Abstract
Skeletal muscle fibres contain ubiquitous and muscle-specific calcium-dependent proteases known as calpains. During normal activity, intracellular [Ca2+] in muscle fibres increases to high levels (∼2–20 μm), and it is not apparent how this can be reconciled with the activation properties of the calpains. Calpains evidently do not cause widespread proteolytic damage within muscle fibres under normal circumstances, but do have a role in necrosis in dystrophic muscle fibres. In this study, we examined the in situ localization and regulation of calpains in muscle fibres in order to identify how they are attuned to normal function. The sarcolemma of individual muscle fibres of the rat was removed by microdissection (fibre ‘skinning’) in order to determine the compartmentalization and diffusibility of the two most Ca2+-sensitive calpains, μ-calpain and calpain-3, and to permit precise manipulation of cytoplasmic [Ca2+] under physiological in situ conditions. Passive force production in stretched fibres, which indicates the patency of the important elastic structural protein titin, was used as a sensitive assay of the amount of diffusible proteolytic activity in individual fibre segments and in muscle homogenates at set [Ca2+]. All calpain-3 is bound tightly within a fibre, whereas most μ-calpain (∼0.2 μm) is initially freely diffusible in the cytoplasm at resting [Ca2+] but binds within seconds at high [Ca2+]. [Ca2+] has to be raised to ≥ 2 μm for ≥ 1 min to initiate detectable autolysis of μ-calpain and to activate appreciable proteolytic activity. If the [Ca2+] is raised sufficiently for long enough to initiate substantial autolysis of μ-calpain, the Ca2+ sensitivity of the proteolytic activity is greatly increased, and it remains active even at 300 nm Ca2+, with activity only ceasing if the [Ca2+] is decreased to ∼50 nm Ca2+, close to the normal resting [Ca2+]. These findings on the Ca2+- and time-dependent binding, autolytic and proteolytic properties of μ-calpain under physiological conditions demonstrate how it is precisely attuned to avoid uncontrolled proteolytic activity under normal circumstances, and indicate why it could lead to substantial proteolytic damage if resting or localized [Ca2+] is elevated, as is likely to occur after eccentric contraction and in dystrophic muscle.
Calpains are non-lysosomal Ca2+-activated cysteine proteases. Skeletal muscle fibres contain both the ubiquitous calpains, μ-calpain and m-calpain, and a muscle-specific calpain, known as calpain-3 or p94 (Goll et al. 2003; Bartoli & Richard, 2005). The precise roles and normal regulation of the calpains in skeletal muscle are currently unclear, though they are likely to be involved in differentiation, atrophy and regeneration of muscle, and it is evident that they have a major involvement in certain types of muscular dystrophy. It is particularly important to understand how these potentially damaging Ca2+-dependent proteases are regulated in skeletal muscle because, inherent to its normal function, a skeletal muscle cell experiences what is probably the largest rise in intracellular free [Ca2+] of all cell types, with such rises typically happening repetitively for extended periods during normal activity. Resting [Ca2+] in a fast-twitch mammalian muscle fibre is in the range ∼20–50 nm and peak free [Ca2+] during tetanic stimulation in murine muscle has been reported to be ∼1–2 μm (Allen & Westerblad, 2001) and even as high as 20 μm very transiently (Baylor & Hollingworth, 2003). This is not readily reconciled with the report by Belcastro (1993) that μ-calpain from quiescent skeletal muscle of the rat displays 10% and 50% of maximal proteolytic activity at 100 nm and 1 μm Ca2+, respectively, because that seemingly implies that there would be a substantial level of proteolytic activity going on throughout normal muscle activity. This highlights the need for a detailed examination of the localization and Ca2+- and time-dependent activation of the calpains, in particular of μ-calpain, under the physiological conditions prevailing in a muscle fibre. Such an examination can also provide insights into the expected activity of calpains at the [Ca2+] prevailing in dystrophic muscle.
In vitro studies of μ-calpain and m-calpain in various tissues indicate that they require ∼3–50 μm and ∼400–800 μm free [Ca2+], respectively, for half-maximal proteolytic activity, but that the presence of phospholipids, small endogenous proteins or even specific substrates can substantially reduce the level of Ca2+ needed for activity (Pontremoli et al. 1990; Melloni et al. 1998; Goll et al. 2003). Importantly, Ca2+-dependent activation is typically accompanied by autolysis of the calpains, which reduces the [Ca2+] required for continued in vitro proteolytic activity to ∼0.5–2 μm for μ-calpain and to ∼50–150 μm for m-calpain (Goll et al. 2003). μ-calpain is autolysed from its 80 kDa full-length form to a 78 kDa and then to a 76/75 kDa form. It has been reported that the 80 kDa form displays proteolytic activity in erythrocyte membranes (Molinari et al. 1994), whereas others have shown that proteolytic activity closely parallels the formation of the autolytic products (Baki et al. 1996; Melloni et al. 1996). In erythrocytes, full-length μ-calpain is primarily cytosolic and in the presence of Ca2+ some partitions to the surface membrane and autolyses to the 78 kDa form and is stabilized there by the membrane, with only a small proportion autolysing further to the 76 kDa form and again becoming readily diffusible (Melloni et al. 1996; Michetti et al. 1996). The proteolytic activities of μ-calpain and m-calpain are inhibited in a Ca2+-dependent manner by the endogenous protein calpastatin (Kapprell & Goll, 1989), which in unstimulated cells is found in bound aggregates rather than in a freely diffusible form (De Tullio et al. 1999). Calpastatin has a much higher affinity for the autolysed forms of μ-calpain than the unautolysed form (Melloni et al. 1996).
In normal rested skeletal muscle, the ubiquitous calpains are found predominantly in their unautolysed form (Goll et al. 2003; Murphy et al. 2006). In the study by Belcastro (1993), the calpains were isolated and purified before examining the Ca2+-dependence of their proteolytic activity; however, the extent of autolysis was not examined, and so it seems quite possible that the observed high Ca2+ sensitivity may have been caused by autolysis of some of the calpain. The ubiquitous calpains do seem to be important in Duchenne muscular dystrophy (DMD). Total Ca2+-activated protease activity is elevated in muscle from humans with DMD (Kar & Pearson, 1976), and overexpression of calpastatin reduces dystrophic pathology in the mdx mouse dystrophic model, indicating that one or both of the ubiquitous calpains is involved in the pathology (Spencer & Mellgren, 2002). However, the role and basis of activation of calpains are currently unclear. The cellular distribution of the ubiquitous calpains in skeletal muscle is also not entirely clear. It has been reported that μ-calpain and m-calpain are present at a higher density in the Z-disk region than in the I-band and A-band regions (Yoshimura et al. 1986; Kumamoto et al. 1992) and are also associated with the sarcolemma, the muscle surface membrane (Dayton & Schollmeyer, 1981), with a higher proportion associating with the sarcolemma in mdx mice than in wild-type mice (Spencer & Tidball, 1996). However, it may be that the ubiquitous calpains are normally quite mobile or differently distributed in fibres in vivo and that they rapidly bind or redistribute during fixation or fractionation and other experimental procedures.
The situation for calpain-3 is understood even less well. It was originally reported that calpain-3 readily autolyses in a Ca2+-independent manner and disappears from muscle (Sorimachi et al. 1993), but subsequent studies reported that it can be stably expressed in vivo (Spencer et al. 2002) and that it autolyses in a Ca2+-dependent manner at micromolar levels of Ca2+ (Branca et al. 1999; Murphy et al. 2006). Calpain-3 binds to titin at the N2A line, adjacent to the extensible PEVK region of this large elastic protein (Sorimachi et al. 1995; Keira et al. 2003) and possibly also binds at the M-line (Ojima et al. 2005). Calpain-3, like μ-calpain and m-calpain, can cleave titin, with cleavage occurring at the PEVK region and the M-line but not at the N2A line where calpain-3 is bound (Kramerova et al. 2004). Absence or decreased activity of calpain-3 leads to the development of limb girdle muscle dystrophy type 2A (Richard et al. 1995), but the actual role of calpain-3 in muscle remains unclear.
In this study, we have examined the compartmentalization and autolysis of μ-calpain in individual fibres from fast-twitch skeletal muscle of the rat, removing the sarcolemma by microdissection in order to gain access and accurately vary the intracellular [Ca2+] throughout the fibre under physiological conditions. In this way, we have been able to show that in contrast to calpain-3, μ-calpain is mostly freely diffusible in the cytoplasm in a resting fibre, and that it binds at high [Ca2+] but does not readily autolyse and increase its Ca2+ sensitivity unless the intracellular [Ca2+] is maintained at a relatively high level (≥ 2 μm Ca2+) for a prolonged time. These findings demonstrate how μ-calpain is precisely attuned to avoid chronic proteolytic activity under normal circumstances, and indicate why there can be substantial proteolytic damage if resting or localized [Ca2+] is elevated, as is likely to occur after eccentric contraction and in dystrophic muscle.
Methods
Preparation
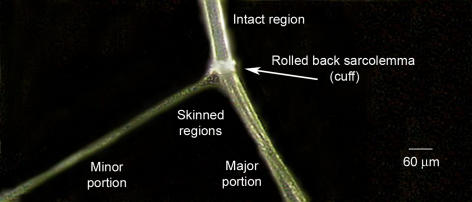
Male Long-Evans hooded rats (∼4–8 months old) were killed by overdose of fluothane (2% v/v) in a restricted air space, and then both extensor digitorum longus (EDL) muscles were swiftly excised. All experimental procedures were approved by the La Trobe University Animal Ethics Committee. Whole muscles used for homogenates were maintained in saline solution containing (mm): NaCl 140, KCl 5, CaCl2 2.5, MgCl2 1 and Hepes 10; pH 7.3 at room temperature and used within 5–30 min. When obtaining single fibres, muscles were pinned at resting length under paraffin oil (Ajax Chemicals, Sydney, Australia) in a Petri dish and kept cool (∼10°C) on an icepack. Individual muscle fibres were mechanically skinned with fine forceps as shown in Fig. 1. By mechanically skinning (or ‘peeling’) single skeletal muscle fibres, it is possible to accurately determine how much of a particular calpain present in a muscle fibre at rest is in close association with the sarcolemma and how much is freely diffusible in the cytoplasm or bound within the fibre. In this procedure the entire sarcolemma from a section of a single fibre is rolled back by microdissection, forming a ‘cuff’, allowing it to be completely removed with little or no contamination with any of the underlying myofibrillar network (Fig. 1). As shown previously, the transverse tubular (T)-system inside the muscle fibre seals off completely when the sarcolemma is rolled back and it remains within the skinned regions as a separate, polarisable compartment (Lamb et al. 1995; Launikonis & Stephenson, 2004) such that the skinned fibre segments fully retain the capacity for normal action potential-mediated excitation–contraction coupling (Posterino et al. 2000; Verburg et al. 2006).
