Abstract
The climbing fibre projection from the motor cortex to the cerebellar cortical C1 zone in the posterior lobe of the rat cerebellum was investigated using a combination of physiological, anatomical and neuropharmacological techniques. Electrical stimulation of the ipsilateral fore- or hindimbs or somatotopically corresponding parts of the contralateral motor cortex evoked climbing fibre field potentials at the same cerebellar recording sites. Forelimb-related responses were located in the C1 zone in the paramedian lobule or lobulus simplex and hindlimb-related responses were located in the C1 zone in the copula pyramidis. Microinjections of anterograde axonal tracer (Fluoro-Ruby or Fluoro-Emerald) were made into the fore- or hindlimb parts of the motor cortex where stimulation evoked the largest cerebellar responses. After a survival period of 7–10 days, the neuraxis was examined for anterograde labelling. No terminal labelling was ever found in the inferior olive, but labelled terminals were consistently found in a well-localized site in the dorso-medial medulla, ventral to the gracile nucleus, termed the matrix region. Pharmacological inactivation of the matrix region (2 mm caudal to the obex) selectively reduced transmission in descending (cerebro-olivocerebellar) but not ascending (spino-olivocerebellar) paths targeting fore- or hindlimb-receiving parts of the C1 zone. Transmission in spino-olivocerebellar paths was either unaffected, or in some cases increased. The identification of a novel pre-olivary relay in cerebro-olivocerebellar paths originating from fore- and hindlimb motor cortex has implications for the regulation of transmission in climbing fibre pathways during voluntary movements and motor learning.
Both anatomical and electrophysiological investigations have shown that climbing fibres impose a precise order on the mammalian cerebellar cortex, dividing it into a series of longitudinally orientated cortical zones. Each zone is targeted by climbing fibres arising from a specific part of the inferior olive that receives signals relayed by a distinct combination of ascending spino-olivocerebellar paths (SOCPs, for a review see Oscarsson, 1980). This arrangement is similar in a range of species, implying conservation of function and underscoring the importance of olivocerebellar pathways in motor control (e.g. cat, Ekerot & Larson, 1979a, b; rat, Atkins & Apps, 1997; ferret, Garwicz, 1997).
A large number of powerful, descending cerebro-olivocerebellar pathways (COCPs) are also known to exist (Armstrong & Harvey, 1966; Provini et al. 1968; Miller et al. 1969; Sasaki et al. 1975; Rowe, 1977a, b; Oka et al. 1976, 1979; Kyuhou, 1992; Baker et al. 2001). These pathways terminate as climbing fibres in the same cortical zones as the SOCPs (Andersson & Eriksson, 1981; Andersson & Nyquist, 1983; Andersson, 1984; Baker et al. 2001; Pardoe et al. 2004). However, by comparison to SOCPs, much less is known about the anatomical connections that mediate these descending signals. In particular, there is disagreement over the pattern and extent of any direct anatomical link between the motor cortex and the inferior olive (Walberg, 1956; Sousa-Pinto, 1969; Sousa-Pinto & Brodal, 1969; Brown et al. 1977; Berkley & Worden, 1978; Saint-Cyr, 1983; Swenson & Castro, 1983; Swenson et al. 1989).
Details of indirect pathways leading from the motor cortex to the inferior olive also remain far from complete, and tract tracing studies have yet to be carried out in relation to COCP projections to individual cerebellar cortical zones. This is a significant gap in our knowledge, because the identity of brain structures that relay signals from higher centres to the olive is an essential prerequisite to a full understanding of the function(s) of such paths, which are generally accepted to be crucial for cerebellar contributions to motor control. Descending climbing fibre pathways are also known to be subject to powerful modulatory influences during active movements (for a review see Apps, 1999). However, the site or sites where this modulation occurs remains unknown, although Pardoe et al. (2004) provided evidence during locomotion in the intact cat that some of the gating is likely to take place prior to the convergence of ascending and descending climbing fibre paths (i.e. prior to the olive). Structures that serve as pre-olivary relay stations in COCPs may therefore play an important role in controlling the signalling capabilities of such paths.
In the present experiments we have used electrophysiological mapping techniques in combination with sensitive anterograde pathway tracing techniques and neuropharmacological methods to chart, in rats, climbing fibre pathways that relay information from the motor cortex to the cerebellar cortical C1 zone. This particular zone was selected for study because the anatomy and physiology of its spino-olivocerebellar inputs and Purkinje cell cortico-nuclear outputs are well characterized (see for example Pardoe & Apps, 2002). Through its connections with the rubrospinal tract via the nucleus interpositus, the C1 zone is likely to be important in the regulation of voluntary and reflex movements of the ipsilateral limbs (for a recent review see Apps & Garwicz, 2005).
Evidence is presented that COCPs mediating information to the cerebellar cortical C1 zone from both fore- and hindlimb parts of the motor cortex do not target the olive directly, but have at least one newly defined pre-olivary relay located in the transitional region between the spinal cord and the medulla oblongata.
Methods
All experimental procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986, and were approved by the University of Bristol institutional animal licence advisory group. A total of 45 adult rats were used (male Wistar, weight 250–400 g). The methods were the same in all experiments except where stated otherwise, and were divided into three interrelated lines of enquiry: (i) electrophysiological mapping (n = 28 rats); (ii) anatomical tract tracing (n = 9 rats); and (iii) pharmacological inactivation (n = 8 rats). All averaged data are presented as mean ± s.e.m.
General experimental procedures
In brief, all animals were anaesthetized with an intraperitoneal injection of sodium pentobarbitone (Sagatal, 60 mg kg−1 Rhone Mérieux, Harlow, UK) and further doses of barbiturate given as required to maintain surgical levels of anaesthesia. Limb-withdrawal and corneal reflexes were tested at regular intervals to ensure sufficient depth of anaesthesia. Rats were placed in a stereotaxic frame and secured with atraumatic ear bars coated with a topical local anaesthetic (Xylocaine, Astra Pharmaceuticals, Kings Langley, UK). Temperature was maintained within physiological limits using a heated blanket controlled by a probe measuring rectal temperature.
A craniotomy exposed the dorsal surface of the posterior lobe of the cerebellum in the region of the paramedian lobule (PML), copula pyramidis (COP) and/or lobulus simplex (LS, nomenclature according to Larsell, 1952) on one or both sides of the brain. A second craniotomy exposed the motor cortex (unilaterally or bilaterally). The dura was carefully removed and the exposed brain surfaces periodically flushed with saline. At the end of each experiment the animals were killed with an intraperitoneal overdose of barbiturate.
Stimulation and recording arrangements
Pairs of percutaneous stimulating needles were inserted into the ipsilateral fore- and hindlimbs, and one or two brief (0.1 ms, 1 kHz) square-wave electrical pulses were given. Bipolar electrodes (World Precision Instruments, FL, USA) were used to stimulate the motor cortex (typically at a depth of 1.6–1.8 mm below the cerebral surface). To identify the fore- and hindlimb regions of the primary motor cortex, trains of 30 square-wave pulses (0.1 ms, 1 kHz) were used to evoke contralateral limb movements. The stimulation typically elicited a small twitch of the forearm or lower half of the hindlimb. Single or double, square-wave electrical pulses (0.1 ms, 1 kHz) were then used to evoke responses in the cerebellum. The final position of the motor cortical stimulating electrode was adjusted to evoke responses in the cerebellar cortical C1 zone with the lowest stimulus intensity (threshold typically 1 mA). The stimulus intensity was expressed as multiples of threshold (T) to evoke a just-detectable cerebellar cortical field potential, and the intensity used for limb or motor cortical stimulation was 2T, unless stated otherwise. Since climbing fibre fields are readily evoked by electrical stimulation of low-threshold peripheral afferents, an intensity of 2T is likely to generate a field (at least for limb stimulation) that is due mainly to activity in A calibre fibres (see for example Ekerot et al. 1987; and Discussion for further comment). The stimulation was delivered once every 1.5 s except in the pharmacological experiments, where the duty cycle was 3 s, with the ipsilateral limb stimulation at time zero and the contralateral motor cortical stimulation 1.5 s later. In one case the duty cycle was 4.5 s.
Extracellular field potentials were recorded from the surface of the cerebellum with a tungsten-in-glass microelectrode (tip diameter ∼50 μm). The cerebellar recording electrode was positioned at approximately the centre of the C1 zone in the PML or COP, i.e. at a site where the largest cerebellar responses could be evoked by limb (and motor cortical) stimulation (cf. Atkins & Apps, 1997; Pardoe & Apps, 2002). In some experiments a second recording electrode was also placed at approximately the centre of the C1 zone within the LS. Responses were recorded differentially, amplified and band-pass filtered (30 Hz–5 kHz), while any 50–60 Hz electrical interference was removed (without signal degradation) by a Humbug device (Quest Scientific, Vancouver, Canada). The responses evoked at one or two cerebellar recording sites were digitized online (sampling rate 10 kHz) using a CED (Cambridge Electronic Design, Cambridge, UK) 1401 A/D converter, and customized trigger-sampled software (Spike 2, CED, Cambridge, UK). In all experiments, the onset latency of evoked responses was measured from the first stimulus pulse.
