mechanoreceptors was likely to be greater during contraction than during stretch. To avoid evoking pain, we limited force evoked by stretch. Second, the central commands during active contraction may contribute to the response. Third, a recent animal study (Hayes et al. 2005) suggests that the muscle afferent fibres engaged by muscle contraction may be different from those engaged by stretch. Thus, we cannot exclude that difference in responses observed were due to the stimulation of different pools of afferents by the different stimuli.
To decrease the influences of the breathing cycle during stretch/contraction protocols, subjects were asked to avoid breath holding during the study. Moreover, both the stretch and contraction protocols were also performed when breathing was controlled. The controlled breathing and the random length of the interval between the stretch/contraction bouts ensured that the breathing cycles were not synchronized with the onset of stretch or contraction. Moreover, stretch/contraction did not induce a clear and definite respiratory pattern in subjects. Importantly, there was no significant difference in the responses of the haemodynamic variables between the two breathing conditions. Therefore, the observed responses in MSNA, heart rate and blood pressure were not evoked by the effects of breathing.
The present findings suggest that in the initial period of exercise, muscle mechanoreceptors are engaged evoking peripheral vasoconstriction and increasing heart rate. This sympathetic activation is overwhelmed sequentially as the baroreflexes are engaged. Thus, the mean MSNA burst rate/total activity during the initial period of exercise is not significantly changed. Therefore, with the protocol used in this report, the general effect of stimulation of mechanoreceptors in evoking sympathetic activation is detectable but not permanent. We speculate that this may play a role in maintaining stable haemodynamic parameters during low intensity exercise in healthy individuals. With sustained exercise, muscle metaboreceptors (group IV) will be activated (Kaufman et al. 1983). Moreover, muscle mechanoreceptor activation may also increase, since the sensitivity of these receptors may increase as muscle metabolite concentrations rise in the interstitium (Herr et al. 1999). Under these circumstances, increased MSNA burst rate can be observed in healthy individuals as demonstrated in previous studies (Mark et al. 1985; Saito et al. 1989; Seals, 1989). The baroreflexes are reset (Ebert, 1986) to a higher pressure level during the exercise. The metaboreceptor engagement can be one of the mechanisms of baroreceptor resetting (Cui et al. 2001). Therefore, as compared to muscle metaboreceptor activation which is seen during fatiguing exercise, muscle mechanoreceptor control of MSNA is seen early in exercise and evokes much smaller change in MSNA. On the other hand, we speculate that pathophysiological conditions in which muscle mechanoreceptor and/or baroreflexes do not function normally may result in an increase in MSNA even with low intensity exercise or perhaps even under rest conditions. For example, congestive heart failure patients have impaired baroreflex function and have high resting MSNA levels (Grassi et al. 1995). Moreover, animal studies (Smith et al. 2003; Li et al. 2004) suggest that the mechanoreflex is accentuated in congestive heart failure. The combination of heightened muscle mechanoreceptor activity and impaired baroreflex function might contribute to the earlier rise in MSNA seen during handgrip (Silber et al. 1998), as well as to the MSNA activation seen during passive exercise in heart failure patients (Middlekauff et al. 2004).
Study limitation
In the present study, the signals were processed on a beat-by-beat basis, while the onset and the end of the 5 s stretch/contraction bouts were not synchronized with a cardiac event (e.g. R wave). This caused two limitations in this study. First, the onset of the force could occur at any time within a cardiac cycle. This might contribute to variations in response latencies. Nevertheless, this influence would not be greater than one heart beat. Second, because the baseline heart rates varied from individual to individual, and the heart rate in each individual also varied with time, the stretch/contraction bouts did not end at the same heart beat number. This caused large variations in the responses noted at the end of the stimulation. Thus, responses during recovery are not discussed in this report.
In conclusion, the haemodynamic responses to activation of mechanosensitive afferents by repetitive short bouts of passive stretch or active contraction in young healthy subjects are measurable with signal averaging analysis. The response is transient, and the magnitude is small. We speculate that the baroreflex buffering contributes to these effects. These results support the concept that mechanoreceptors in muscles contribute to evoking sympathetic responses during exercise, although its role in regulating MSNA under the condition of this protocol may be limited.
Acknowledgments
We are grateful to the subjects for their enthusiastic participation. This project was supported by National Institutes of Health Grants P01 HL077670, M01 RR010732, and The American Heart Association Grants 0565399U, 0635245N.