Figure 1. Skinning a skeletal muscle fibre by microdissection.
A single skeletal muscle fibre was dissected away from a rat extensor digitorum longus muscle under paraffin oil. Individual myofibrils within the fibre run axially, and a group of myofibrils (‘minor portion’) were pulled away from the fibre, causing the entire sarcolemma along the fibre segment to roll back as a ‘cuff’ of membrane, which then could be dissected away and analysed separately from the resulting ‘skinned’ fibre portions that remain (major and minor portions pooled together as required).
In some experiments, a segment of the skinned fibre (length, ∼3 mm; diameter, ∼50 μm) was attached to a force transducer (AME801, SensoNor, Norway) at resting length or stretched as described in the text, and then placed in various solution baths (2.0 ml) or alternately moved under paraffin oil between different solution droplets (5–20 μl). In other cases, the skinned fibre segment was transferred through sequences of various solutions (5–10 μl) in 0.6-ml microfuge tubes. Where required, the sarcolemma (< 1% of fibre mass) and the associated skinned fibre segments were analysed separately using SDS-PAGE and Western blotting. All experiments were conducted at room temperature (24 ± 2°C) unless stated otherwise. All data show mean values with the standard error of the mean (s.e.m.) in the text and figures.
Solutions
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA), unless specified otherwise. Purified μ-calpain (referred to as exogenous μ-calpain) was purchased from Calbiochem (San Diego, CA, USA) and was derived from human erythrocytes. As previously described (Posterino et al. 2000; Verburg et al. 2005, 2006), the solutions were designed to mimic the major aspects of the normal cytoplasmic conditions, with K+ (126 mm) as the major cation (with 36 mm Na+) and with impermeant divalent anions rather than Cl−, and 1 mm free Mg2+, 90 mm Hepes (pH 7.1) and osmolality of 295 ± 5 mosmol l−1. The total divalent anion concentration was 68 mm and was composed predominantly of either hexamethylene-diamine-tetraacetate (HDTA2−; Fluka, Buchs, Switzerland) or EGTA2−–CaEGTA2− mixtures. Solutions were made with 8 mm ATP and 10 mm creatine phosphate, or without any ATP or creatine phosphate (‘rigor’ solutions) so as to prevent all Ca2+ uptake. Solutions with various [Ca2+] were made by altering the ratio of EGTA to CaEGTA and in some cases up 5 mm fast Ca2+ buffer BAPTA was also present. The free [Ca2+] in solutions (for 50 nm and above) was measured with a Ca2+-sensitive electrode (Orion Research, Cambridge, MA, USA). The caffeine–Ca2+ solution used for very brief dipping was similar to the CaEGTA rigor solution though not isosmotic as it also contained 5 mm additional Ca2+ and 30 mm caffeine.
Passive force measurements
Force responses were recorded simultaneously on both a chart recorder (Linear) and a computer with Powerlab series 4/20 amplifier/AD converter card and Chart 5 software (AD Instruments, Sydney, Australia). Fibres were stretched on the transducer to twice the resting length, reaching the final level within ∼3 s. Peak force was defined as the force 30 s after applying the stretch, as this was highly reproducible and depended on the final length and not on the rate at which the stretch was applied. For calculations of rates of decline of passive force (in percentage per minute), the 100% level was defined as the level on the slow declining phase following the initial 2 min of treatment in control rigor solution (or the equivalent time when kept only in oil); this was the passive force level immediately before applying the test treatment.
Exposure of fibres to muscle homogenate
Muscle homogenates made with free [Ca2+] heavily buffered to a given level were applied to stretched skinned fibres to test whether the raised [Ca2+] activated a readily diffusible compound that disrupted the patency of titin and reduced passive force production. Muscle samples were homogenized in Ca2+-containing rigor solution (with 50 mm total EGTA/BAPTA) with 2–20 μm free Ca2+ (0.25 g tissue per 1 ml). In some preparations, the [Ca2+] was raised to 20 μm for 5 min to allow for initiation of any Ca2+-activated processes, and then lowered to a new set level. These whole homogenates were then immediately used to treat a skinned fibre. The effect on passive force was tested as above, but with the skinned fibres being immersed for 1–3 min in the homogenate instead of Ca2+-containing rigor solution. This was compared to control treatments using homogenates prepared without raising the [Ca2+] or with 1 mm leupeptin added.
Collection and treatment of fibres
All fibres were collected into a total volume of 15 μl, containing a control rigor solution and sample loading buffer (0.125 m Tris-Cl (pH 6.8), 4% SDS, 10% glycerol, 4 m urea, 10% mercaptoethanol and 0.001% bromophenol blue). For fibres undergoing specific treatments, 10 μl aliquots of solution were placed in 0.6-ml microfuge tubes. Skinned fibres were transferred into the solution and then removed after the required time and treated as detailed above. Sample loading buffer was then added to the solution. Samples were heated to 95°C for 4 min before being stored at −20°C until required for analysis by Western blotting. Treatment solutions consisted of either control rigor solution or a rigor solution containing 1, 2, 5, 8 or 40 μm free [Ca2+] for times indicated in results (solutions described above).
Western blotting
μ-calpain and calpain-3 were analysed by Western blotting as previously described (Murphy et al. 2006). In brief, denatured protein from single fibres or muscle homogenates was separated on 8% SDS-PAGE gels (100 V for 15 min, followed by 160 V for 60 min) and transferred to nitrocellulose (15 V for 60 min). Following transfer, gels were stained with BioSafe Coomassie (Bio-Rad, Hercules, CA, USA). After a series of washes, membranes were exposed overnight to either mouse anti-calpain-3 (1 : 100, Novocastra monoclonal 12A2, Newcastle, UK) or mouse anti-μ-calpain (1 : 1000, Sigma), both diluted in 1% bovine serum albumin in phosphate-buffered saline with 0.025% Tween (PBST). Following a number of washes in blocking buffer (5% skimmed milk powder in tris-buffered saline with 0.025% Tween (TBST)), the membranes were exposed to secondary antibody (goat anti-mouse horseradish peroxidase, Bio-Rad), diluted 1 : 20 000 in blocking buffer for 60 min. Following a further series of washes in TBST, bands were visualized using either colorimetric (Opti-4 CN substrate, Bio-Rad) or chemiluminescent (Pierce, IL, USA) detection. Images were collected using a CCD camera attached to a ChemiDoc XRS chemiluminescent detection system (Bio-Rad) and using Quantity One software (Bio-Rad). Densitometry was performed with the Quantity One software.
To verify that the detection of μ-calpain by Western blotting showed a linear response for amounts in the range of a single skeletal muscle fibre segment and below, a series of dilutions equivalent to 1, 0.5, 0.25 and 0.13 fibre segments were analysed as described above. Linear regression analysis indicated a near linear relationship between band density and fibre quantity over the range examined (data not shown; r2= 0.9996, x-intercept = 0.04 fibre segment). As total protein could not be determined for the same individual muscle fibre segments used in the Western blotting, where relevant the band intensities on a given Western blot were normalized by the amount of myosin heavy chain (MHC) present in the analysed segment as seen by Coomassie staining of the gel following transfer. To validate this approach, we examined the linearity of the amount of MHC remaining in a gel following transfer. As for μ-calpain, a linear response was observed for MHC (data not shown; r2= 0.9858, x-intercept =− 0.15 fibre segment) indicating that the amount of MHC left in the gel after transfer was a valid indicator of the amount of protein loaded. Similar responses were seen for μ-calpain and MHC in three separate analyses.
Titin analyses
For analysis of titin, treated single fibres were added to 5 μl titin extraction buffer (8.7% SDS, 0.1 m Tris-Cl (pH 8.8), 5 mm EGTA and 50 mm dithiotreitol), heated at 65°C for 5 min and left at room temperature (RT) for 90–120 min before being spun at 13 000 g for 20 min. The supernatant was then mixed 1 : 1 with 50% glycerol in PBS with bromophenol blue and stored at −20°C until required for analysis within 1 week. Low percentage (2.8%) acrylamide gels with silver staining were used to detect titin, as previously described (Verburg et al. 2005). Electrophoresis was performed at RT (10 mA for 30 min, 20 mA for 3 h and 30 mA for ∼90 min). Gels were silver stained using GelCode SilverSNAP Stain Kit II (Pierce, IL, USA). To determine the ratio of full-length titin (T1) to degraded titin (T2), densitometry of the bands was quantified using Quantity One software (Bio-Rad).
