Abstract
Alterations in synaptic strength are thought to represent the cellular basis of learning and memory. While such processes appear to be fundamental to all synapses, until recently there has been a relative dearth of information regarding synaptic ‘memory’ processes in autonomic circuits. Here we examine recent advances in our understanding of plasticity at glutamatergic synapses onto magnocellular neurosecretory cells in the hypothalamus, paying particular attention to the contributions of noradrenaline in coding long-lasting pre- and postsynaptic changes in efficacy. We also highlight recent work demonstrating that glial cells play a crucial role in the induction of long-term potentiation. Based on the work reviewed here, we have a clearer picture of the synaptic and cellular mechanisms that allow autonomic pathways to learn and remember.
It is generally accepted that alterations in the output of a neural circuit require alterations in the strength of the individual synapses that comprise that circuit (Bains et al. 1999; Malenka & Bear, 2004). This concept, which can involve long-term changes in synaptic signalling, forms the basis of how the brain learns and remembers. Synaptic plasticity of this type is probably ubiquitous throughout the nervous system yet its description has been limited largely to circuits responsible for motor, cognitive and behavioural processes with few descriptions of long-lasting synaptic changes in neural pathways involved in restoring homeostatic set points (e.g. osmotic balance) or pathways that respond quickly to essential physiological demands (e.g. lactation).
What would be the advantages of learning and remembering in autonomic circuits? If one considers that changes in the internal or external environment can occur swiftly and endure for prolonged periods of time, long-term alterations in synaptic strength may increase the gain and/or sensitivity of system output for the duration of the physiological challenge so that the demands of the organism are met more efficiently. For example, in vivo studies demonstrating augmented release of the neurohormones vasopressin (VP) and corticotropin releasing hormone (CRH) from the pituitary gland in response to repetitive hypovolaemic challenges (Lilly et al. 1983, 1986; Thrivikraman et al. 1997) hints at underlying learning and memory processes that may reside in autonomic circuitry. We will focus here on synaptic mechanisms that may contribute to learning and memory at glutamate synapses on the magnocellular neurosecretory cells (MNCs) of the hypothalamus which are responsible for the synthesis and secretion of VP and oxytocin (OT). MNCs from the paraventricular nucleus (PVN) and supraoptic nucleus (SON) send their axons to the posterior pituitary to release VP or OT directly into the blood. OT is released into the blood during parturition to facilitate uterine contractions and in response to suckling behaviour during lactation to promote milk letdown from the mammary glands. VP is released in response to changes in body fluid homeostasis (for review see Leng et al. 1999). These cells represent the final integration step for both local changes in osmolarity and afferent signals before neurohormone is secreted. This property makes the MNCs ideally suited for the study and interpretation of changes in synaptic strength as such alterations can be directly related to changes in system output (neurohormone release).
A number of other studies have provided the framework to better understand the fundamentals of glutamatergic signalling in this system. These will not be reviewed here, but briefly, it is evident that excitatory postsynaptic signals require the activation of AMPA/KA receptors and NMDA receptors, while presynaptic metabotropic glutamate receptors (mGluRs) are also present to decrease transmitter release. Additionally, there is an important role for glial cells in regulating transmitter spillover and synapse independence. Here we will review some recent advances in how presynaptic, postsynaptic and glial-mediated mechanisms confer long-lasting plasticity at excitatory glutamatergic synapses on the MNCs. We will examine activity-dependent and -independent synaptic modifications induced by neuro and gliotransmitters, including glutamate, ATP and d-serine, with a particular focus on the neuromodulator noradrenaline (NA), which is crucial in regulating MNC responses during lactation (Michaloudi et al. 1997), parturition (Meddle et al. 2000) and challenges to fluid homeostasis (Day et al. 1984).