In the pharmacological inactivation experiments cerebellar responses were recorded for at least 2 min, to obtain control responses prior to microinjection of the drug, and for about 70 min thereafter. Individual responses were measured offline by manual positioning of computer cursors at the onset and offset of each response, and using customized Spike scripts to calculate total response area (mV.ms).
Anatomical tract tracing
In experiments to investigate anatomical connections, a small injection (total volume 100–400 nl) of one of two different types of anterograde fluorescent tracer material (Fluoro-Ruby or Fluoro-Emerald, in both cases 20% solution, Molecular Probes, Eugene, OR, USA) was made into the contralateral motor cortex. In six animals, only one type of tracer was used but in three animals a double anterograde tracing strategy was employed, with one tracer injected into the forelimb motor cortex and the other tracer injected into the hindlimb motor cortex, yielding a total of 12 cases (see Table 1 for further details). In each case the injection was made where stimulation evoked the largest cerebellar responses, corresponding to approximately the centre of the fore- or hindlimb parts of the motor cortex.
Table 1.
Summary of injection details
| Case | Tracer | Volume (nl) | r–c (μm) | m–l (μm) | d–v (μm) | Depth (mm) | Injection site (mm3) |
|---|---|---|---|---|---|---|---|
| DP1 HL | FR | 100 | 300 | 190 | 265 | 1.6 | 0.063 |
| DP11 HL | FR | 3 × 100 | 300 | 270 | 115 | 1.2 | 0.039 |
| DP13 FL | FR | 300 + 100 | — | — | — | — | — |
| DP14 HL | FR | 350 | 300 | 475 | 165 | 1.7 | 0.098 |
| DP23 FL | FR | 3 × 100 | 300 | 270 | 175 | 1.7 | 0.059 |
| DP24 HL | FR | 3 × 100 | 300 | 105 | 300 | 1.5 | 0.039 |
| DP25 FL | FR | 3 × 100 | 400 | 135 | 175 | 1.5 | 0.039 |
| DP25 HL | FE | 3 × 100 | 200 | 70 | 300 | 1.6 | 0.018 |
| DP26 FL | FE | 3 × 100 | 100 | 90 | 210 | 1.6 | 0.008 |
| DP26 HL | FR | 3 × 100 | 300 | 50 | 585 | 1.7 | 0.037 |
| DP27 FL | FR | 3 × 100 | 300 | 70 | 475 | 1.8 | 0.042 |
| DP27 HL | FE | 3 × 100 | 200 | 155 | 140 | 1.6 | 0.018 |
From left to right: case number (HL and FL refer to injections into hindlimb and forelimb motor cortex, respectively); tracer (FE, Fluoro-Emerald, FR, Fluoro-Ruby); volume of tracer; rostrocaudal (r–c); mediolateral (m–l); dorsoventral (d–v) extent of the injection site core; depth of the centre of the injection site from the cerebral cortical surface; volume of injection site; – histological data not available.
For each injection, a glass micropipette (∼20 μm tip diameter) attached to a 1 μl Hamilton syringe (Precision Sampling Corp, Baton Rouge, Louisiana, USA) was inserted perpendicular to the cerebral cortical surface to a depth of about 1.6 mm (see Table 1), where from one to three hydraulic microinjections of tracer were made. The micropipette was then immediately removed, the surface washed thoroughly with sterile saline, and the exposed cortex covered with gelfoam (Sterispon, Allen and Hanbury, London, UK). In some experiments, three different sites in close proximity to one another in the same fore- or hindlimb part of the motor cortex were injected to increase tracer uptake.
In every case a small hydraulic injection of retrograde tracer (fluorescent-tagged beads, total volume approximately 50 nl, about 0.4 mm below the cerebellar cortical surface), was also made at the recording site in the C1 zone, where limb and motor cortical stimulation evoked the largest cerebellar responses. The retrograde tracer was used to mark the location within the inferior olive where neurones are the source of climbing fibres to the cortical zone under study. As in previous studies (see for example Apps, 1998) the beads produced distinctive granular labelling confined to cell bodies. This cell labelling could be readily differentiated from any axonal or terminal labelling arising from injections of anterograde tracer in the same experiments (see Fig. 2B). After the cerebral and cerebellar injections had been made, the wound was closed in layers, and upon recovery, a long-acting analgesic was given as a precautionary measure (Temgesic, 0.1 mg kg−1s.c., Schering-Plough, Welwyn Garden City, UK). All animals were monitored daily and recovered uneventfully.
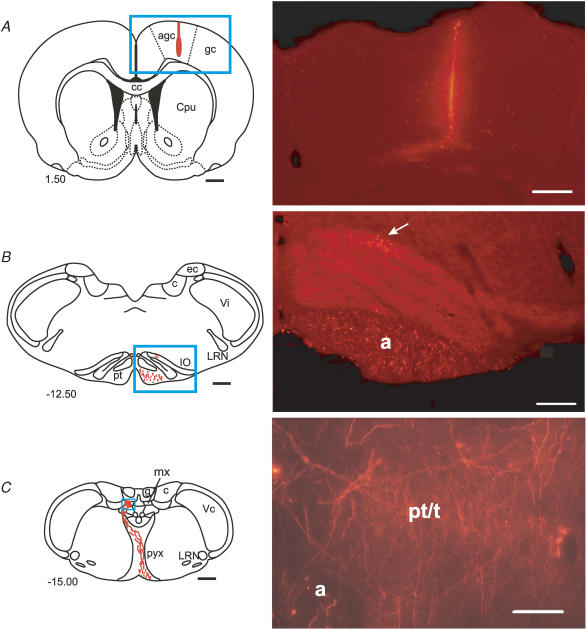
Figure 2. Example injection site and anatomical labelling.
A, standard transverse outline of the cerebral cortex at the level of the forelimb motor cortex. Box indicates the location of the high-power photomicrograph to the right, showing the core of a Fluoro-Ruby injection site in the agranular cortex (case DP26). B, standard transverse outline of the brainstem at the level of the rostral inferior olive. Box indicates the location of the high-power photomicrograph to the right, showing anterogradely labelled axons in the pyramidal tract and retrogradely labelled olive cells in the rostral dorsal accessory olive (arrow), but no evidence of terminal labelling in the olive (case DP11). C, standard transverse outline of the brainstem at the level of the pyramidal decussation. Box indicates the location of the high-power photomicrograph to the right, showing anterogradely labelled axons and a dense plexus of labelled preterminals and terminal fibres in the matrix region (case DP11). Standard maps are adapted from Paxinos & Watson (2005). Values to the left of each map indicate rostrocaudal distance (in mm) from bregma. Scale bars: photomicrographs: A, 500 μm; B, 200 μm, C, 20 μm; 1 mm for all standard maps. a, axons; agc, agranular cortex; c, cuneate nucleus; cc, corpus callosum; Cpu, caudate putamen; ec, external cuneate nucleus; g, gracile nucleus; gc, granular cortex; LRN, lateral reticular nucleus; mx, medial matrix region; IO, inferior olive; pt/t, preterminals and terminals; pyx, pyramidal decussation; Vc, caudal trigeminal nucleus; Vi, interpolar trigeminal nucleus.
Survival time, perfusion and histological processing
For the anatomical tract tracing experiments the animals were kept for a survival period of 7–10 days to allow for sufficient axonal transport of tracer material. The rats were then deeply anaesthetized using sodium pentobarbitone (60 mg kg−1, i.p., Sagatal, Rhône Mérieux, Harlow, UK) and transcardially perfusion-fixed. A heparinized saline flush (approximately 500 ml, 0.9% saline) was immediately followed by fixative (approximately 500–1000 ml 4% paraformaldehyde in phosphate buffered solution, PBS), and finally with sucrose PBS (approximately 500 ml, 10% sucrose). The brain and spinal cord were dissected out, stored in 30% sucrose PBS at 4°C and allowed to sink. The tissue was then cut into three blocks (cerebral, cerebellar and caudal brainstem (including rostral spinal cord)). All blocks were sectioned transversely on a freezing microtome, at 50 or 100 μm, and alternate sections were mounted for analysis. The second series was kept as a spare. Some cerebral blocks were embedded in gelatine. In one case (DP11), the whole brain was embedded in gelatine and sectioned as one block to enable analysis of the entire neuraxis in one continuous series of sections.
In the pharmacological inactivation experiments, the brainstem was carefully removed at the end of the experiment and postfixed in 4% paraformaldehyde. The block of tissue was sectioned transversely on a freezing microtome at 100 μm thickness, and mounted as one complete series. Sections were inspected using light microscopy for histological verification of the site of injection of the drug (see below).