Results
Sarcolemmal, diffusible and bound pools of calpains in muscle fibres
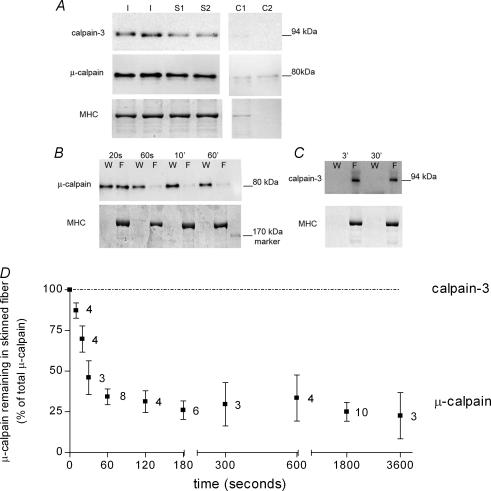
Examination of individual skinned fibres from freshly dissected EDL muscles of the rat showed that the majority of the μ-calpain is present in the bulk of the fibre (skinned parts) and only a small proportion is closely associated with the sarcolemma. In the six cases examined where no detectable myosin heavy chain (MHC) was present with the excised sarcolemma (i.e. no myofibrillar contamination, see lane C2 in Fig. 2A), the percentage of the total μ-calpain found with the sarcolemma was 16 ± 6% (all data are means ±s.e.m.). In accord, the amount of μ-calpain in skinned fibre segments was on average 90 ± 20% of that found in intact (i.e. unskinned) fibre segments, consistent with relatively little of the total μ-calpain being associated with the sarcolemma (n = 12 skinned and 16 intact segments, first normalizing density of μ-calpain to MHC in each fibre segment, and then comparing values for skinned and intact fibres). It is important to note that the μ-calpain present in intact and skinned fibres was found to be almost exclusively in its unautolysed (80 kDa) form, with < 5% of the total in the partially autolysed 78 kDa form. It is interesting that the relative proportion of total μ-calpain in the 78 kDa form was noticeably higher in the sarcolemma than in the rest of the fibre in every case examined (e.g. Fig. 2A). This activation was not attributable to exposure to extracellular Ca2+ during the skinning procedure, as the skinning was always done with the fibre in paraffin oil and also was no different if the muscle was presoaked in a 0 Ca2+ external solution (data not shown).
Figure 2. Diffusible and bound pools of μ-calpain in single muscle fibres at rest.
A, Western blots for calpain-3 and μ-calpain, and corresponding Coomassie stain of myosin heavy chain (MHC), in an intact section of each of two extensor digitorum longus (EDL) fibres (I) and in the total skinned segments (S1 and S2) and the associated sarcolemmal cuffs (C1 and C2) of two other EDL fibres. Calpain-3 and μ-calpain were present predominantly in their unautolysed full-length forms (94 and 80 kDa, respectively). B, μ-calpain and corresponding MHC remaining in the skinned fibre (F) or lost to the wash solution (W), after washing single skinned EDL fibre segments for the indicated time. (Each W on left side of corresponding F). Wash solution was a K+-based physiological solution with free [Ca2+] buffered at 20 nm. C, similar experiment with calpain-3 in skinned fibres exposed to 20 nm Ca2+ wash solution for 3 or 30 min. Calpain-3 is not lost from the fibre even with extensive washing. D, time course of washout of μ-calpain from single skinned EDL fibres; values are the mean (±s.e.m.) μ-calpain remaining in the skinned segment (expressed as a percentage of the total present in the skinned segment and wash solution) in indicated number of fibre segments.
The μ-calpain present within the skinned parts of a muscle fibre could be further subdivided into cytosolic and bound pools by determining how much of the calpain readily diffused out of the skinned segments when they were bathed in a comparatively large volume of solution mimicking the normal intracellular environment (K+-based solution with ∼20 nm free Ca2+; solution volume 5 or 10 μl compared to ∼6 nl for skinned fibre segments). Because the fibres were skinned under paraffin oil, they initially contained all normal cytoplasmic constituents, including any calpain. When transferred to the bathing solution, ∼70% of the μ-calpain initially present in the skinned fibre segments was rapidly lost from the fibre with a time constant of ∼25 s, consistent with free diffusion (Nguyen et al. 1998), with the other ∼30% being bound and diffusing only relatively slowly out of the fibre over up to 60 min (Fig. 2B and D). The extent of washout of μ-calpain was not noticeably different at any free [Ca2+] in the range 1–200 nm, nor was it different with in the presence or absence of ATP.
In contrast to μ-calpain, there was no detectable calpain-3 associated with the sarcolemma (Fig. 2A), and all of the calpain-3 remained bound within the skinned fibre segments in its 94 kDa unautolysed form over a 60-min washout period in 20 nm free Ca2+ (Fig. 2C).
Absolute amount of μ-calpain in a muscle fibre
An estimate of the absolute amount of μ-calpain present in a segment of an EDL fibre was ascertained from Western blotting by comparing the density of bands from fibre segments with that for known amounts of purified μ-calpain (three repetitions). There was ∼150 pg μ-calpain in a fibre segment, typically ∼3 mm long and ∼50 μm in diameter. This means that the total amount of μ-calpain in EDL fibres (expressed relative to total fibre volume) is ∼0.2 μm. Single intact fibres from rat soleus muscle contained on average 79 ± 8% (n = 11) of the μ-calpain present in EDL fibres (values normalized to amount of MHC in each fibre segment).
Binding and autolysis of diffusible μ-calpain pool upon raising cytosolic [Ca2+]
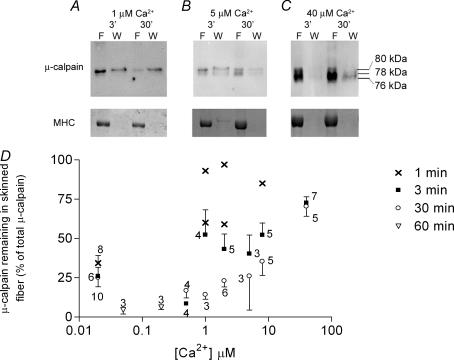
The effect of cytoplasmic [Ca2+] on the distribution and activation of μ-calpain was examined by transferring freshly skinned single fibres from the paraffin oil straight into a rigor solution with free [Ca2+] buffered in the range 0.5–40 μm. In contrast to the results at 20 nm cytosolic [Ca2+], the majority of the μ-calpain remained in the skinned fibre during the first 1–3 min of washing when the [Ca2+] was ≥ 1 μm (e.g. Fig. 3). This retention of the μ-calpain in the skinned fibre may have been due to some extent to the fibre segment contracting (as endogenous ATP was still initially present in the fibre), which could have interfered with the diffusional loss. However, this seems unlikely to fully account for the substantially slower diffusional loss seen at 1 μm Ca2+, a [Ca2+] that triggers only submaximal contraction in these rat EDL fibres (Lamb & Posterino, 2003), nor would it account for the high retention of μ-calpain even over 30 min at 40 μm Ca2+ (70 ± 6% retained, n = 7). Furthermore, when fibre segments were fixed at resting length on the force transducer the majority of the μ-calpain remained in the fibre when it was treated with 40 μm Ca2+ for 3 min (72 ± 10% retained, n = 3). Given that the diffusible cytoplasmic pool of μ-calpain is normally able to wash out very rapidly (see above), this slow loss suggests that a substantial part of the diffusible pool of μ-calpain must bind within seconds to sites within the skinned fibre when the cytosolic [Ca2+] is raised to high levels (> 10 μm).
Figure 3. Diffusible pool of μ-calpain rapidly binds in presence of raised cytosolic [Ca2+].
A, Western blot of μ-calpain and corresponding Coomassie stain of myosin heavy chain (MHC) for individual skinned fibres that were first exposed to a rigor wash solution (W) with the free [Ca2+] buffered at 1 μm. The majority of the μ-calpain remained in the skinned fibre (F) when the wash was for only 3 min (left-hand pair of lanes), but most was lost from a fibre with a 30-min wash (right-hand pair of lanes). B and C, similar experiments with wash solution containing 5 μm and 40 μm free Ca2+, respectively. The proportion of μ-calpain lost from the fibre decreased at higher [Ca2+], and the extent of autolysis of μ-calpain increased. The total of all μ-calpain in the fibre and wash (normalized to MHC) remained approximately the same as in untreated skinned fibres. D, percentage of μ-calpain remaining in a skinned fibre when the fibre was washed in a rigor solution with indicated free [Ca2+] for 1, 3 or 30 min (or 60 min at 50 nm and 200 nm Ca2+). Values are based on the total amount of μ-calpain present in the fibre and wash, irrespective of whether it was autolysed. Values are means (± s.e.m.) with number of fibres indicated, except for five cases at 1–8 μm Ca2+ where values for individual fibres are shown.
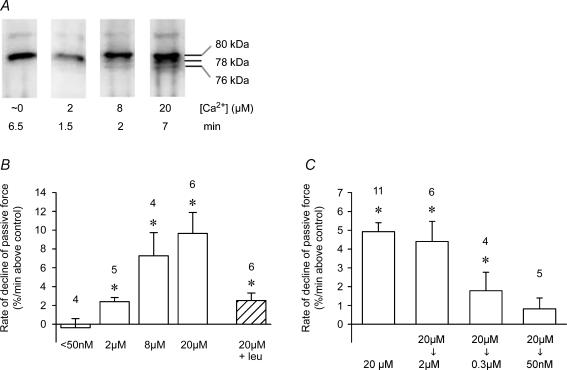
There was no detectable autolysis of μ-calpain when a skinned fibre was kept in a solution with either 50 or 200 nm free [Ca2+] even for 60 min (n = 3 fibres each, data not shown). When the free [Ca2+] was maintained at 1 μm, it still did not trigger substantial autolysis of the μ-calpain, as seen by the large proportion that remained in the unautolysed 80 kDa form even over 30 min (e.g. Fig. 3A). However, when the cytoplasmic [Ca2+] was maintained at a higher level (5–40 μm), a appreciable proportion of the μ-calpain was autolysed to the 78 kDa and 76 kDa forms (Fig. 3B and C), with the amount of autolysis increasing in both a Ca2+-dependent and time-dependent manner (e.g. ∼50% autolysed after 30 min at 5 μm Ca2+; > 50% within 3 min at 40 μm Ca2+).
Ca2+-dependent autolysis of diffusible and bound pools of μ-calpain
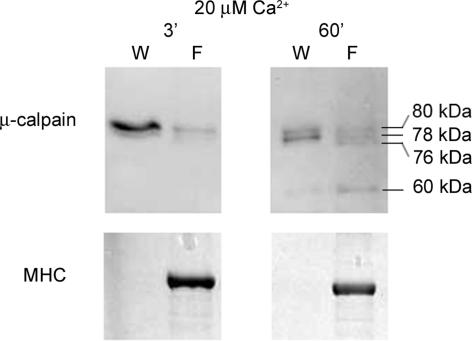
In order to examine whether the diffusible and bound pools of μ-calpain within a skinned fibre segment (∼60% and 25% of total in a fibre, respectively) are each individually autolysed in the presence of elevated [Ca2+], the two pools of calpain were separated by washing skinned fibres in 20 nm Ca2+ for 60 s and then the [Ca2+] was raised separately in the wash solution (containing the freely diffusible cytosolic constituents) and in the fibre. As expected from Fig. 2, the majority of the μ-calpain was found in the cytosolic pool (i.e. in the wash solution). When the [Ca2+] was raised to 20 μm, noticeable autolysis occurred in both pools within 3 min, and more than 50% autolysis occurred within 60 min (e.g. Fig. 4). With the longer exposure, the majority of the μ-calpain in both pools was autolysed to either the 78- or 76 kDa forms, and some 60 kDa product was also apparent in a number of cases (e.g. Fig. 4).