References
- Baum K, Selle K, Leyk D, Essfeld D. Comparison of blood pressure and heart rate responses to isometric exercise and passive muscle stretch in humans. Eur J Appl Physiol Occup Physiol. 1995;70:240–245. doi: 10.1007/BF00238570. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Shibasaki M, Hodges NA, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J Appl Physiol. 2001;91:1679–1686. doi: 10.1152/jappl.2001.91.4.1679. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2004;286:H1101–H1106. doi: 10.1152/ajpheart.00790.2003. [DOI] [PubMed] [Google Scholar]
- Donadio V, Kallio M, Karlsson T, Nordin M, Wallin BG. Inhibition of human muscle sympathetic activity by sensory stimulation. J Physiol. 2002a;544:285–292. doi: 10.1113/jphysiol.2002.019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio V, Karlsson T, Elam M, Wallin BG. Interindividual differences in sympathetic and effector responses to arousal in humans. J Physiol. 2002b;544:293–302. doi: 10.1113/jphysiol.2002.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert TJ. Baroreflex responsiveness is maintained during isometric exercise in humans. J Appl Physiol. 1986;61:797–803. doi: 10.1152/jappl.1986.61.2.797. [DOI] [PubMed] [Google Scholar]
- Eckberg D, Sleight P. Efferent baroreflex responses. In: Eckberg D, Sleight P, editors. Human Baroreflexes in Health and Diesase. New York: Oxford University Press; 1992. pp. 216–254. [Google Scholar]
- Fisher JP, Bell MP, White MJ. Cardiovascular responses to human calf muscle stretch during varying levels of muscle metaboreflex activation. Exp Physiol. 2005;90:773–781. doi: 10.1113/expphysiol.2005.030577. [DOI] [PubMed] [Google Scholar]
- Gelsema AJ, Bouman LN, Karemaker JM. Short-latency tachycardia evoked by stimulation of muscle and cutaneous afferents. Am J Physiol. 1985;248:R426–R433. doi: 10.1152/ajpregu.1985.248.4.R426. [DOI] [PubMed] [Google Scholar]
- Gladwell VF, Coote JH. Heart rate at the onset of muscle contraction and during passive muscle stretch in humans: a role for mechanoreceptors. J Physiol. 2002;540:1095–1102. doi: 10.1113/jphysiol.2001.013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol. 2001;280:H2153–H2161. doi: 10.1152/ajpheart.2001.280.5.H2153. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kaufman MP. MLR stimulation and exercise pressor reflex activate different renal sympathetic fibers in decerebrate cats. J Appl Physiol. 2002;92:1628–1634. doi: 10.1152/japplphysiol.00905.2001. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–1896. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol. 1999;86:767–772. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- Hill JM, Adreani CM, Kaufman MP. Muscle reflex stimulates sympathetic postganglionic efferents innervating triceps surae muscles of cats. Am J Physiol. 1996;271:H38–H43. doi: 10.1152/ajpheart.1996.271.1.H38. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38:272–278. doi: 10.1152/jappl.1975.38.2.272. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Li J, Sinoway AN, Gao Z, Maile MD, Pu M, Sinoway LI. Muscle mechanoreflex and metaboreflex responses after myocardial infarction in rats. Circulation. 2004;110:3049–3054. doi: 10.1161/01.CIR.0000147188.46287.1B. [DOI] [PubMed] [Google Scholar]
- McClain J, Hardy JC, Sinoway LI. Forearm compression during exercise increases sympathetic nerve traffic. J Appl Physiol. 1994;77:2612–2617. doi: 10.1152/jappl.1994.77.6.2612. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25:37–41. [PubMed] [Google Scholar]
- Rowell LB. Central circuitry adjustments to dynamic exercise. In: Rowell LB, editor. Human Cardiovascular Control. New York: Oxford University Press; 1993. pp. 162–203. [Google Scholar]
- Saito M, Mano T, Iwase S. Sympathetic nerve activity related to local fatigue sensation during static contraction. J Appl Physiol. 1989;67:980–984. doi: 10.1152/jappl.1989.67.3.980. [DOI] [PubMed] [Google Scholar]
- Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Silber DH, Sutliff G, Yang QX, Smith MB, Sinoway LI, Leuenberger UA. Altered mechanisms of sympathetic activation during rhythmic forearm exercise in heart failure. J Appl Physiol. 1998;84:1551–1559. doi: 10.1152/jappl.1998.84.5.1551. [DOI] [PubMed] [Google Scholar]
- Sinoway L, Prophet S, Gorman I, Mosher T, Shenberger J, Dolecki M, Briggs R, Zelis R. Muscle acidosis during static exercise is associated with calf vasoconstriction. J Appl Physiol. 1989;66:429–436. doi: 10.1152/jappl.1989.66.1.429. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci L, Pryor S, Nunnally R. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest. 1988;82:1301–1305. doi: 10.1172/JCI113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res. 1989;64:592–599. doi: 10.1161/01.res.64.3.592. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res. 1991;69:228–238. doi: 10.1161/01.res.69.1.228. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol. 1988;50:565–576. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Mitchell JH, Olesen HL, Raven PB, Secher NH. Reflex increase in blood pressure induced by leg compression in man. J Physiol. 1994;475:351–357. doi: 10.1113/jphysiol.1994.sp020076. [DOI] [PMC free article] [PubMed] [Google Scholar]