Tailoring mGluR activity to suit physiological demand
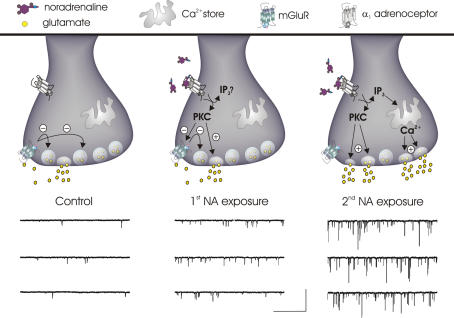
One of the initial demonstrations of synaptic learning and memory in MNCs came from examining the effectiveness of mGluRs after repetitive challenges with NA. mGluRs are a heterogeneous family of G-protein-coupled receptors categorized into three subfamilies (Conn & Pin, 1997). There is extensive evidence that group III mGluRs (mGluR 4, 7 and 8) regulate glutamate release onto MNCs (Schrader & Tasker, 1997; Gordon & Bains, 2003; Panatier et al. 2004). These receptors are located presynaptically and function both as autoreceptors, which act as short loop negative feedback receptors to mitigate the release of glutamate, and heteroreceptors, which decrease the release of GABA from neighbouring inhibitory terminals (Piet et al. 2004). mGluRs are targeted (Sorensen et al. 2002) and regulated (Macek et al. 1998; Peavy et al. 2002) by intracellular messengers, including protein kinase C (PKC). In the PVN, NA elicits a robust increase in the frequency of miniature excitatory postsynaptic currents (mEPSC) that requires Gq-linked α1-adrenoceptors and the subsequent activation of PKC (Gordon & Bains, 2003). This ‘physiological’ activation of PKC inactivates presynaptic group III mGluRs, effectively removing autoinhibition from glutamatergic synapses (Gordon & Bains, 2003). The functional consequences of this dis-inhibition include a more robust increase in glutamate release in response to subsequent applications of NA (Fig. 1). The inactivation of mGluRs may partially explain in vivo data demonstrating synergistic effects between glutamate and NA on MNC excitability (Parker & Crowley, 1993). The application of glutamate alone would increase MNC activity, but at the same time temper the release of synaptic glutamate by activating presynaptic mGluRs. In the presence of NA, however, the inactivation of mGluRs would remove the target by which exogenous glutamate would curtail synaptic glutamate release. A mechanism such as this might serve as one means by which autonomic pathways, once primed by an initial challenge that releases NA in the hypothalamus, can respond more effectively to additional stressors.
Figure 1. Priming of mESPC frequency and amplitude by NA.
Left panel: in control conditions, synaptic glutamate activates presynaptic mGluRs keeping mEPSC frequency low. Middle panel: initial α1-adrenoceptor activation increases PKC activity causing an enhancement of mEPSC frequency without changing mEPSC amplitude. Although the facilitating effect on mEPSC frequency is still limited by the activity of functional mGluRs, during this time PKC is working to inactivate these autoreceptors. Right panel: the consequences of mGluR inactivation become apparent when an additional NA challenge is administered in which an increase in mEPSC frequency is observed that is substantially larger than the first. Successive α1-adrenoceptor activation also results in dramatically larger mEPSCs that arise from the rapid release of stored calcium. The mechanism underlying amplitude priming, however, has not been elucidated. The voltage clamp traces of mEPSCs are adapted from Gordon & Bains (2003). Scale bars, 50 pA and 1 s.
Inactivation of mGluRs can endure for at least 1 h in the brain slice (author's unpublished observation) but the persistence of mGluR inactivation in the intact animal remains incompletely understood. In control conditions, mGluRs are tonically active on glutamate terminals (Schrader & Tasker, 1997) and this level of activity is further enhanced during dehydration (Boudaba et al. 2003) and lactation (Oliet et al. 2001) when the retraction of glial processes from synaptic elements (Tweedle & Hatton, 1977; Hatton & Tweedle, 1982) increases the accessibility of synaptic glutamate to presynaptic mGluRs (Oliet, 2002). These findings argue against persistent mGluR inactivation in response to dehydration and lactation, but do not rule out the possibility that an acute, intense stressor such as hypovolaemia may be better suited to unmask this effect in the intact animal.