Figure 4. Cytosolic and bound pools of μ-calpain are both autolysed at elevated [Ca2+].
Western blot showing that μ-calpain residing in both the cytosolic and bound pools in quiescent fibres is autolysed in a Ca2+- and time-dependent manner. Skinned extensor digitorum longus fibre segments (examined in pairs for better detection) were washed for 60 s in 20 nm Ca2+ solution to separate cytosolic pool of μ-calpain, and then cytosolic constituents (W) and fibre segments (F) were separately exposed for 3 or 60 min to a solution at 20 μm free [Ca2+].
Passive force measurements and proteolysis of titin
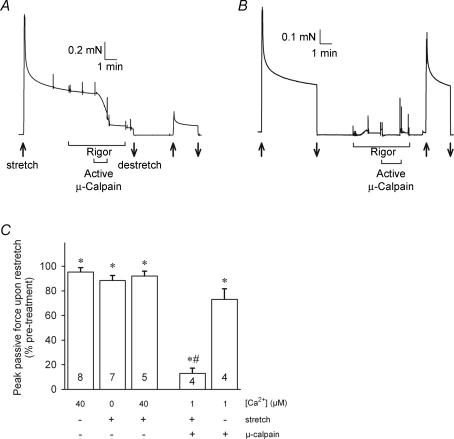
The proteolytic activity of muscle μ-calpain was assayed by measuring its effect on passive force production, which indicates the patency (i.e. functional integrity) of the important muscle structural protein, titin. Skinned fibres have no surface membrane or extracellular matrix and consequently the passive force produced by a stretch is predominantly dependent on the extent of titin elongation (Horowits et al. 1986). Figure 5A shows the passive force produced in a rat skinned EDL fibre that was mounted on a force transducer and stretched (over a few seconds) to twice its resting length. As typically observed with a rapid stretch of this order, force transiently peaked at a high level (similar in magnitude to the maximum Ca2+-activated force level that could be generated in such a fibre) and then declined rapidly over the first minute and then much more slowly in the following minutes. This force is entirely ‘passive’, resulting from the stretch of elastic elements, and it does not involve any active force production by myosin cross-bridges. When a stretched fibre was returned to its resting length for several minutes and then re-stretched to twice the resting length again, the passive force showed very similar behaviour, although the peak force was usually decreased by ∼5% if the first stretch was brief and by ∼10% if the fibre had been kept stretched for ∼10 min.
Figure 5. Passive force changes with activated exogenous μ-calpain.
A, stretching a skinned fibre (in solution with < 1 nm Ca2+) to twice its resting length (at upwards arrow) elicited a force response with a large transient peak followed by a gradual further reduction over many minutes. This ‘passive force’ is due to stretching the elastic structural protein, titin. During the slowly declining phase, the fibre was transferred to two successive baths containing rigor solution (0 ATP, 10 nm Ca2+), which had no effect on force production (except for force artefacts when transferring the fibre between solutions). Exposure for 1 min to pre-activated exogenous μ-calpain (0.25 μm, in the presence of 1 μm Ca2+) produced a rapid decline in passive force. Slackening the fibre to the original resting length (downwards arrow) and then reapplying the stretch to twice the resting length produced only a small passive force response. B, when another skinned fibre was exposed to activated μ-calpain while at its resting length (in rigor solution, causing small rigor force development), there was subsequently only a relatively small decrease in the level of passive force produced in response to a stretch to twice the resting length. C, mean (±s.e.m.) passive force production following indicated treatment, measured by reapplying a stretch to twice the resting length. Number of fibres shown on each bar. *Significantly different from pretreatment level (P < 0.05). #Effect of activated calpain treatment significantly different in stretched and unstretched fibres.
We used this method to examine the proteolytic action of both endogenous and exogenous μ-calpain. As the skinned fibre segment was stretched to twice its normal resting length, there was no overlap of the actin and myosin at the level of the sarcomere, and consequently there could be no active force production, nor could there be any rigor force if ATP was removed. The baseline rate of passive force decline in each skinned fibre was established by bathing the fibre in solution with [Ca2+] buffered to 20 nm (or lower), and changing this to a ‘rigor’ solution (i.e. one with no ATP) had no effect on the force, as expected (Fig. 5A). The absence of ATP ensured that there could be no Ca2+ pump activity within the skinned fibre that might alter the free [Ca2+] set by the buffering, and hence that all regions within the fibre would be subjected to that same [Ca2+].
In contrast to our previous findings in toad fibres (Verburg et al. 2005), once a rat skinned fibre had been washed for several minutes, raising the [Ca2+] to 40 μm had little if any effect on passive force production, causing no noticeable change in the rate of force decline (data not shown), nor any more reduction in peak passive force (measured upon subsequent re-stretch; Fig. 5C, left-hand column) than seen in untreated fibres stretched for a similar time (see above). A similar lack of effect on passive force was found when treating a fibre in open solution at any [Ca2+] in the range 1–125 μm (data not shown). This demonstrates that neither calpain-3, nor the ∼25% of μ-calpain that remains bound within the fibre after washing in low [Ca2+], are readily able to disrupt titin, even when the fibre is stretched. (As described later in Fig. 8C, a very small but significant decline in passive force is detected if a washed fibre is exposed to 20 μm Ca2+ and then placed back in oil so as to entrap any of the remaining endogenous proteases that may have become active and diffusible).
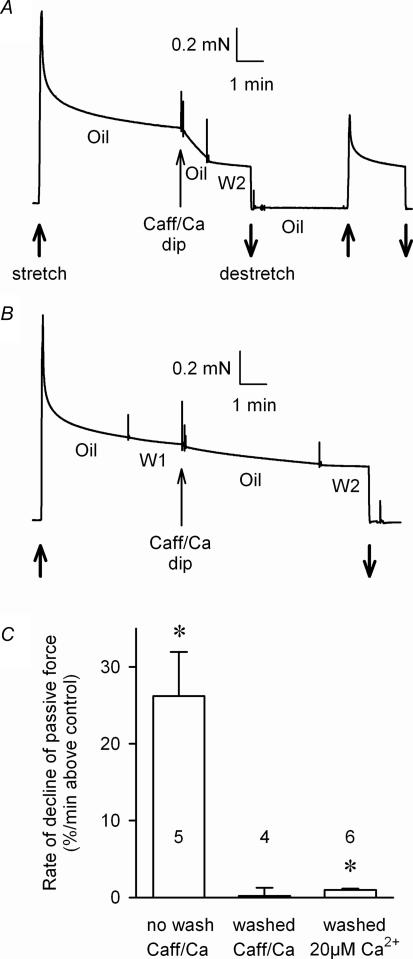
Figure 8. Washout of cytoplasm reduces endogenous Ca2+-dependent proteolytic activity.
A, a skinned fibre kept in paraffin oil and stretched to twice resting length (upwards arrow) displayed a steep decline in passive force in the period following a very brief (< 1 s) dip in a solution designed to raise the cytoplasmic [Ca2+] (30 mm caffeine solution with added Ca2+); most of the normal cytoplasmic constituents would have been still present in the fibre after such a brief dip. The force decline was subsequently stopped by transferring the fibre out of the oil and into a solution with the [Ca2+] strongly buffered at < 1 nm (W2). A subsequent re-stretch to twice resting length (second upwards arrow) confirmed the reduction in passive force production. B, when the normal cytoplasmic constituents in a skinned fibre were first washed out (2 min in solution with [Ca2+] weakly buffered at 20 nm (W1)), raising intracellular [Ca2+] by a dip in the caffeine–Ca2+ solution had little if any effect on the passive force response. C, mean (±s.e.m.) rate of decline in passive force immediately after caffeine–Ca2+ dip in skinned fibres that had no prior wash or a 15-s or 2-min wash (n = 3 and 1 fibres, respectively). Right-hand column shows data for six fibres that were washed for 2 min and then equilibrated for 15 s in a solution with 20 μm Ca2+ before being transferred back to oil to monitor the resultant decline in passive force. Values were determined after subtracting the rate of force decline in the same fibre immediately before treatment. *Significantly different from zero (P < 0.05).
When a stretched skinned fibre was exposed to exogenous active μ-calpain (0.25 μm), passive force declined steeply, decreasing over a 1-min exposure to only ∼25% of the level just before the exposure to μ-calpain (Fig. 5A). In accord, when the fibre was returned to resting length and then re-stretched to twice the resting length again, peak passive force production was also greatly reduced. (In all experiments reported here there was invariably close correlation in the results when assessing passive force either by the change in the steady level of passive force or by the change in the peak force upon re-stretch). It is important to note that if the skinned fibre was not stretched during the application of the exogenous calpain, the reduction in passive force production was relatively small (Fig. 5B and C). In these experiments, the exogenous μ-calpain was pre-activated by a 5-min exposure to 20 μm Ca2+, which was sufficient to cause substantial autolysis of the μ-calpain (see Fig. 7), and this calpain was then applied to the skinned fibre in the presence of 1 μm free Ca2+. The method utilizes the fact that once μ-calpain has undergone Ca2+-dependent autolysis it shows proteolytic activity at relatively low [Ca2+] (∼0.3 μm and above) (Goll et al. 2003). The procedure was designed to minimize Ca2+-dependent activation of any endogenous calpain still present in the skinned fibre, though it is apparent from the data above that the endogenous calpains that remained in the fibres after washing did not cause a significant decrease in passive force production. The rate of passive force decline was approximately linearly related to concentration of exogenous μ-calpain added over this range, with the rate of decline with 0.1 μm calpain being on average 24 ± 8% (n = 3) of that in fibres examined in the same experiment with 0.5 μm calpain (and all other conditions unchanged), indicating that the decline in passive force could indeed be used as an assay of μ-calpain activity. Furthermore, the effect of exogenous μ-calpain on passive force was inhibited by leupeptin (see next section) and entirely Ca2+-dependent, there being no detectable decrease in passive force at < 10 nm Ca2+ and with the rate of decline in force at 50 nm Ca2+ on average being 3 ± 1% (n = 4) of that at 1 μm Ca2+. After washout of exogenous μ-calpain for 1–2 min with rigor solution (20 nm Ca2+), raising [Ca2+] to 1 or 40 μm caused no detectable decline in passive force.