Anatomical mapping
Sections were examined and mapped using a Leica DMRB microscope (Wetzlar, Germany) or a Zeiss Axioskop II Mot microscope (Oberkochen, Germany). For the Leica microscope, green fluorescence was scrutinized using an H3 filter block (dichroic mirror (DM) 510 nm, band-pass (BP) 420–490 nm, long-pass (LP) 520 nm), and red fluorescence was viewed with an N2.1 filter block (DM 580 nm, BP 515–560 nm, LP 580 nm). For the Zeiss microscope, green fluorescence was viewed using filter set no. 09 (DM 510 nm, BP 450–490 nm, LP 515 nm), and red fluorescence was viewed using filter set no. 15 (DM 580 nm, BP 546/12 nm, LP 590 nm). No difference was found in the pattern or extent of labelling plotted using the two different microscopes.
Slides were temporarily coverslipped using PBS to improve visualization of labelling. In every case the whole rostro-caudal extent of the brain was carefully examined for anterogradely labelled fibres and terminal labelling. Standard transverse maps were used to chart the location of any labelling (maps based on Paxinos & Watson, 2005). Axon terminals anterogradely labelled with Fluoro-Ruby or Fluoro-Emerald were indicated on standard transverse outlines (see for example Fig. 3). Axonal labelling was recorded as wavy lines, and preterminal and terminal labelling by filled areas.
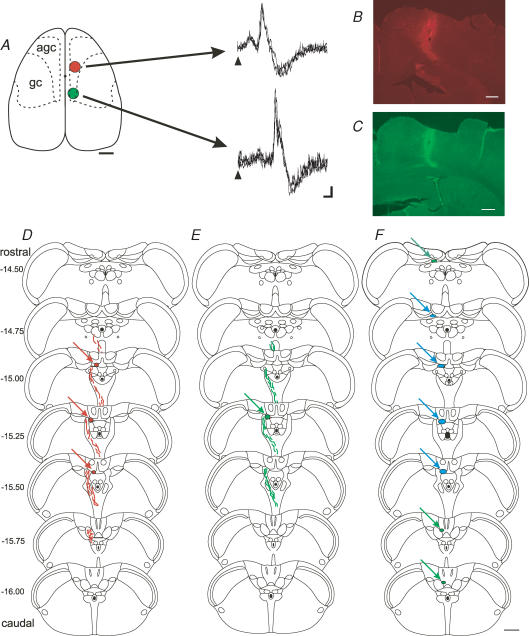
Figure 3. Distribution of anterograde labelling in the caudal brainstem.
A–E, anatomical and physiological documentation of a double tracer experiment (DP25). A, dorsal view of the cerebral cortex indicating the location of two injection sites (sites not to scale). In this experiment a microinjection of Fluoro-Emerald was made into the hindlimb motor cortex (shown in green), and a microinjection of Fluoro-Ruby was also made into the forelimb motor cortex (shown in red). To the right are shown example climbing fibre field responses evoked in the fore- and hindlimb parts of the cerebellar cortical C1 zone as a result of microstimulation where the injections were made in the somatotopically corresponding parts of the contralateral motor cortex. Stimulus was delivered at time of arrowhead (3 sweeps superimposed in each case). Calibration: 5 ms, 0.1 mV. B and C, photomicrographs showing the core of the injection sites in transverse sections of the cerebral cortex. D and E, distribution of anterogradely labelled fibres (wavy lines) and preterminal and terminal fibres (red and green filled areas, see arrows), plotted on standard transverse outlines of the caudal brainstem. F, same as D and E, but only showing terminal fields and for all cases pooled (n = 10). Regions of overlap between fore- and hindlimb cases are shown in blue, hindlimb areas in green, see arrows). Labelling in the gracile nucleus (n = 3) not shown. Values to the left indicate rostrocaudal distance (in mm) from bregma. Scale bars: A, 2 mm, B–F, 0.5 mm.
Injections and injection sites
The injection sites of axonal tracer in the cerebral cortex could be readily identified by the presence of residual tracer and track damage. In each experiment (except experiment DP13 in which histological verification of the injection site was not obtained), the depth to the central core of the injection site from the cerebral cortical surface, the mediolateral and rostro-caudal dimensions of the core, and the total volume of the injection site were estimated by making cursor measurements on digital photomicrographs using image analysis software (Axiovision, Zeiss, Oberkochen, Germany, see Table 1). The cytoarchitecture immediately surrounding each injection site was also compared with the cytoarchitectonic areas described by Donoghue & Wise (1982) to confirm that in each case the injection was confined to the agranular cortex. Note that two forelimb motor cortical areas have been identified in the rat – a large caudal area close to bregma and a small rostral area near the frontal pole (see for example Neafsey et al. 1986). The present experiments are confined to investigation of the caudal area.
In the pharmacological inactivation experiments one of two different drugs was used to reduce neural activity: either the local anaesthetic xylocaine which blocks sodium channels and eliminates generation or conduction of action potentials (10% lidocaine; Astra Pharmaceuticals Ltd, Kings Langley, UK), or a solution of the inhibitory neurotransmitter γ-aminobutyric acid (GABA, 0.1 m in artificial cerebrospinal fluid (ACSF), Tocris, Bristol (UK) mixed with the non-selective glutamate receptor antagonist kynurenic acid (KA, 0.001 m in ACSF; Sigma, St Louis, MO, USA). The GABA/KA combination meant that cell body inhibition was increased, while major excitatory inputs were blocked, producing a comprehensive neuronal cell block. Xylocaine and GABA have been shown to have similar onset latencies and duration of effect (e.g. Lomber, 1999; Malpeli, 1999). In every case the drug was delivered hydraulically via a glass micropipette (tip diameter ∼20 μm) attached to 1 μl Hamilton syringe. A single injection (25–150 nl) was delivered 0.5–0.7 mm below the surface of the brainstem. Prior to injection, small amounts of pontamine sky blue were added to the xylocaine or GABA/KA to colour the solution and thereby aid the accurate positioning of the injection pipette tip at the time of the experiment, and also to mark the injection site for subsequent histological reconstruction.
Results
Electrophysiological responses
Evoked field potentials were recorded from the surface of the cerebellar cortex (see Fig. 1) at a total of 47 different recording sites in 28 adult rats. In accordance with previous studies in rats (Atkins & Apps, 1997; Teune et al. 1998; Jörntell et al. 2000; Baker et al. 2001; Pardoe & Apps, 2002), individual sites were identified as located within the forelimb-receiving parts of the C1 zone in the paramedian lobule (PML, n = 23 rats) or lobulus simplex (LS, n = 8 rats), or within the hindlimb-receiving part of the C1 zone in the copula pyramidis (COP, n = 16 rats), by their location in the medial part of the paravermal cortex and by the presence of relatively short-latency field potentials to ipsilateral fore- or hindlimb stimulation (onset latency mean 12.3 ± 0.2 ms and 16.3 ± 0.3 ms, respectively). Since responses recorded at forelimb sites in the PML or LS produced very similar findings, the data are considered together.
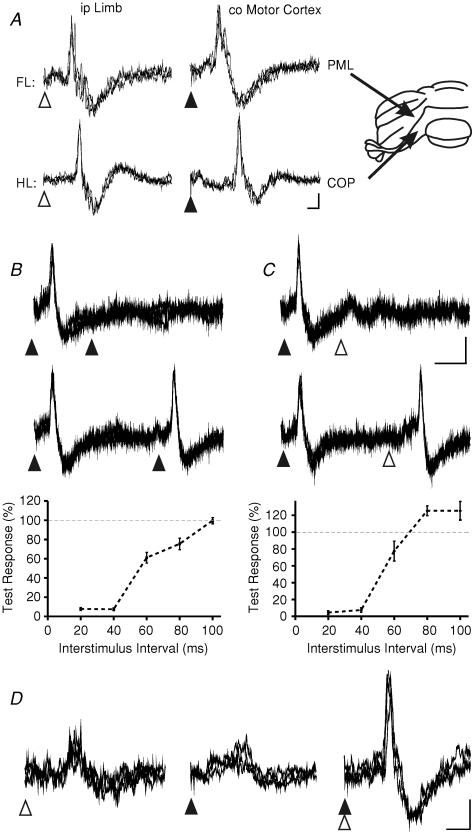
Figure 1. Sample climbing fibre field potentials.
Extracellular recordings from the surface of the cerebellar cortex in the anaesthetized rat evoked by limb or motor cortical stimulation. A, to the right is shown a posterior view of the rat cerebellum with arrows pointing to the centre of the fore- and hindlimb-receiving parts of the C1 zone in the PML and COP. To the left, top and bottom rows show sample responses evoked in the two different parts of the zone. Calibrations: 5 ms, 0.1 mV. B, example climbing fibre field responses to illustrate the effect of the delivery of paired stimuli on the amplitude of the evoked responses (paired pulse test, see Results). Graph shows for the same data the size of the test responses (expressed as a percentage of control) plotted as a function of different interstimulus intervals. Each value is an average of three trials (± s.e.m.). C, same as B but showing the effects of conditioning on a test response. In the case illustrated, the motor cortex is the conditioning stimulus and the forelimb is the test stimulus. B and C, calibrations: 20 ms, 0.1 mV. D, the effect of conjunctive limb and motor cortical stimulation on responses evoked by stimuli delivered at an intensity just above threshold for a detectable cerebellar response. Calibrations: 10 ms; 0.1 mV. All traces show three consecutive sweeps. Open arrowheads, time of delivery of ipsilateral limb stimulation; filled arrowheads, time of delivery of contralateral motor cortical stimulation; PML, paramedian lobule; COP, copula pyramidis; FL, forelimb-receiving part of the C1 zone; HL, hindlimb- receiving part of the C1 zone; ip, ipsilateral; co, contralateral.