Increasing signal strength through multi-vesicular release
In addition to the priming effects of NA on mEPSC frequency, NA also causes a robust increase in the amplitude of mEPSCs in PVN. This effect requires calcium from presynaptic stores which synchronizes the release of multiple glutamatergic vesicles (Fig. 1) (Gordon & Bains, 2005). Detailed quantal analysis combined with pharmacological tools revealed that large mEPSCs observed during NA were associated with a greater concentration of glutamate in the synaptic cleft when compared with smaller mEPSCs. How the release of additional vesicles and thus more glutamate contributes to large amplitude mEPSCs during NA is still unexplored. Possibilities include: (1) true multi-vesicular release (MVR) at a single active zone or (2) MVR in which single vesicles are synchronized across closely apposed active zones, where accumulation of glutamate at a single postsynaptic site occurs via transmitter crosstalk (Wadiche & Jahr, 2001). A qualitatively similar increase in the amplitude of mEPSCs has also been reported following brief high-frequency stimulation of MNC afferents (Kombian et al. 2000), but a mechanism contributing to this observation has yet to be defined. Similar to mEPSC frequency priming, NA-mediated MVR becomes more evident with subsequent applications of NA in some cells (Fig. 1) (Gordon & Bains, 2003). While the specific mechanisms of this process have not yet been elucidated, it is clear that some form of trial-to-trial information storage is occurring for MVR at glutamatergic synapses in MNCs.
Glial cells and long-term postsynaptic memory
These long lasting changes in presynaptic release properties may interact with changes in postsynaptic strength, similar to those described at synapses throughout the nervous system (Malenka & Bear, 2004). This process, termed long-term potentiation (LTP) or long-term depression (LTD), has best been described under conditions where coincident presynaptic and postsynaptic glutamatergic activity provides sufficient transmitter release and relief of magnesium block from NMDA receptors, to increase calcium influx and alter the number of postsynaptic AMPA receptors available to bind glutamate (Malinow & Malenka, 2002). In the SON, glutamate afferents, and in particular those originating in the organum vasculosum of the lamina terminalis (OVLT), exhibit NMDA receptor-dependent LTP and LTD (Panatier et al. 2006a). Interestingly, the induction of plasticity is precisely controlled by the astrocytes (a type of glial cell) that ensheath the synaptic contacts. In the SON, astrocytes serve as the sole source of d-serine (Panatier et al. 2006b), the endogenous ligand for the glycine binding site on the NMDA receptor. These experiments reveal that when the concentration of d-serine is relatively high there is robust NMDA activation resulting in reproducible LTP in response to high-frequency stimulation of afferents. Compromising the availability of d-serine, either by inhibiting its synthesis or by decreasing the physical interposition between glial cells and synapses, causes an LTD of synapses in response to the same stimulation parameters. The ability to induce LTP in the absence of glial cells could be recovered by applying a more intense stimulation protocol, suggesting that glial cells use d-serine to not only activate NMDA receptors, but also to set the threshold for plasticity in this system (Panatier et al. 2006b).
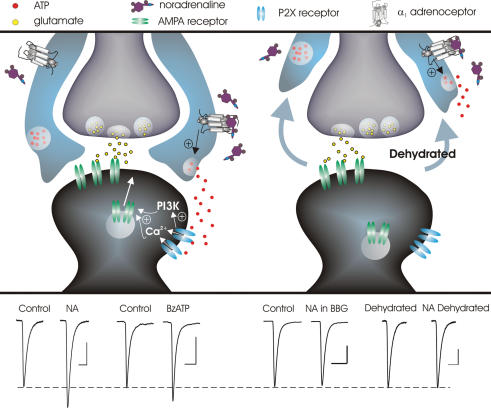
In addition to this activity-dependent form of synaptic plasticity, recent work has described an activity-independent form of long-term potentiation that also relies on glial cells. While the end result, an insertion of AMPA receptors to increase postsynaptic strength, is the same, the steps by which this point is reached are quite different. This latter form of plasticity critically depends on the release of ATP in response to NA (Gordon et al. 2005) and provides the first demonstration linking calcium-permeable ATP-gated P2X channels to AMPA receptor trafficking into the postsynaptic receptor field. Even more striking, however, is that ATP was not released by the neurons, but rather by glial cells which possess α1-adrenoceptors (Fig. 2). This was confirmed by demonstrating that (1) pure glial cell cultures released ATP in the response to NA and (2) NA was unable to increase the amplitude of mEPSCs when contact between glial cells and synaptic elements was decreased following dehydration. Collectively, these data suggest that the physical relationship between neurons and glial cells can allow, disallow or alter the threshold for the glial-mediated induction of long-lasting postsynaptic plasticity and thus synaptic memory in the MNCs.
Figure 2. Induction of long-term synaptic strengthening by NA-mediated release of glial ATP depends on the physical neuro-glia relationship.