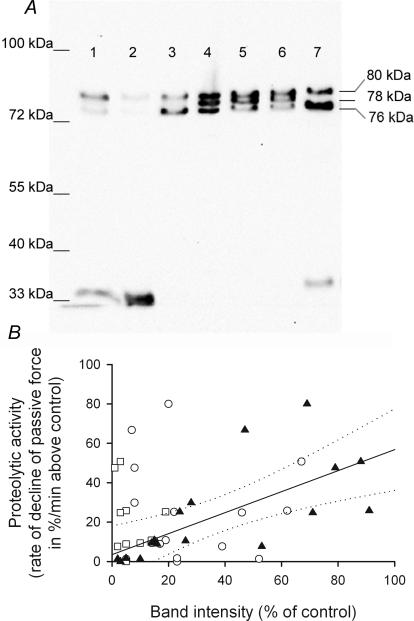
Figure 7. Activation and proteolytic activity of exogenous μ-calpain.
A, Western blot of μ-calpain following treatment with 0.5 μm exogenous μ-calpain with 1 or 20 μm free Ca2+ for different times. Exogenous μ-calpain was initially present in almost equal proportions in the 80-, 78- and 76 kDa forms (lane 4). Exposure to 20 μm Ca2+ for 5 min (lane 3), 30 min (lane 7), 4 h (lane 1), 24 h (lane 2) or to 1 μm Ca2+ for 7 min (lane 5) or 39 min (lane 6). B, proteolytic activity (assayed as in Figure 5A by rate of decline of passive force in the presence of 1 μm Ca2+) compared with the amounts of 80 kDa (^), 78 kDa (□) and 76 kDa (▴) μ-calpain present. The amount of each form present (band intensity) was expressed as a percentage of total initially added (i.e. total of all forms present in untreated exogenous μ-calpain). Linear regression analysis of data for 76 kDa form gave a best fit line (continuous line) with a gradient significantly different from zero, indicating a direct relationship between amount of 76 kDa isoform present and proteolytic activity (at 1 μm Ca2+) of that sample (r2= 0.48, P < 0.01, dotted lines indicate 95% confidence limits of fit).
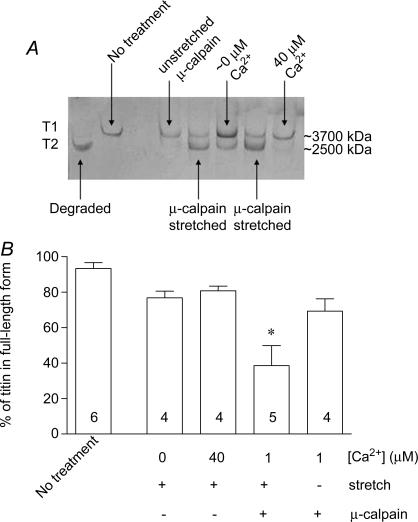
The extent of proteolysis of titin was also directly visualized by silver staining of SDS-PAGE gels of the proteins in a subset of the fibres used in the passive force experiments. In freshly isolated fibres, most titin is in its full-length form (T1, ∼3700 kDa). It could be readily proteolysed to its degraded form (T2, ∼2500 kDa) (Kimura et al. 1992) by warming a whole muscle in vitro for a prolonged period (Fig. 6). The amount of titin in the degraded form was increased significantly in the skinned fibres that had been exposed to exogenous active μ-calpain while stretched on the transducer, but in the remaining treatment groups it was not significantly different from that in untreated fibres (Fig. 6).
Figure 6. Proteolysis of titin by activated exogenous μ-calpain.
A, 2.8% SDS-PAGE gel showing silver staining of titin in rat extensor digitorum longus fibres following passive force measurements and treatments as in Figure 5. Fibres with ‘no treatment’ were skinned but not mounted on a transducer. Degraded titin was produced by incubating fibres at 30°C for 60 min. B, mean percentage (±s.e.m.) of total titin in full-length form in fibres given indicated treatments. One of the stretched fibres exposed to μ-calpain broke during re-stretch and could not be included in the results in Figure 5C. *Significant difference in proportion of full-length titin remaining compared with other treatments (one way ANOVA with Newman–Keuls post hoc test, P < 0.05).
In summary, the above data demonstrate that titin is proteolysed by exogenous μ-calpain, provided the fibre is stretched, and that the passive force decline is indicative of the patency of the titin and is a useful assay of μ-calpain activity. The data also show that in rat skinned fibres, after washout of the freely diffusible cytoplasmic constituents, raising the cytoplasmic [Ca2+] results in little or no proteolysis of titin by the bound endogenous μ-calpain (and calpain-3) still present in the fibre.
Proteolytically active forms of μ-calpain at 1 μM Ca2+
We also examined which of the unautolysed and autolysed forms of the exogenous μ-calpain were proteolytically active under the conditions used. The exogenous μ-calpain obtained from the supplier (see Methods) consisted of approximately equal proportions of the 80-, 78- and 76 kDa forms (Fig. 7A, lane 4). By exposing this calpain to physiological solution with various [Ca2+] for up to 24 h, it was possible to obtain a whole range of different combinations of μ-calpain forms (Fig. 7A) and then to apply each of these to a skinned fibre under the same final conditions (e.g. at 1 μm free [Ca2+]) in order to determine its level of proteolytic activity. Exposure to 20 μm Ca2+ caused relatively slow autolysis of the full-length (80 kDa) protein, but any partially autolysed 78 kDa calpain rapidly autolysed further to the 76 kDa form (Fig. 7A, lanes 3 and 7), where it accumulated before eventually autolysing even further (to ∼35 kDa and possibly to other undetected products) if the exposure continued for 4–24 h (Fig. 7A, lanes 1 and 2). Autolytic products of μ-calpain smaller than 76 kDa had no detectable proteolytic activity in the passive force assay at either 1 or 20 μm Ca2+. Although it was not possible to obtain conditions where only the 76 kDa form was present, linear regression analysis of the combined data over the whole range of combinations indicated that the level of proteolytic activity in the presence of 1 μm Ca2+ was directly related to the amount of the 76 kDa autolytic product present and showed no apparent relationship with the amount of the full-length (80 kDa) form present (Fig. 7B). The range of conditions where there was substantial amounts of the 78 kDa product present was too limited to make any conclusion about whether the 78 kDa product did or did not contribute significantly to the total proteolytic activity at 1 μm Ca2+. With exogenous μ-calpain that had been pre-activated at 20 μm Ca2+ for 5–30 min, addition of 1 mm leupeptin stopped all detectable proteolytic activity at 1 μm Ca2+ and reduced activity at 20 μm Ca2+ 10-fold but did not completely eliminate it (n = 6 fibres).
Ca2+-activation of diffusible proteolytic factors present in the normal cytoplasm
We next examined whether the normal endogenous cytoplasm in a muscle fibre contained diffusible Ca2+-dependent proteolytic factors. This was achieved by very briefly (< 1 s) dipping a freshly skinned muscle fibre into a solution with caffeine and high Ca2+ in order to raise the cytoplasmic [Ca2+] without major loss of the normal cytoplasmic constituents (the fibre was kept under oil before and after the dip). This brief caffeine–Ca2+ exposure immediately caused a large and rapid decline in passive force production in every fibre examined (Fig. 8A and C), demonstrating that the normal cytoplasm contains a large amount of a proteolytic factor that can be rapidly activated by raising the free [Ca2+]. It was further found that almost all of this activity was lost with washout of the normal cytoplasm for ∼15–120 s; the same brief dip in caffeine–Ca2+ solution caused no significant reduction in passive force when the normal cytoplasm had already been washed out (Fig. 8B and C). Because the actual change in cytoplasmic [Ca2+] was not controlled in the caffeine–Ca2+ dip experiment, it might be argued that in the washout case the rise in [Ca2+] was not sufficient to activate proteolysis. However, this seems very unlikely given the very high concentrations of Ca2+ and caffeine present in the dip solution (5 mm and 30 mm, respectively) and the fact that the initial wash solution had only weak Ca2+ buffering (0.5 mm total EGTA). Furthermore, when the normal cytoplasm was washed out for 2 min and the fibre exposed for 15 s to a solution heavily buffered at a known high free [Ca2+] (20 μm) before moving the fibre into oil, the rate of passive force decline in the oil was very much smaller than when raising the [Ca2+] in the caffeine–Ca2+ dip experiment with the normal cytoplasm still present (Fig. 8C). Thus, it is clear that most of the important diffusible Ca2+-dependent proteolytic factor normally present in the cytoplasm (i.e. that capable of disrupting passive force production) is readily diffusible at resting Ca2+ levels and is easily washed out of the fibre. At least some part of this factor is likely to be the diffusible pool of μ-calpain that is present endogenously in a resting fibre (Fig. 2).
It is also worth noting that the small but significant decrease in passive force seen in the fibres exposed to 20 μm Ca2+ and then returned to oil (right-hand column in Fig. 8C) indicates that some finite amount of a Ca2+-dependent proteolytic factor is retained within the fibre even after a 2-min washout of the normal cytoplasm and that it is able to disrupt passive force production when the [Ca2+] is raised. As raised [Ca2+] had no detectable effect on passive force in fibres open to the bathing solution, one possible interpretation of the data is that the disruption to passive force comes about because some of the remaining μ-calpain within the fibre dissociates from its binding site and produces a detectable level of titin proteolysis because it remains trapped within the cytoplasmic space.