At all the cerebellar recording sites responses could also be reliably evoked by stimulation of the contralateral motor cortex, with an onset latency for PML/LS sites that was, on average, 12.6 ± 0.2 ms (evoked by forelimb motor cortical stimulation, n = 31 sites obtained from a total of 27 animals), or 21.2 ± 0.6 ms in COP (evoked by hindlimb motor cortical stimulation, n = 16 animals). The difference in onset latency between COCPs arising from fore- and hindlimb contralateral motor cortex was statistically highly significant (Student's paired t test P < 0.0001). Stimulation of the somatotopically ‘non-corresponding’ region of the contralateral motor cortex always failed to evoke a detectable response at a given cerebellar recording site. In eight of the animals (n = 11 sites), responses were also recorded at the same PML/LS forelimb-receiving C1 zone cerebellar sites evoked by stimulation of the forelimb part of the ipsilateral motor cortex. Onset latency was significantly longer than for responses evoked by stimulation of the forelimb part of the contralateral motor cortex (mean 15.3 ± 0.5 ms, n = 11 sites, Student's paired t test P < 0.0001). The responses evoked by ipsilateral motor cortical stimulation were not studied further.
All the cerebellar responses were recorded on the cortical surface and displayed the following features typical of climbing fibre field potentials (Fig. 1A): (i) an onset latency that was always greater than 9 ms; (ii) a highly characteristic waveform that was initially positive-going, including in some cases one or two secondary peaks on the falling phase at approximately 2 ms intervals; and (iii) a duration (∼5 ms) that was always shorter than responses attributable to activity in longer-latency mossy fibre paths (cf. Kennedy et al. 1966; Morissette & Bower, 1996). The pattern of the responses to a paired-pulse test also helped distinguish them further from responses evoked by shorter-latency mossy fibre paths (Fig. 1B). When two supramaximal stimuli are delivered at interstimulus intervals ranging from 20 to 100 ms, the second climbing fibre response always exhibits a reduction in size, while responses attributable to activation by short-latency mossy fibre paths usually remain unaffected (Eccles et al. 1966; Armstrong & Harvey, 1968).
In the present experiments it was also important to provide evidence that the climbing fibre field potentials evoked at a given cerebellar recording site by limb or motor cortical stimulation were the result of activation of a common group of olivary neurones. Each Purkinje cell receives a single climbing fibre afferent which is synaptically highly secure (e.g. Eccles et al. 1966). Thus, conditioning stimuli can be used to indicate whether a similar population of olive cells is activated by both the peripheral and cerebral stimulation (cf. Leicht et al. 1972, 1973; Rowe, 1977a, b; Allen et al. 1974). This is because a climbing fibre field evoked by one source of stimulation will leave the parent olive cells unresponsive to the second source of stimulation if this is delivered within an interstimulus interval of 100 ms. This possibility was tested at three recording sites. Figure 1C shows representative data in which the conditioning response was evoked by ipsilateral forelimb stimulation, while the test response at the same recording site was evoked by contralateral (forelimb) motor cortex stimulation. The test response was depressed relative to control at short interstimulus intervals, but exhibited recovery at interstimulus intervals greater than 60 ms. Consistent with climbing fibre responses, a rebound facilitation was also present at longer interstimulus intervals. The reverse combination of stimuli (i.e. the test response was evoked by peripheral stimulation) displayed a similar pattern of response.
An additional method to determine whether there is convergence of ascending and descending olivary paths is to deliver the peripheral and cerebral stimuli at the same time (if they have similar latencies), but deliver both stimuli near threshold intensity for evoking a cerebellar response (cf. Allen et al. 1974). If the two paths converge on the same olive cells, and thus provide a common climbing fibre input to the recording site under study, they should summate to evoke a larger cerebellar response. In every animal tested (n = 21) this was found to be the case (see for example, Fig. 1D).
In summary, the electrophysiological data indicate that the responses recorded in the rat cerebellar cortical C1 zone evoked by both limb and motor cortical stimulation were mainly (if not exclusively) climbing fibre in origin. Furthermore, the responses recorded at the same cerebellar recording site evoked by ascending (spino-olivocerebellar) and descending (cerebro-olivocerebellar) paths were likely to be the result of activation of a common group of olivary neurones.
Anatomical findings
The presence of large climbing fibre responses in the C1 zone in the rat cerebellar cortex evoked by contralateral motor cortical stimulation implies that there are powerful pathways leading from this region of the cerebral cortex to the inferior olive. Previous anatomical tract tracing studies in rats have shown that the forelimb-receiving parts of the C1 zone in the PML and LS both have climbing fibre projections that are entirely crossed, and that these arise from the medial part of the rostral dorsal accessory olive (rDAO), and also from the middle part of the contralateral medial accessory olive (MAO; Atkins and Apps, 1997; Pardoe & Apps, 2002). The hindlimb-receiving part of the C1 zone in the COP receives its climbing fibre input from the lateral part of the contralateral rDAO (Atkins & Apps, 1997). If there is a direct projection from the fore- and hindlimb areas of the motor cortex to the olive, then it would be expected that cerebro-fugal pathways would terminate in these particular subdivisions of the olive.
In a total of nine animals, this possibility was tested directly by placing microinjections of anterograde tracer (Fluoro-Ruby or Fluoro-Emerald, see Methods and Table 1 for further details) into the motor cortex on the right-hand side of the brain (contralateral to the cerebellar recordings). In seven animals, a microinjection was made into approximately the centre of the electrophysiologically identified hindlimb motor cortex, and in five animals a microinjection was made into approximately the centre of the electrophysiologically identified forelimb motor cortex (see Fig. 2A for an example injection site).
In all 12 cases, axons were traced from the motor cortical injection site via the internal capsule through the cerebral peduncle and into the pyramidal tract. A plexus of terminal labelling was always found in the pons, mainly on the ipsilateral side in the basilar pontine nuclei. However, these data are not considered further here because the present study is confined to consideration of the anatomical connections underlying the cerebro-olivary projection. In every case, careful inspection of the inferior olive (bilaterally) failed to reveal any terminal labelling in any of its subdivisions, including those parts known to be the source of olivocerebellar projections to the C1 zone in the PML, LS and COP (see Fig. 2B).
The absence of terminal labelling in the olive was not likely to be the result of insufficient transport of tracer, because labelled axons in the pyramidal tract could always be traced caudal to the olive, as far as the beginning of the spinal cord (see Discussion for further consideration). In most cases more caudal segments of the spinal cord were not collected so it was not possible to trace the labelled axons further. However, in 10 cases labelled fibres were noted as far caudal as spinal segment C1, and in each of these a highly localized plexus of usually fine-calibre terminal labelling was found in the medulla just caudal to the olive (Fig. 2C). The terminal field was located ventral to the gracile nucleus on the contralateral side to the injection site, at and caudal to the pyramidal decussation (centred approximately 2 mm caudal to obex, about −15 mm from bregma, in the medial part of the region termed the ‘matrix’ by Paxinos & Watson, 2005). In the two additional cases in which terminal labelling was absent in this region of the caudal medulla, only very weak axonal labelling was found in the pyramidal tract. In three of the cases with labelling in the matrix, a separate region of terminal labelling was also noted in the gracile nucleus (in one case on the left side, in the other two cases bilaterally).
In Fig. 3 one experiment (DP25) is illustrated in detail to depict results representative of the material as whole. In Fig. 3A, the dorsal view of the cerebral cortex shows the approximate locations in the right motor cortex where microstimulation evoked contralateral fore- or hindlimb movements, and also evoked large-climbing fibre field potentials in the contralateral (left) cerebellar cortical C1 zone in the PML and COP, respectively. In this animal the electrophysiological mapping guided a microinjection of Fluoro-Ruby into the forelimb motor cortex, and of Fluoro-Emerald into the hindlimb motor cortex. Transverse views of the centre of the two injection sites are shown in Fig. 3B and C and in Fig. 3D and E each series of standard transverse outlines of the caudal medulla maps the distribution of anterogradely labelled axons and terminals. The two terminal fields are located in similar territories (arrowed). The pooled data (Fig. 3F) emphasize that for both fore- and hindlimb cases the terminal labelling was located in mainly overlapping areas of the matrix (centred approximately 2 mm caudal from the obex), although the labelling in hindlimb cases was more extensive rostro–caudally.
In summary, the anatomical tract tracing experiments failed to identify any terminal labelling in the inferior olive arising from anterograde tracer injections into the fore- or hindlimb motor cortex. However, in the majority of available cases a well-localized terminal field was usually centred caudal to the olive, in the dorso-medial medulla, corresponding to the medial aspect of the matrix region of Paxinos & Watson (2005).