Proteolytic activity of muscle homogenates at controlled [Ca2+]
The Ca2+-dependence of the proteolytic activity present in quiescent muscle was determined by homogenizing EDL muscles in K+-based physiological solution with the [Ca2+] very heavily buffered with EGTA and BAPTA at levels in the range < 10 nm to 20 μm. We have recently shown that this results in autolysis of both μ-calpain and calpain-3 in a Ca2+- and time-dependent manner, with slight though detectable autolysis of μ-calpain occurring within 1 min at a [Ca2+] of ∼2.5 μm (Murphy et al. 2006). Freshly made homogenates were applied here within 10 min to a skinned fibre and passive force monitored as in Fig. 5. Homogenates prepared with [Ca2+] maintained at < 50 nm showed no proteolytic activity (i.e. no decline in passive force, Fig. 9B). Homogenates made with [Ca2+] set at 2 μm showed a small but significant level of proteolytic activity even within 2 min of being produced, and the amount of activity increased in a graded manner with [Ca2+] up to 20 μm (Fig. 9B). Dilution of a given homogenate in a larger volume of the same solution caused an approximately proportional decrease in measured proteolytic activity. Addition of 1 mm leupeptin to a homogenate that had been made and kept in 20 μm Ca2+ for 5 min, reduced its proteolytic activity 4-fold but did not completely abolish it (Fig. 9B) and 100 μm E64c another calpain inhibitor similarly caused only partial inhibition of the activity (data not shown). Other homogenates were made with the free [Ca2+] at 20 μm for 5 min and then the [Ca2+] reduced to a lower level. Proteolytic activity was little if at all affected by reducing the [Ca2+] to 2 μm, and importantly it was still present even at 0.3 μm Ca2+ (∼30% of that at 20 μm Ca2+), but there was no significant activity when the [Ca2+] was reduced to 50 nm (Fig. 9C), which is close to the normal cytoplasmic [Ca2+] in a resting fibre.
Figure 9. Ca2+-dependence of proteolytic activity in muscle homogenates.
A, Western blot of μ-calpain present in extensor digitorum longus muscle homogenized with free [Ca2+] strongly buffered at particular levels for indicated time. Autolysis becomes more apparent at higher [Ca2+]. B, rate of decline in passive force when muscle homogenates made at indicated [Ca2+] were applied within 1–5 min to a stretched skinned fibre (as in Figure 5A). Right-hand column shows rate with 1 mm leupeptin and 20 μm Ca2+. C, rate of decline in passive force when muscle homogenates were made at 20 μm Ca2+ and then [Ca2+] decreased after 5 min to indicated level before applying the homogenate to skinned fibre. *Significantly greater than zero (i.e. greater than rate of decline measured in same fibre segment in control solution immediately before and after applying the homogenate). Note that rate of decline at 20 μm Ca2+ is very similar in B and C if data are normalized by final level of homogenate dilution (5-fold and 9-fold dilution, respectively).
Progression of μ-calpain autolysis in muscle homogenates
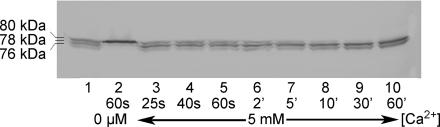
Finally, noting that when exogenous μ-calpain in free solution was exposed to 20 μm Ca2+ the 78 kDa product rapidly autolysed further to the 76 kDa product (Fig. 7A), whereas when muscle homogenates or single fibres were exposed to 20 μm Ca2+ there was accumulation of μ-calpain in the intermediate 78 kDa product (e.g. Fig. 4), we examined what happened when muscle homogenates were exposed to even higher [Ca2+]. As seen in Fig. 10, in the presence of 5 mm Ca2+ a large fraction of the full-length μ-calpain rapidly autolyses to the 78 kDa product within 25 s, but its further autolysis to the 76 kDa product is very slow, with the result that much of the μ-calpain is found in the 78 kDa form even after 30–60 min. The finding that a small amount of 76 kDa product was evidently formed quite rapidly (within 25 s in 5 mm Ca2+; lane 3 in Fig. 10) may have been due to some unbound full-length μ-calpain quickly autolysing all the way to the 76 kDa form without becoming membrane-associated; association of μ-calpain with membranes probably slows its transition from the 78 kDa to the 76 kDa form (see Introduction).
Figure 10. Time course of μ-calpain autolysis in extensor digitorum longus (EDL) muscle homogenate at 5 mM Ca2+.
Western blot of μ-calpain isoforms present at indicated time in an EDL muscle homogenate made with 5 mm free Ca2+ (lanes 3–10) or with 5 mm EGTA (< 1 nm free Ca2+; lane 2). Lane 1 contains a mixture of homogenates exposed to 5 mm Ca2+ or to EGTA, in order to show all three bands together. Approximately half of the full-length (80 kDa) μ-calpain autolyses to the 78 kDa product within 25 s, but further autolysis to the 76 kDa product occurs with a much slower time course.
Discussion
Amount, distribution and properties of μ-calpain in a muscle fibre
In this study we have characterized the amount, compartmentalization and Ca2+-dependent autolytic and proteolytic properties of μ-calpain in skeletal muscle fibres under the physiological conditions prevailing in situ. The results show how the properties of μ-calpain are very well attuned to the [Ca2+] changes occurring in skeletal muscle, explaining why μ-calpain does not readily cause generalized proteolytic damage with normal activity, and why it could do so in certain circumstances, such as following eccentric exercise and in some dystrophic conditions.
The total amount of μ-calpain present in a rat fast-twitch fibre was found to be ∼0.2 μm (expressed relative to total fibre volume). At the normal resting [Ca2+] in a quiescent fibre (∼20–50 nm), ∼15% of the total was closely associated with the sarcolemma, ∼60% was freely diffusible in the cytoplasm and ∼25% was tightly bound or constrained within the fibre (Fig. 2). The concentration of freely diffusible μ-calpain is also equivalent to ∼0.2 μm when expressed relative to cytoplasmic water volume. In comparison, the concentrations of phosphocreatine kinase and pyruvate kinase in the cytoplasm are ∼100 μm and ∼80 μm, respectively, (Maughan et al. 2005) and the equivalent value for titin is ∼5–8 μm (total titin molecules expressed relative to fibre volume). Thus, μ-calpain is present in a fibre at almost 500-fold lower levels than key enzymes involved in maintaining cellular energy levels, and also at a 30-fold lower level than titin, one of its important substrates.
Almost all of the μ-calpain in a quiescent fibre exists in the unautolysed (80 kDa) form (Fig. 2), and exposure under physiological conditions to tightly controlled [Ca2+] produced no detectable autolysis even over 1 h if the [Ca2+] was maintained at ≤ 200 nm. There was also little if any autolysis of μ-calpain even if the [Ca2+] was kept (i) for 30 min at ∼1 μm (e.g. Fig. 3A), a level sufficient to elicit submaximal force, or (ii) for 1–2 min at 2 μm, a level producing near maximal force in the rat EDL fibres here in these ionic conditions (Lamb & Posterino, 2003). However, if the [Ca2+] was held at 5 μm for a number of minutes, a fraction of the μ-calpain was autolysed in both single EDL fibres (Fig. 3A) and muscle homogenates (Fig. 9A), with the extent and rate of autolysis increasing at higher [Ca2+], consistent with our previous observations in EDL muscle homogenates (Murphy et al. 2006). In addition, the mobility of the diffusible pool of μ-calpain in the skinned fibres was greatly reduced when the [Ca2+] was raised (Fig. 3D). This may have been due to Ca2+-dependent binding of the unautolysed μ-calpain to membranes (e.g. those of the sarcoplasmic reticulum (SR) or transverse T-system, not the sarcolemma, which had been removed), with its subsequent autolysis and stabilization there in the 78 kDa form; this would be analogous to membrane-dependent events observed with μ-calpain in erythrocytes (Melloni et al. 1996; Michetti et al. 1996; see Introduction). Such a scenario could explain why in the presence of Ca2+ the μ-calpain was found to accumulate in the 78 kDa form in skinned fibres and muscle homogenates (Figs 3 and 10), whereas isolated μ-calpain in solution autolysed rapidly to the 76 kDa form (Fig. 7). It is also interesting to note that Raynaud et al. (2003) observed that exogenous μ-calpain bound to (but did not proteolyse) skeletal muscle α-actinin in a Ca2+-dependent manner and their electron micrograph of a permeabilized bovine fibre (their Fig. 6A) showed that the μ-calpain bound in a Ca2+-dependent manner not only near the Z-disk (possibly to α-actinin, or to titin itself; Raynaud et al. 2005) but also seemingly clustered at the triad junctions, a membrane-dense region where the SR abuts the T-system. Gilchrist et al. (1992) have also reported calpain binding to the SR and triad junctions.
Passive force assay of proteolytic activity and the patency of titin
We have used changes in passive force production in stretched muscle fibres as an assay of diffusible proteolytic activity. This assay is a relevant measure because it indicates how much diffuse cellular damage might be expected from the proteolytic activity being assayed, as it depends on the ability of the proteolytic factor(s) to reach fixed sites (irrespective of whether or not the factor then becomes largely fixed). If instead one were to assay proteolytic activity using a diffusible substrate, it would not distinguish between diffusible and fixed proteases, and yet it is the diffusible proteases that could be expected to have the wider ranging effects. This is not to say that bound proteases are not of very considerable functional importance in particular localized regions (also see below). Using exogenous μ-calpain, it was demonstrated that the decline in passive force production accurately indicated the relative level of proteolytic activity present, in that (i) it showed an approximately linear relationship with the amount of μ-calpain added, (ii) the effect was totally Ca2+-dependent and operated over the expected range for activated μ-calpain (Goll et al. 2003) and (iii) it was inhibited by leupeptin. Passive force production in stretched skinned fibres depends predominantly on the extent of titin elongation (Horowits et al. 1986) and hence the irreversible reductions in the passive force assay here should reflect disruptions in the patency of titin. It was also shown here that titin was cleaved following exogenous μ-calpain treatment under the same circumstances as those where passive force was disrupted (Figs 5 and 6), and the large titin fragment remaining was the size expected with cleavage by calpain (Goll et al. 2003). Certainly too, it is known that titin is very readily proteolysed by calpains (Goll et al. 2003). It is nevertheless quite possible that calpain first proteolyses some other structural protein in the fibre, such as in the Z-disk, and that this then disrupts passive force production by titin and also allows titin to be cleaved. However, if this is the case, it would still mean the passive force measure used here was a useful assay of the relevant proteolytic activity of calpain.
The size of the degraded titin relative to the full-length titin observed here is consistent with cleavage in the PEVK region of titin (Kimura et al. 1992; Kramerova et al. 2004). This cleavage site is located in the I-band, slightly closer to the M-line than the N2A line. The triad junction is also located in this general region in the vicinity of the N2A line. Calpain-3 is normally bound to titin at the N2A line, but significantly it does not proteolyse titin at this site (Kramerova et al. 2004). The situation is likely to be similar for μ-calpain, in that it could be expected to be able to reach the PEVK site and proteolyse titin when in a readily diffusible form, but probably cannot do so when bound on membranes such as at the triad junction or on other sites on titin itself.
The assay of proteolysis used here is also of obvious direct relevance to muscle function, because the substrates are normal muscle proteins rather than an exogenous unmuscle substrate which perhaps might have atypical susceptibility to proteolysis. Furthermore, the ability of the factor to disrupt the patency of titin in particular (either directly or secondarily) is a relevant aspect of the assay, because dismantling of the normal sarcomeric structure is a precursor to further proteolytic degradation (Kramerova et al. 2004; Bartoli & Richard, 2005; Costelli et al. 2005). The use of highly stretched fibres in the passive force assay should not be mistaken as meaning that whole muscles would ever undergo such stretches in vivo. The in vitro use of such fibres in the assay is valid nonetheless, and it also yielded the additional interesting finding that titin in rat muscle fibres is considerably more susceptible to proteolysis by calpain when the sarcomere is stretched compared to when it is not stretched (Figs 5 and 6). This is similar to what is seen in toad fibres (Verburg et al. 2005). The likely physiological importance of this is that it would mean that ‘popped’ sarcomeres (Morgan & Proske, 2004) and other damaged sarcomeres where the myosin filaments are grossly misaligned, and hence where titin must be overextended at least on one side, would be more susceptible to calpain proteolysis, possibly aiding the dismantling or remodelling of such sarcomeres.
Proteolytic activity in muscle homogenates and fibres
It was found that muscle homogenates made with the free [Ca2+] set at 2 μm displayed a significant though relatively small degree of diffusible proteolytic activity, and that the extent of the proteolytic activity increased in a graded manner with [Ca2+] in the homogenate at least up to 20 μm Ca2+ (Fig. 9B). If the [Ca2+] in the homogenate was kept at < 50 nm, the homogenate showed no detectable proteolytic activity. It is important to note that if the homogenate was made at 20 μm Ca2+ for 5 min and then the [Ca2+] decreased to 2 μm, the activity was little if at all reduced, and the activity still remained at ∼30% of that at 20 μm Ca2+ even when the free [Ca2+] was decreased to 0.3 μm (Fig. 9C). Thus, if the [Ca2+] was raised to levels where there was significant autolysis of μ-calpain (Fig. 9B and also see Murphy et al. 2006), the homogenate showed heightened proteolytic activity at low [Ca2+] (0.3–2 μm). This is fully consistent with the known increase in Ca2+ sensitivity for proteolytic activity of μ-calpain over this same [Ca2+] when the μ-calpain is autolysed by exposure to high [Ca2+] (see Introduction and Goll et al. 2003). Furthermore, it was found here that muscle fibres initially contain a large amount of diffusible Ca2+-activated proteolytic factor that is almost completely lost by washout of the cytoplasm for 15–120 s (Fig. 8), which is consistent with the rapid washout of the diffusible cytoplasmic pool of μ-calpain initially present in a quiescent fibre (Fig. 2). In contrast, calpain-3 remained tightly bound in the skinned fibres at 20 nm Ca2+ and showed no detectable washout over 30–60 min (Fig. 2), strongly suggesting that it could not be responsible for the observed proteolytic activity. In confirmation of this, we also found no difference in proteolytic activity in muscle from calpain-3-knockout mice compared to wild-type mice (authors' unpublished observations). It is quite possible, however, that m-calpain, the other ubiquitous calpain present in skeletal muscle, contributes to the observed activity. We presume that its contribution is substantially smaller than that of μ-calpain because in vitro studies indicate that it is proteolytically active only at > 100 μm Ca2+ even after autolysis (Goll et al. 2003), but there may be factors present in muscle fibres that increase its sensitivity further. It nevertheless seems likely that a substantial proportion of the Ca2+-dependent proteolytic activity present in the muscle homogenates and in the normal fibre cytoplasm is attributable to μ-calpain activity.
This is also consistent with the level of proteolytic activity expected for the amount of μ-calpain present endogenously in the fibres; 0.5 μm of exogenous μ-calpain pre-activated in 20 μm Ca2+ resulted in the passive force declining at ∼220% min−1, or ∼440% min−1 per μmμ-calpain. In the experiments where the cytoplasmic [Ca2+] was elevated (though to an unknown level) in a single fibre with most of the endogenous μ-calpain (∼0.2 μm) still present, passive force declined at ∼23% min−1 (see Fig. 8), or ∼120% min−1 per μmμ-calpain. When endogenous μ-calpain was applied in a fresh muscle homogenate activated in 20 μm Ca2+, passive force declined ∼10% min−1 and ∼5% min−1 when the endogenous calpain (∼0.2 μm) was diluted in the homogenate by a factor of 5-fold and 9-fold, respectively (Fig. 9B and C), equivalent to a passive force decline of ∼220–250% min−1 per μmμ-calpain. Thus, the proteolytic activity found in a muscle fibre at 20 μm Ca2+ can be accounted for by the activity of the μ-calpain present, because this activity was totally Ca2+-dependent (Fig. 9B) and was ∼50% of that measured when applying a similar concentration of pre-activated exogenous μ-calpain.
Calpain activity in muscle fibres in various circumstances
The findings here help explain how the presence of calpains is compatible with normal muscle function. Even though the cytoplasmic [Ca2+] reaches 1–2 μm or perhaps even higher during a tetanus, it is typically only at such levels for the order of a second or less (Allen & Westerblad, 2001) and not maintained at a sufficiently high level for significant autolysis of μ-calpain to occur, which requires a [Ca2+] > 1 μm for several minutes, and even a large number of successive brief rises in [Ca2+] may be relatively ineffective. This conclusion is strongly supported by our finding that there is no significant autolysis of μ-calpain after sprint exercise in untrained humans or with sustained exercise to exhaustion at a lower power level in trained subjects (Murphy et al. 2006). It is important to note that proteolytic activity of unautolysed μ-calpain is low and short-lived, ceasing if [Ca2+] is < 1 μm. Even if some μ-calpain were autolysed, its proteolytic activity still stops if the [Ca2+] drops back to normal resting levels (20–50 nm) (Fig. 9C). The very high Ca2+ sensitivity of proteolytic activity in rested muscle reported by Belcastro (1993) (see Introduction) may reflect unintended autolysis during calpain isolation or may be due to the non-physiological conditions used in the proteolysis assay, and may not be truly indicative of the situation in functioning muscle. Another key point about μ-calpain activity is that when the [Ca2+] reaches high levels, much or all of the freely diffusible pool of μ-calpain rapidly binds to sites within the fibre, and it is no longer free to readily move, which would greatly reduce its ability to cause diffuse proteolytic damage. Even if the μ-calpain is autolysed, it appears to be stabilized to some extent in the 78 kDa form and does not readily autolyse further to the putatively more diffusible 76 kDa form (Fig. 10). Finally, though the calpastatin endogenously present in muscle fibres evidently did not prevent all proteolytic activity when the cytoplasmic [Ca2+] was raised, given the very high affinity of calpastatin for autolysed forms of the ubiquitous calpains, it could well be that any generalized proteolytic activity of autolysed μ-calpain is eventually controlled or reduced by calpastatin. This is one explanation of why the muscle homogenates used here showed greatly reduced proteolytic activity at times longer than 30 min after homogenization even though Western blotting showed that the μ-calpain was not autolysed beyond the 76 kDa form (data not shown). In summary, the Ca2+ dependence of μ-calpain autolysis, binding and proteolytic activity are attuned quite perfectly such that the range of [Ca2+] occurring in a muscle at rest and during normal activity would not readily lead to uncontrolled μ-calpain activity.
However, there are certain situations in muscle where the operating range of intracellular [Ca2+] could lead to excessive μ-calpain activity. Firstly, subjecting normal muscles to eccentric (or lengthening) contractions, leads to appreciable entry of extracellular Ca2+ (Allen et al. 2005) and a long-term rise in the resting intracellular [Ca2+] (Lynch et al. 1997). Secondly, when dystophin is missing from the sarcolemma, as in human DMD and mouse mdx muscle, there is also increased influx of extracellular Ca2+ which is particularly marked with eccentric contractions (Allen et al. 2005), and the resting free [Ca2+] in the subsarcolemmal region of mdx muscle is estimated to be raised 3-fold over levels in normal muscle (115 nm and 40 nm, respectively; Mallouk et al. 2000). Thus, in these cases with increased Ca2+ influx augmenting the Ca2+ released during normal contraction, there is quite likely to be increased autolysis of μ-calpain, and furthermore the rise in resting [Ca2+] would mean that any autolysed μ-calpain present would be proteolytically active to some extent for a much larger proportion of the time than occurs in muscle normally. Thus, the properties of μ-calpain described in the present study help in understanding the mechanistic basis of the deleterious role of calpains which may occur with eccentric contraction and in certain dystrophies (Kar & Pearson, 1976; Spencer & Mellgren, 2002; Allen et al. 2005).
Finally, we emphasize that the constraints on widespread μ-calpain activity in normal muscle by no means precludes μ-calpain or the other calpains from having vital proteolytic roles in specialized local regions. Indeed, we suggest that calpains probably mediate the disruption of the normal coupling of the T-system excitation to SR Ca2+ release that evidently occurs at the triad junction if intracellular [Ca2+] is maintained at levels of 2 μm for several minutes (Lamb et al. 1995; Verburg et al. 2005) and occurs very rapidly if the local [Ca2+] near the triad junction reaches very high levels (Verburg et al. 2006). This phenomenon may play an important feedback role by terminating Ca2+ release from the SR if it becomes excessive, and seems likely to be the cause of long-duration muscle weakness after excessive exercise and eccentric contractions (Lamb et al. 1995; Westerblad et al. 2000; Allen et al. 2005; Verburg et al. 2006). The calpains involved might be normally fixed close to the triad junction or might be part of a diffusible pool that becomes bound near there owing to the high local [Ca2+] occurring with normal Ca2+ release. In either case, they could become proteolytically active on adjacent triadic substrates if or whenever the local [Ca2+] rises to an appropriate level.