Pharmacological inactivation
To provide evidence that the labelled terminals in the caudal medulla could be a site of synaptic relay for cerebro-olivocerebellar paths arising from the motor cortex and targeting the fore- and hindlimb parts of the C1 zone, a series of additional electrophysiological experiments was carried out. In a total of eight animals, local anaesthetic (xylocaine) or neuronal cell body blocker (a mix of GABA and kynurenic acid, KA, see Methods for further details) was microinjected into the brainstem on one side, at coordinates that correspond to where labelled terminals were consistently found in the anatomical experiments. Since xylocaine and GABA/KA produced very similar results, the data are considered together.
In each animal, cerebellar responses were evoked in the C1 zone by stimulation of the hind- or forelimb areas of the contralateral motor cortex. In one animal cerebellar responses were recorded simultaneously from both the fore- and hindlimb parts of the C1 zone, while in a second animal cerebellar responses were recorded simultaneously from the forelimb parts of the C1 zone in the PML and LS, yielding a total of 10 different cerebellar recording sites (4 hindlimb and 6 forelimb C1 zone sites). Since the time course and magnitude of effect of the drugs was similar for both fore- and hindlimb cases they are considered together.
Figure 4A illustrates example raw data obtained from one experiment in which cerebellar responses were recorded at a forelimb-receiving C1 zone site in the PML (left side). After microinjection on the same side of the brainstem of GABA/KA into the matrix region (2 mm caudal from obex, Fig. 4B), the responses evoked by stimulation of the contralateral motor cortex (left hand column) are abolished but recover over time. By marked contrast, the responses evoked at the same cerebellar recording site by ipsilateral forelimb stimulation (right hand column) remain essentially unchanged after drug delivery. Note that in both cases a small short-latency response is also present. This is most probably due to activation of mossy fibre paths (cf. Provini et al. 1968; Allen et al. 1974). Since barbiturate was used in the present experiments and is known to severely depress such responses (Gordon et al. 1973), mossy fibre-related activity was observed in only 4/10 cases. When present these responses were always small in size and did not display any statistically significant change in amplitude after the microinjection of blocker (ANOVA, P > 0.05 in n = 4 comparisons).
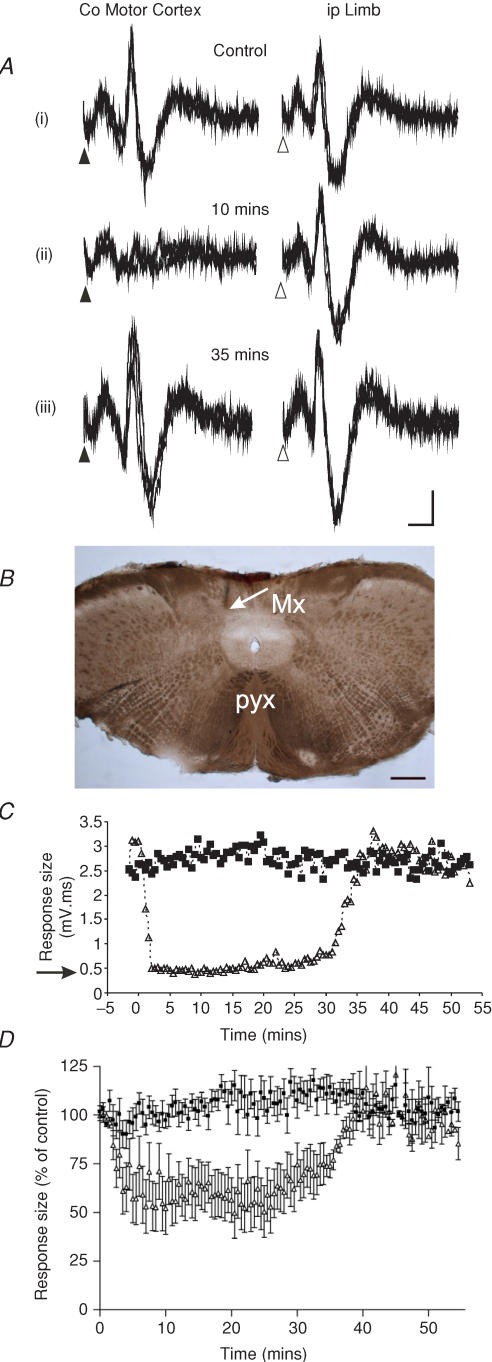
Figure 4. Selective and reversible inactivation of cerebro-olivocerebellar paths.
A, representative responses (3 sweeps superimposed) of climbing fibre field potentials recorded at the same cerebellar cortical C1 zone site and evoked either by contralateral motor cortex (left hand column) or ipsilateral forelimb stimulation (right hand column), before (i), during (ii) and after recovery (iii) from a microinjection of GABA/KA into the contralateral matrix region (experiment DPE28, see Methods for further details). Note that COCP responses evoked by motor cortical stimulation are reversibly abolished, while SOCP responses are similar or larger in size. See Results regarding the small, short-latency responses. Calibration: 5 ms, 0.1 mV. B, histological verification of the site of injection of GABA/KA into the medial matrix region in the experiment shown in A. The arrow indicates the location of the ventral extent of the pipette track. Calibration: 0.5 mm. C, data from a different experiment (DPE26), plotting the size (mV ms) of evoked climbing fibre responses as a function of time before and after injection (at time zero) of local anaesthetic into the matrix region. ▵, COCP responses, ▪, SOCP responses recorded at the same (hindlimb) C1 zone site. Each value is the average of 10 trials. Arrow indicates mean size of baseline noise for a similar time window as the duration of the evoked responses (5 ms). D, same as C but for all cases pooled (n = 10 cases) and response size expressed as a percentage of control size pre-injection (local anaesthetic or GABA/KA). Each value is the mean ± s.e.m. Mx, matrix; pyx, pyramidal decussation.
The mean size of cerebellar responses recorded in a different experiment (DPE26) are plotted as a function of time in Fig. 4C. In this case the responses were evoked at a hindlimb-receiving C1 zone site in the COP. After microinjection of xylocaine into the matrix region 2 mm caudal from the obex, there was a very pronounced reduction in the size of the responses evoked by motor cortical stimulation. For example, 10 min after the injection, the responses evoked by contralateral hindlimb motor cortical stimulation were significantly reduced to the extent that they were similar in size to baseline noise for a similar time window (ANOVA, Dunnett's post hoc test P < 0.001, Fig. 4C, see arrow). The responses recovered after ∼35 min (ANOVA, Dunnett's post hoc test P > 0.05). In marked contrast, at the same 10 min time point after the injection, the responses evoked at the same cerebellar recording site by ipsilateral hindlimb stimulation were not statistically different from control values (ANOVA, Dunnett's post hoc test P > 0.05).
In all 10 cases, the mean size of response at four different time points after drug delivery (5, 10, 15 and 20 min) was compared statistically to control values. In eight of these cases, a statistically significant reduction in the size of responses evoked by motor cortical stimulation occurred at two or more of these time points (ANOVA, Dunnett's post hoc test P < 0.05 or P < 0.001 for different comparisons). The most common time point was 10 min post-injection, when a statistically significant reduction in size of responses evoked by contralateral motor cortical stimulation occurred (range 15–78% of control size (ANOVA, Dunnett's post hoc test P < 0.05). The two exceptions were one experiment in which the responses evoked at a forelimb cerebellar recording site were reduced in size at 10 min (to 87%) and also at later time points (to 40% of control), but the reduction only reached statistical significance at the later time intervals (e.g. at 15 min post-injection, ANOVA, Dunnett's post hoc test P < 0.001). In the second experiment there was no significant change in size 10 min post-injection (responses were 110% of control, ANOVA, Dunnett's post hoc test P > 0.05). However, in this animal a second injection was targeted at the same brainstem location over an hour later, and this resulted in a highly statistically significant reduction in size of the cerebrally evoked responses 5 min after drug delivery (reduced to 47% of control, ANOVA, Dunnett's post hoc test P < 0.001).
By marked contrast to the effects on cerebrally evoked responses, seven of the same ten recording sites displayed no statistically significant difference in the size of responses evoked by limb stimulation at the 10 min time point post-injection (ANOVA, Dunnett's post hoc test P > 0.05). The exceptions were three sites where there was a statistically significant increase (to 120–142% of control, ANOVA, Dunnett's post hoc test P < 0.001). The data are pooled in Fig. 4D which emphasizes the finding that cerebellar responses evoked by contralateral motor cortical stimulation were reduced in size from about 2 min post-injection (on average to 60 ± 9% of control) and recovered about 40 min later, while at the same recording sites responses evoked by limb stimulation were slightly increased in size during the same time period (on average to 107 ± 6% of control). The difference in mean size of COCP responses as compared to SOCP responses was statistically significant (n = 10, Student's paired t test, P < 0.01).
Note that the effects of both xylocaine and GABA/KA on motor cortical projections to the cerebellum but not on corresponding fore- and hindlimb sensory paths suggests that the action of both sets of drugs was not due to spread to areas neighbouring the matrix (notably the dorsal column nuclei). Localization of this differential effect to 2 mm caudal to the obex supports this interpretation.