In conclusion, the present findings demonstrate how the properties of μ-calpain are well attuned to avoid uncontrolled proteolytic activity in muscle under normal circumstances, and why they could lead to substantial proteolytic damage after eccentric contraction and in dystrophic muscle.
Acknowledgments
We thank Maria Cellini and Aida Yousef for assistance and Professor D. George Stephenson for helpful discussions. This project was supported by Australian National Health and Medical Research Council grant 280623. R.M. is supported by Australian National Health and Medical Research Council grant 380842.
References
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Whitehead NP, Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol. 2005;567:723–735. doi: 10.1113/jphysiol.2005.091694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baki A, Tompa P, Alexa A, Molnar O, Friedrich P. Autolysis parallels activation of mu-calpain. Biochem J. 1996;318:897–901. doi: 10.1042/bj3180897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli M, Richard I. Calpains in muscle wasting. Int J Biochem Cell Biol. 2005;37:2115–2133. doi: 10.1016/j.biocel.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcastro AN. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. J Appl Physiol. 1993;74:1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- Branca D, Gugliucci A, Bano D, Brini M, Carafoli E. Expression, partial purification and functional properties of the muscle-specific calpain isoform p94. Eur J Biochem. 1999;265:839–846. doi: 10.1046/j.1432-1327.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM. Ca2+-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol. 2005;37:2134–2146. doi: 10.1016/j.biocel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Dayton WR, Schollmeyer JV. Immunocytochemical localization of a calcium-activated protease in skeletal muscle cells. Exp Cell Res. 1981;136:423–433. doi: 10.1016/0014-4827(81)90022-7. [DOI] [PubMed] [Google Scholar]
- De Tullio R, Passalacqua M, Averna M, Salamino F, Melloni E, Pontremoli S. Changes in intracellular localization of calpastatin during calpain activation. Biochem J. 1999;343:467–472. [PMC free article] [PubMed] [Google Scholar]
- Gilchrist JS, Wang KK, Katz S, Belcastro AN. Calcium-activated neutral protease effects upon skeletal muscle sarcoplasmic reticulum protein structure and calcium release. J Biol Chem. 1992;267:20857–20865. [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Horowits R, Kempner ES, Bisher ME, Podolsky RJ. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986;323:160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- Kapprell HP, Goll DE. Effect of Ca2+ on binding of the calpains to calpastatin. J Biol Chem. 1989;264:17888–17896. [PubMed] [Google Scholar]
- Kar NC, Pearson CM. A calcium-activated neutral protease in normal and dystrophic human muscle. Clin Chim Acta. 1976;73:293–297. doi: 10.1016/0009-8981(76)90175-3. [DOI] [PubMed] [Google Scholar]
- Keira Y, Noguchi S, Minami N, Hayashi YK, Nishino I. Localization of calpain 3 in human skeletal muscle and its alteration in limb-girdle muscular dystrophy 2A muscle. J Biochem (Tokyo) 2003;133:659–664. doi: 10.1093/jb/mvg084. [DOI] [PubMed] [Google Scholar]
- Kimura S, Matsuura T, Ohtsuka S, Nakauchi Y, Matsuno A, Maruyama K. Characterization and localization of alpha-connectin (titin 1): an elastic protein isolated from rabbit skeletal muscle. J Muscle Res Cell Motil. 1992;13:39–47. doi: 10.1007/BF01738426. [DOI] [PubMed] [Google Scholar]
- Kramerova I, Kudryashova E, Tidball JG, Spencer MJ. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum Mol Genet. 2004;13:1373–1388. doi: 10.1093/hmg/ddh153. [DOI] [PubMed] [Google Scholar]
- Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE. Localization of the Ca2+-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec. 1992;232:60–77. doi: 10.1002/ar.1092320108. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Osmotic properties of the sealed tubular system of toad and rat skeletal muscle. J Gen Physiol. 2004;123:231–247. doi: 10.1085/jgp.200308946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium. 1997;22:373–383. doi: 10.1016/s0143-4160(97)90022-1. [DOI] [PubMed] [Google Scholar]
- Mallouk N, Jacquemond V, Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc Natl Acad Sci U S A. 2000;97:4950–4955. doi: 10.1073/pnas.97.9.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan DW, Henkin JA, Vigoreaux JO. Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol Cell Proteomics. 2005;4:1541–1549. doi: 10.1074/mcp.M500053-MCP200. [DOI] [PubMed] [Google Scholar]
- Melloni E, Michetti M, Salamino F, Minafra R, Pontremoli S. Modulation of the calpain autoproteolysis by calpastatin and phospholipids. Biochem Biophys Res Commun. 1996;229:193–197. doi: 10.1006/bbrc.1996.1779. [DOI] [PubMed] [Google Scholar]
- Melloni E, Michetti M, Salamino F, Pontremoli S. Molecular and functional properties of a calpain activator protein specific for mu-isoforms. J Biol Chem. 1998;273:12827–12831. doi: 10.1074/jbc.273.21.12827. [DOI] [PubMed] [Google Scholar]
- Michetti M, Salamino F, Tedesco I, Averna M, Minafra R, Melloni E, Pontremoli S. Autolysis of human erythrocyte calpain produces two active enzyme forms with different cell localization. FEBS Lett. 1996;392:11–15. doi: 10.1016/0014-5793(96)00775-2. [DOI] [PubMed] [Google Scholar]
- Molinari M, Anagli J, Carafoli E. Ca2+-activated neutral protease is active in the erythrocyte membrane in its nonautolyzed 80 kDa form. J Biol Chem. 1994;269:27992–27995. [PubMed] [Google Scholar]
- Morgan DL, Proske U. Popping sarcomere hypothesis explains stretch-induced muscle damage. Clin Exp Pharmacol Physiol. 2004;31:541–545. doi: 10.1111/j.1440-1681.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Snow RJ, Lamb GD. mu-Calpain and calpain-3 are not autolyzed with exhaustive exercise in humans. Am J Physiol Cell Physiol. 2006;290:C116–C122. doi: 10.1152/ajpcell.00291.2005. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Stephenson DG, Stephenson GM. Microfluorometric analyses of glycogen in freshly dissected, single skeletal muscle fibres of the cane toad using a mechanically skinned fibre preparation. J Muscle Res Cell Motil. 1998;19:631–638. doi: 10.1023/a:1005377030193. [DOI] [PubMed] [Google Scholar]
- Ojima K, Ono Y, Hata S, Koyama S, Doi N, Sorimachi H. Possible functions of p94 in connectin-mediated signaling pathways in skeletal muscle cells. J Muscle Res Cell Motil. 2005;26:409–41. doi: 10.1007/s10974-005-9023-8. [DOI] [PubMed] [Google Scholar]
- Pontremoli S, Viotti PL, Michetti M, Sparatore B, Salamino F, Melloni E. Identification of an endogenous activator of calpain in rat skeletal muscle. Biochem Biophys Res Commun. 1990;171:569–574. doi: 10.1016/0006-291x(90)91184-t. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud F, Bonnal C, Fernandez E, Bremaud L, Cerutti M, Lebart MC, Roustan C, Ouali A, Benyamin Y. The calpain 1-alpha-actinin interaction. Resting complex between the calcium-dependent protease and its target in cytoskeleton. Eur J Biochem. 2003;270:4662–4670. doi: 10.1046/j.1432-1033.2003.03859.x. [DOI] [PubMed] [Google Scholar]
- Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, Gautel M, Benyamin Y, Ouali A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J. 2005;272:2578–2590. doi: 10.1111/j.1742-4658.2005.04683.x. [DOI] [PubMed] [Google Scholar]
- Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K, Suzuki K. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem. 1995;270:31158–31162. doi: 10.1074/jbc.270.52.31158. [DOI] [PubMed] [Google Scholar]
- Sorimachi H, Toyama-Sorimachi N, Saido TC, Kawasaki H, Sugita H, Miyasaka M, Arahata K, Ishiura S, Suzuki K. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J Biol Chem. 1993;268:10593–10605. [PubMed] [Google Scholar]
- Spencer MJ, Guyon JR, Sorimachi H, Potts A, Richard I, Herasse M, Chamberlain J, Dalkilic I, Kunkel LM, Beckmann JS. Stable expression of calpain 3 from a muscle transgene in vivo: immature muscle in transgenic mice suggests a role for calpain 3 in muscle maturation. Proc Natl Acad Sci U S A. 2002;99:8874–8879. doi: 10.1073/pnas.132269299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet. 2002;11:2645–2655. doi: 10.1093/hmg/11.21.2645. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Tidball JG. Calpain translocation during muscle fiber necrosis and regeneration in dystrophin-deficient mice. Exp Cell Res. 1996;226:264–272. doi: 10.1006/excr.1996.0227. [DOI] [PubMed] [Google Scholar]
- Verburg E, Dutka TL, Lamb GD. Long-lasting muscle fatigue: partial disruption of excitation-contraction coupling by elevated cytosolic Ca2+ concentration during contractions. Am J Physiol Cell Physiol. 2006;290:C1199–C1208. doi: 10.1152/ajpcell.00469.2005. [DOI] [PubMed] [Google Scholar]
- Verburg E, Murphy RM, Stephenson DG, Lamb GD. Disruption of excitation-contraction coupling and titin by endogenous Ca2+-activated proteases in toad muscle fibres. J Physiol. 2005;564:775–789. doi: 10.1113/jphysiol.2004.082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Allen DG, Lannergren J. Functional significance of Ca2+ in long-lasting fatigue of skeletal muscle. Eur J Appl Physiol. 2000;83:166–174. doi: 10.1007/s004210000275. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Murachi T, Heath R, Kay J, Jasani B, Newman GR. Immunogold electron-microscopic localisation of calpain I in skeletal muscle of rats. Cell Tissue Res. 1986;244:265–270. doi: 10.1007/BF00219201. [DOI] [PubMed] [Google Scholar]