Injections into a similar dorso-medial location in the brainstem but at loci 0.5–1.5 mm caudal from the obex produced no significant difference in response size. For example, 10 min after a similar volume of blocker was injected (see Methods) the mean size of response evoked by peripheral or motor cortical stimulation was, respectively, 102 ± 7% and 98 ± 8%, n = 15 cases, Student's paired t test P > 0.05). For injections made into a similar dorso-medial location at 2.5–4.0 mm caudal to the obex, the corresponding mean values for peripheral and motor cortical stimulation were 69 ± 14% and 77 ± 13%, n = 9 cases, Student's paired t test P > 0.05). The similar reductions in transmission in both SOCPs and COCPs at more caudal injection sites presumably reflects intervention where at least some convergence of the associated ascending and descending climbing fibre paths has taken place.
In summary, the pharmacological inactivation experiments demonstrate that microinjection of neural blocker into the caudal brainstem can reliably reduce transmission in COCPs but not SOCPs arising, respectively, from the contralateral motor cortex and the ipsilateral limbs when the injection is made at a dorso-medial site 2 mm caudal to the obex. This differential effect occurs despite the fact that the relevant ascending and descending paths share a common climbing fibre input to the C1 zone in the posterior lobe of the rat cerebellum, providing strong evidence that the two pathways are independent at this level of the caudal brainstem. The dorso-medial location of the site corresponds closely to the matrix region where labelled cerebro-fugal terminals were identified in the tract-tracing experiments.
Discussion
The present study provides physiological, anatomical and pharmacological evidence for the existence of a newly defined pre-olivary relay in the projection from the contralateral motor cortex to the C1 zone in the posterior lobe of the rat cerebellum. The location of this relay corresponds to the medial aspect of the ‘matrix’ region as depicted in the atlas of Paxinos & Watson (2005).
Methodological considerations
In our anatomical tract-tracing experiments it might be argued that the absence of terminal labelling in the inferior olive was due to insufficient transport time and/or an inability to detect fine-calibre labelled terminals. However, this is unlikely to fully explain our findings because survival time was sufficient to label fibres as far as the dorsal columns in the spinal cord, and other studies in rats have used similar survival times for the same anterograde tracers to chart cortico-spinal projections to thoracic segments (Coleman et al. 1997). In addition, in our study fine-calibre preterminal and terminal labelling was found in other caudal brainstem structures, notably the matrix region but also the pons, suggesting that if direct projections to the olive from fore- and/or hindlimb areas of the contralateral motor cortex are present, then it should have been possible to detect them.
This is not to imply that a direct cerebro-olivary projection does not exist; clearly other regions of cerebral cortex not investigated in the present experiments may provide such a projection, and/or pyramidal cells with olivary projections may be scattered rather diffusely so only larger injections might label them. In the present experiments the most effective labelling of cerebro-bulbar fibres was obtained when several small injections of tracer were made into the motor cortex in close proximity to one another. In cats the cerebro-olivary projection from the motor cortex may arise from layer V pyramidal cells that are distributed diffusely (Bishop et al. 1976), suggesting that it would be difficult to identify such a projection with small injection sites. However, this would imply that any direct motor cortical projection to the olive is not substantial, which is at odds with physiological studies that have shown that COCPs originating from the motor cortex are functionally powerful (e.g. Provini et al. 1968; Miller et al. 1969; Andersson & Nyquist, 1983; results from the present study). To account for this apparent discrepancy it would seem reasonable to suggest that at least one additional relay may be present in the path from the motor cortex to the inferior olive.
Comparison with previous anatomical experiments
While some studies charting the connections between the motor cortex and the inferior olive have emphasized the importance of relays within the midbrain (Saint-Cyr & Courville, 1980, 1982; Saint-Cyr, 1987; Rutherford et al. 1989), others have suggested there may be a direct link (e.g. Kuypers, 1958; Sousa-Pinto & Brodal, 1969; Bishop et al. 1976; Brown et al. 1977; Berkley & Worden, 1978; Swenson & Castro, 1983; Saint-Cyr, 1983). However, these studies used anterograde degeneration or retrograde tracer techniques which are difficult to interpret because many cerebro-fugal fibres pass through the olive on their way to other brainstem structures.
By contrast, Swenson et al. (1989) used WGA-HRP to anterogradely chart olivary projections from the sensorimotor cortex in rats. In disagreement with our findings they reported a substantial direct ipsilateral olivary projection after injections into the fore- or hindlimb motor cortex. Moreover, the terminal labelling included those olivary regions that provide climbing fibres to the C1 zone. The discrepancy with our findings is puzzling, but it may be related to the fact that their motor cortical injections were larger and were made more superficially than ours. They also mapped projections arising from motor cortical regions where microstimulation elicited wrist and/or digit movements. In the present experiments, microstimulation usually evoked whole forearm or lower hindlimb movements, raising the additional possibility that in rats, control of multijoint limb movements requires a pre-olivary relay, while control of distal movements requires a direct cerebro-olivary link.
It may also be relevant to note that McCurdy et al. (1992, 1998) used WGA-HRP injections into the rDAO (the source of climbing fibres to the C1 and C3 zones) to chart retrograde projections to the cuneate nucleus and upper cervical cord in cats. No clear topography was found in the cuneo-olivary projection; nevertheless, projections to proximal and distal forelimb-receiving areas of the rDAO were located mainly in the caudal cuneate and spinal segments C1/C2, respectively. The absence of a clear topography is also reminiscent of our finding that large overlaps were present in the matrix region between projections from fore- and hindlimb motor cortex. Clearly a topography may be revealed by using higher-resolution techniques to map motor cortical inputs to the matrix. Alternatively, the cells targeted by these different inputs could be genuinely intermingled, but their outputs organized to generate the somatotopy present within the rDAO (cf. Gellman et al. 1983).
Comparison with previous physiological experiments
The ability to record large responses in the cerebellar cortex evoked by contralateral motor cortical stimulation that correlate closely with the pattern of limb inputs, extends to a different species a feature of the climbing fibre system that has been described in the monkey (Snider & Eldred, 1952) and cat (Snider & Eldred, 1951; Allen et al. 1974; Provini et al. 1968; Miller et al. 1969). The convergence of ascending and descending climbing fibre inputs onto the same population of Purkinje cells located within a particular cortical zone is also consistent with findings by Andersson & Nyquist (1983). However, a species difference may be present here, because in cats COCP inputs to the C1 zone were found to arise almost exclusively from the contralateral motor cortex, whereas in rats responses could also be reliably evoked from the ipsilateral motor cortex, although at longer latency. In this respect it may be relevant to note that rats tend to use both forelimbs during skilled movements such as food handling, while cats tend to use only one limb.
Despite these differences, the shortest-latency responses in forelimb-receiving parts of the cerebellar cortical C1 zone evoked by contralateral motor cortex stimulation are similar in both the rat and cat (∼13 ms), suggesting that in both species these signals are conveyed primarily via slow-conducting pyramidal tract fibres (cf. Kitai et al. 1969; Baker et al. 2001). These previous physiological studies also suggested that, even after taking slow conduction velocities into account, there was still sufficient time to include one or more pre-olivary synaptic relays for the associated COCPs. Moreover, Baker et al. (2001) provided evidence that this putative relay(s) was likely to be located in the caudal brainstem. The present experiments confirm and extend this suggestion by providing both anatomical and physiological evidence for the presence of a pre-olivary relay located within the matrix region for COCP paths that target the C1 zone. With regard to latencies it is also noteworthy that the fastest-conducting SOCPs targeting forelimb parts of the C1 zone have a similar latency to the corresponding COCPs (∼12 ms), implying that precise timing of the integration of ascending and descending climbing fibre inputs is important for cerebellar contributions to the control of forelimb movements.
By comparison to forelimb paths, ascending and descending climbing fibre pathways that target hindlimb-receiving parts of the C1 zone have considerably longer onset latencies (∼16 ms for SOCPs and ∼21 ms for COCPs). For ascending hindlimb paths, this presumably reflects the greater conduction distances involved. However, this is unlikely to account for the substantial difference in latency between forelimb and hindlimb COCPs (a difference of ∼9 ms, see Results). Either descending hindlimb paths involve a much greater number of intercalating synapses between the matrix and the olive, and/or the responses are mediated by considerably slower-conducting cortico-fugal fibres. The latter possibility gains indirect support from ultrastructrural studies that indicate that the pyramidal tract in rats consists of many fine-calibre fibres, including a high proportion of unmyelinated axons (Leenen et al. 1985). However, to resolve this issue will require further experiments, including electrophysiological studies in which recording and microstimulation methods are used in the matrix.
A new relay for COCPs
Our pharmacological inactivation experiments provide direct evidence that COCP input from the contralateral motor cortex to the C1 zone in rats is mediated, at least in part, by a pathway that includes a newly defined relay in the medial part of the brainstem region termed the ‘matrix’ by Paxinos & Watson (2005). The differential effects of reversible blockade of this region on COCP and SOCP responses evoked at the same cerebellar recording sites indicates that this synaptic relay must be located prior to convergence of the associated ascending and descending climbing fibre paths.
Little is known about the matrix, except that it is defined as the residual region after the solitary, trigemino-solitary, gracile, cuneate and parvicellular reticular nuclei are accounted for (Paxinos & Watson, 2005). It was first described in humans by Paxinos & Huang (1995), and there is evidence that motor cortical projections may target a similar region in cats (Kuypers, 1958). Whether a direct projection exists from the matrix to the inferior olive remains to be determined. However, this does not alter our principal finding that the region of the matrix located 2 mm caudal to the obex serves (at least in rats) as an important pre-olivary relay for descending COCPs but not ascending SOCPs targeting the same parts of the cerebellar cortical C1 zone.
Possible functional significance
A general feature of the climbing fibre system is that the central pathways mediating these signals to the cerebellum are subject to powerful regulatory influences during active movements (for a review see Apps, 1999). During locomotion, for example, both COCPs and SOCPs targeting the C1 zone are subject to step-related modulation, but the pattern of this ‘gating’ differs markedly (Pardoe et al. 2004). The functional significance of these systematic differences in pathway transmissibility remain poorly understood, but one possibility is that they reflect the changing usefulness during the step cycle of climbing fibre inputs arising from ascending and descending sources as training signals to modify cerebellar operation. The site or sites where this modulation occurs remains unknown, but since the pattern of gating differs for COCPs and SOCPs targeting the same C1 zone sites, it is likely that some (perhaps all) of the modulation occurs prior to the convergence of the two pathways, i.e. prior to the olive (cf. Weiss et al. 1990).
The present experiments provide evidence for the existence of a pre-olivary relay where modulation of COCPs targeting the C1 zone could take place. In cats, topical application of local anaesthetic to the caudal cuneate nucleus abolished SOCP and short-latency COCP responses in the C1 and C3 zones (Andersson, 1984). Andersson concluded that climbing fibre responses in these zones evoked by sensorimotor cortical stimulation were mediated through a pathway relaying in this part of the cuneate. However, the present results raise the additional possibility that the reduction in COCP transmission that Andersson (1984) observed was due partly (perhaps entirely), to spread of local anaesthetic to affect the underlying equivalent of the matrix region in cats. This gains support from the WGA-HRP tract-tracing study of McCurdy et al. (1992), who showed that a rostro-caudally extended column of cells in the ventral part of the caudal cuneate nucleus was an important source of input to the forelimb-receiving parts of the rDAO. They also found that the territory in the ventro-caudal cuneate occupied by olivary projection neurones overlapped heavily with inputs from forelimb parts of the motor cortex and red nucleus, suggesting that this is where convergence of forelimb-related ascending and descending climbing fibre paths to the C1 and C3 zones takes place. In the present experiments, injections of blocker in more caudal parts of the matrix (2.5–4 mm caudal to the obex) reduced both SOCP and COCP transmission, consistent with this possibility. Thus, the matrix may comprise at least two rostro-caudally separated regions: one region ∼2 mm caudal to the obex that serves as a relay for COCPs but not SOCPs targeting the same parts of the C1 zone, and a second more caudal region (2.5–4 mm caudal to the obex) where SOCPs and COCPs conveying climbing fibre signals to the C1 zone converge.
Inactivation of the matrix 2 mm caudal to the obex also led in some of our cases to significant increases in SOCP responses. This implies that descending signals mediated by this route can inhibit ascending climbing fibre paths. It is well known that ascending pathways are subject to inhibitory control by the sensorimotor cortex at the level of the dorsal column nuclei (e.g. Towe & Jabbur, 1961; Gordon & Jukes, 1964). The present results introduce the additional possibility that part of this inhibition may be mediated by interposing an inhibitory neurone in a link between the matrix and the dorsal column nuclei. In this way the matrix could play a role in the reductions in SOCP transmission that occur after cerebral cortical stimulation (Fennel & Rowe, 1973; Rowe, 1977b; Leicht et al. 1972, 1973) or that occur during associative conditioning (Hesslow & Ivarsson, 1996; Apps & Lee, 2002), and the potentially related phenomenon that climbing fibre activity declines over the time course required to learn a new motor task (Gilbert & Thach, 1977).
Acknowledgments
We thank Miss Rachel Bissett for expert technical assistance. This work was supported by the Medical Research Council (UK).
References
- Allen GI, Azzena GB, Ohno T. Somatotopically organised inputs from fore- and hindlimb areas of sensorimotor cortex to cerebellar Purkyne cells. Exp Brain Res. 1974;20:255–272. doi: 10.1007/BF00238316. [DOI] [PubMed] [Google Scholar]
- Andersson G. Demonstration of a cuneate relay in a cortico-olivo-cerebellar pathway in the cat. Neurosci Lett. 1984;46:47–52. doi: 10.1016/0304-3940(84)90197-6. [DOI] [PubMed] [Google Scholar]
- Andersson G, Eriksson L. Spinal, trigeminal, and cortical climbing fibre paths to the lateral vermis of the cerebellar anterior lobe in the cat. Exp Brain Res. 1981;44:71–81. doi: 10.1007/BF00238750. [DOI] [PubMed] [Google Scholar]
- Andersson G, Nyquist J. Origin and sagittal termination areas of cerebro-cerebellar climbing fibre paths in the cat. J Physiol. 1983;337:257–285. doi: 10.1113/jphysiol.1983.sp014623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R. Input-output connections of the ‘hindlimb’ region of the interior olive in cats. J Comp Neurol. 1998;399:513–529. [PubMed] [Google Scholar]
- Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Prog Neurobiol. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Apps R, Lee S. Central regulation of cerebellar climbing fibre input during motor learning. J Physiol. 2002;541:301–317. doi: 10.1113/jphysiol.2002.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ. Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol. 1966;187:553–574. doi: 10.1113/jphysiol.1966.sp008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Harvey RJ. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol. 1968;194:147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MJ, Apps R. Somatotopical organisation within the climbing fibre projection to the paramedian lobule and copula pyramidis of the rat cerebellum. J Comp Neurol. 1997;389:249–263. [PubMed] [Google Scholar]
- Baker MR, Javid M, Edgley SA. Activation of cerebellar climbing fibres to rat cerebellar posterior lobe from motor cortical output pathways. J Physiol. 2001;536:825–839. doi: 10.1111/j.1469-7793.2001.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, Worden IG. Projections to the inferior olive of the cat. I. Comparisons of input from the dorsal column nuclei, the lateral cervical nucleus, the spino-olivary pathways, the cerebral cortex and the cerebellum. J Comp Neurol. 1978;180:237–251. doi: 10.1002/cne.901800204. [DOI] [PubMed] [Google Scholar]
- Bishop GA, McCrea RA, Kitai ST. A horseradish peroxidase study of the cortico-olivary projection in the cat. Brain Res. 1976;116:306–311. doi: 10.1016/0006-8993(76)90908-2. [DOI] [PubMed] [Google Scholar]
- Brown JT, Chan-Palay V, Palay SL. A study of afferent input to the inferior olivary complex in the rat by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1977;176:1–22. doi: 10.1002/cne.901760102. [DOI] [PubMed] [Google Scholar]
- Coleman KA, Baker GE, Mitrofanis J. Topography of fibre organisation in the corticofugal pathways of rats. J Comp Neurol. 1997;381:143–157. doi: 10.1002/(sici)1096-9861(19970505)381:2<143::aid-cne3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the purkinje cells of the cerebellum. J Physiol. 1966;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Larson B. The dorsal spino-olivocerebellar system in the cat. I. Functional organization and termination in the anterior lobe. Exp Brain Res. 1979a;36:201–217. doi: 10.1007/BF00238905. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Larson B. The dorsal spino-olivocerebellar system in the cat. II. Somatotopical organization. Exp Brain Res. 1979b;36:219–232. doi: 10.1007/BF00238906. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Oscarsson O, Schouenborg J. Stimulation of cat cutaneous nociceptive C fibres causing tonic and synchronous activity in climbing fibres. J Physiol. 1987;386:539–546. doi: 10.1113/jphysiol.1987.sp016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell E, Rowe MJ. Sensorimotor cortical influences on the climbing fibre input to cerebellar Purkyne cells. Brain Res. 1973;60:263–266. doi: 10.1016/0006-8993(73)90868-8. [DOI] [PubMed] [Google Scholar]
- Garwicz M. Sagittal zonal organization of climbing fibre input to the cerebellar anterior lobe of the ferret. Exp Brain Res. 1997;117:389–398. doi: 10.1007/s002210050233. [DOI] [PubMed] [Google Scholar]
- Gellman RS, Houk JC, Gibson AR. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983;215:228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–328. doi: 10.1016/0006-8993(77)90997-0. [DOI] [PubMed] [Google Scholar]
- Gordon G, Jukes MGM. Descending influences on the exteroceptive organizations of the cat's gracile nucleus. J Physiol. 1964;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon M, Rubia FJ, Strata P. Effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973;17:50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Hesslow G, Ivarsson M. Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Exp Brain Res. 1996;110:36–46. doi: 10.1007/BF00241372. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot C, Garwicz M, Luo XL. Functional organization of climbing fibre projection to the cerebellar anterior lobe of the rat. J Physiol 522. 2000;2:297–309. doi: 10.1111/j.1469-7793.2000.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy TT, Grimm RJ, Towe AL. The role of cerebral cortex in evoked somatosensory activity in cat cerebellum. Exp Neurol. 1966;14:13–32. doi: 10.1016/0014-4886(66)90021-5. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Oshima T, Provini L, Tsukahara N. Cerebro-cerebellar connections mediated by fast and slow conducting pyramidal tract fibres of the cat. Brain Res. 1969;15:267–271. doi: 10.1016/0006-8993(69)90329-1. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. An anatomical analysis of cortico-bulbar connections to the pons and lower brainstem in the cat. J Anat. 1958;92:198–217. [PMC free article] [PubMed] [Google Scholar]
- Kyuhou S. Cerebro-cerebellar projections from the ventral bank of the anterior ectosylvian sulcus in the cat. J Physiol. 1992;451:673–687. doi: 10.1113/jphysiol.1992.sp019185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol. 1952;97:281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- Leenen LP, Meek J, Posthuma PR, Nieuwenhuys R. A detailed morphometrical analysis of the pyramidal tract of the rat. Brain Res. 1985;359:65–80. doi: 10.1016/0006-8993(85)91413-1. [DOI] [PubMed] [Google Scholar]
- Leicht R, Rowe MJ, Schmidt RF. Inhibition of cerebellar climbing fibre activity by stimulation of precruciate cortex. Brain Res. 1972;43:640–644. doi: 10.1016/0006-8993(72)90421-0. [DOI] [PubMed] [Google Scholar]
- Leicht R, Rowe MJ, Schmidt RF. Cortical and peripheral modification of cerebellar climbing fibre activity arising from cutaneous mechanoreceptors. J Physiol. 1973;228:619–635. doi: 10.1113/jphysiol.1973.sp010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG. The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Meth. 1999;86:109–117. doi: 10.1016/s0165-0270(98)00160-5. [DOI] [PubMed] [Google Scholar]
- Malpeli JG. Reversible inactivation of subcortical sites by drug injection. J Neurosci Meth. 1999;86:119–128. doi: 10.1016/s0165-0270(98)00161-7. [DOI] [PubMed] [Google Scholar]
- McCurdy ML, Gibson AR, Houk JC. Spatial overlap of rubrospinal and corticospinal terminals with input to the inferior olive. Neuroimage. 1992;1:23–41. doi: 10.1016/1053-8119(92)90005-8. [DOI] [PubMed] [Google Scholar]
- McCurdy ML, Houk JC, Gibson AR. Organization of ascending pathways to the forelimb area of the dorsal accessory olive in the cat. J Comp Neurol. 1998;392:115–133. [PubMed] [Google Scholar]
- Miller S, Nezlina N, Oscarsson O. Projection and convergence patterns in climbing fibre paths to cerebellar anterior lobe activated from cerebral cortex and spinal cord. Brain Res. 1969;14:230–233. doi: 10.1016/0006-8993(69)90045-6. [DOI] [PubMed] [Google Scholar]
- Morissette J, Bower JM. Contribution of somatosensory cortex to responses in the rat cerebellar granule cell layer following peripheral tactile stimulation. Exp Brain Res. 1996;109:240–250. doi: 10.1007/BF00231784. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res Rev. 1986;11:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Oka H, Jinnai K, Yamamoto T. The parieto-rubro-olivary pathway in the cat. Exp Brain Res. 1979;37:115–125. doi: 10.1007/BF01474258. [DOI] [PubMed] [Google Scholar]
- Oka H, Yasuda T, Jinnai K, Yoneda Y. Reexamination of cerebellar responses to stimulation of sensorimotor areas of the cerebral cortex. Brain Res. 1976;118:312–319. doi: 10.1016/0006-8993(76)90717-4. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of olivary projection to cerebellar anterior lobe. In: Courville J, de Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven Press; 1980. pp. 279–289. [Google Scholar]
- Pardoe J, Apps R. Structure-function relations of two somatotopically corresponding regions of the rat cerebellar cortex: olivo-cortico-nuclear connections. Cerebellum. 2002;1:165–184. doi: 10.1080/14734220260418402. [DOI] [PubMed] [Google Scholar]
- Pardoe J, Edgley SA, Drew T, Apps R. Changes in excitability of ascending and descending inputs to cerebellar climbing fibres during locomotion. J Neurosci. 2004;24:2656–2666. doi: 10.1523/JNEUROSCI.1659-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F. Atlas of the Human Brainstem. San Diego, CA, USA: Academic Press; 1995. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Co-Ordinates. San Diego, CA, USA: Academic Press; 2005. [Google Scholar]
- Provini L, Redman S, Strata P. Mossy and climbing fibre organization of the anterior lobe of the cerebellum activated by forelimb and hindlimb areas of the sensorimotor cortex. Exp Brain Res. 1968;6:216–233. doi: 10.1007/BF00235125. [DOI] [PubMed] [Google Scholar]
- Rowe MJ. Cerebral cortical areas associated with the activation of climbing fibre input to cerebellar Purkinje cells. Arch Ital Biol. 1977a;115:79–93. [PubMed] [Google Scholar]
- Rowe MJ. Topography of inhibitory actions from the cerebral cortex on the climbing fibre input to cerebellar Purkinje cells. Arch Ital Biol. 1977b;115:95–107. [PubMed] [Google Scholar]
- Rutherford JG, Zuk-Harper A, Gwyn DG. A comparison of the distribution of the cerebellar and cortical connections of the nucleus of Darkschewitsch (ND) in the cat: a study using anterograde and retrograde HRP tracing techniques. Anat Embryol (Berl) 1989;180:485–496. doi: 10.1007/BF00305124. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA. The projection from the motor cortex to the inferior olive in the cat. An experimental study using axonal transport techniques. Neuroscience. 1983;10:667–684. doi: 10.1016/0306-4522(83)90209-9. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA. Anatomical organization of cortico-mesencephalo-olivary pathways in the cat as demonstrated by axonal transport techniques. J Comp Neurol. 1987;257:39–59. doi: 10.1002/cne.902570105. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Courville J. Projections from the motor cortex, midbrain and vestibular nuclei to the inferior olive in the cat. anatomical organization and functional correlates. In: Courville J, de Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven Press; 1980. pp. 97–214. [Google Scholar]
- Saint-Cyr JA, Courville J. Descending projections to the inferior olive from the mesencephalon and superior colliculus in the cat. An autoradiographic study. Exp Brain Res. 1982;45:333–348. doi: 10.1007/BF01208593. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Oka H, Matsuda Y, Shimono T, Mizuno N. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp Brain Res. 1975;23:91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- Snider R, Eldred E. Electro-anatomical studies on cerebro-cerebellar connections in the cat. J Comp Neurol. 1951;95:1–16. doi: 10.1002/cne.900950102. [DOI] [PubMed] [Google Scholar]
- Snider RS, Eldred E. Cerebrocerebellar relationships in the monkey. J Neurophysiol. 1952;15:27–40. doi: 10.1152/jn.1952.15.1.27. [DOI] [PubMed] [Google Scholar]
- Sousa-Pinto A. Experimental anatomical demonstration of a cortico-olivary projection from area 6 (supplementary motor area?) in the cat. Brain Res. 1969;16:73–83. doi: 10.1016/0006-8993(69)90086-9. [DOI] [PubMed] [Google Scholar]
- Sousa-Pinto A, Brodal A. Demonstration of a somatotopical pattern in the cortico-olivary projection in the cat. An experimental-anatomical study. Exp Brain Res. 1969;8:364–386. doi: 10.1007/BF00234382. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats. An anterograde study using autoradiographic and axonal degeneration techniques. Neuroscience. 1983;8:259–275. doi: 10.1016/0306-4522(83)90064-7. [DOI] [PubMed] [Google Scholar]
- Swenson RS, Sievert CF, Terreberry RR, Neafsey EJ, Castro AJ. Organization of cerebral cortico-olivary projections in the rat. Neurosci Res. 1989;7:43–54. doi: 10.1016/0168-0102(89)90036-9. [DOI] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Towe AL, Jabbur SJ. Cortical inhibition of neurons in dorsal column nuclei of cat. J Neurophysiol. 1961;24:488–498. doi: 10.1152/jn.1961.24.5.488. [DOI] [PubMed] [Google Scholar]
- Walberg F. Descending connections to the inferior olive; an experimental study in the cat. J Comp Neurol. 1956;104:77–173. doi: 10.1002/cne.901040107. [DOI] [PubMed] [Google Scholar]
- Weiss C, Houk JC, Gibson AR. Inhibition of sensory responses of cat inferior olive neurons produced by stimulation of red nucleus. J Neurophysiol. 1990;64:1170–1185. doi: 10.1152/jn.1990.64.4.1170. [DOI] [PubMed] [Google Scholar]