Upper panel, left: in the control state, where there is a relative abundance of glial processes surrounding synaptic elements, glial cell α1-adrenoceptor activation triggers the release of ATP which can then activate postsynaptic P2X channels on MNCs. P2X channel activation results in calcium influx and the activation of phosphotidyl inositol 3-kinase (PI3K) leading to the insertion of AMPA receptors which is manifested as a long lasting increase in mEPSC amplitude. Upper panel, right: during states of chronic dehydration or lactation where there is a withdrawal of glial processes from around synaptic elements, NA fails to elicit changes in mEPSC amplitude. Lower panel: average mEPSC traces taken during control and 30 min after treatment. Left: NA causes a long-lasting increase in mEPSC amplitude, an effect that is mimicked by the P2X receptor agonist BzATP. Right: the long-lasting enhancement of mEPSC amplitude caused by NA is blocked either by the P2X7 antagonist Brilliant Blue G (BBG) or by withdrawal of glial processes. Scale bars: 10pA, 5 ms. Data adapted from Gordon et al. (2005).
The utilization of glia ATP and P2X channels by NA or afferent neuronal signalling and NMDA channels is interesting as each type of plasticity possesses specific properties. Unlike NMDA channels, P2X channels are not regulated by magnesium in a voltage-sensitive manner and thus do not require the same local depolarization to become activated (North, 2002). Therefore, while NMDA-mediated plasticity occurs at specific activated synapses, P2X-mediated plasticity has no requirement for coincidence detection to trigger synaptic strengthening, a property that may be particularly useful for potentiating hormone output in the absence of concerted afferent glutamatergic activity. Additionally, glial cell processes can also form physical communication points with adjacent glia, resulting in a lattice of interconnected cells capable of propagating short- and long-range signals (Charles et al. 1991). This raises the possibility of glial influence over spatial neuronal ‘domains’. That NA utilizes glial ATP for the induction of long-term strengthening of excitatory synapses, a molecule central to the propagation of long-range glial signals (Anderson et al. 2004), suggests that NA may also target glial cell networks to control synaptic efficacy globally.
Plasticity and system behaviour
A critical aspect of MNC physiology is their ability to undergo distinct patterns of action potential discharge in vivo to elevate neurohormone secretion. Specifically, individual VP cells produce a phasic bursting pattern, whereas OT cells display coordinated milk ejection bursts. Although the mechanisms responsible for these phenomena have remained largely elusive, the current belief is that both the release of neurohormone itself from the dendrites of the MNCs (Moos et al. 1998) combined with intrinsic membrane properties (Bourque et al. 1998) are responsible. Notably, NA has been shown to enhance the somatodendritic release of these peptides (Armstrong et al. 1986), which in turn can facilitate further NA release (Ludwig et al. 2000), and NA has been shown to facilitate MNC firing by altering K+ conductances (Dudek et al. 1989). Whether the types of aforementioned plasticity recruit the dendritic release of hormone and potentially regulate the firing behaviour of VP and OT cells requires further investigation. It is likely that NA and ATP, while important for augmenting system excitability and, along with d-serine, putatively important for learning and memory in the MNCs, are probably part of a more complex network of signals that generates the totality of MNC behaviour.
Together, the types of plasticity described here (Fig. 3) may be important for MNC system behaviour by constituting a novel method for selectively inducing or limiting changes in synaptic efficacy depending on the relative abundance or absence of glial cell processes surrounding MNC synapses. Long-term strengthening of excitatory synapses by glial ATP or d-serine may be utilized at the onset of dehydration or lactation to combat the ensuing challenge and only after they persist will astrocytic processes retract to ‘lock’ synapses in the potentiated state. It follows then that after retraction, plasticity requiring glial cells will not be induced as easily while other types of plasticity not requiring glial cells are permissible. These ideas are supported by: (1) the observation in dehydrated animal in which mEPSCs display larger amplitudes (Di & Tasker, 2004), which might have resulted from earlier actions of NA and ATP or d-serine; (2) the demonstration that NA is incapable of inducing further postsynaptic strengthening (Gordon et al. 2005), while the d-serine effect requires greater afferent activation (Panatier et al. 2006b) when glial cell processes have withdrawn; and (3) that NA can still elicit MVR, which does not rely on glial cells (author's unpublished observation).
Figure 3. Long-lasting plasticity at glutamatergic synapses on MNCs.
Summary of different types of enduring plasticity at glutamatergic synapses on MNCs, which incorporate presynaptic, postsynaptic and glial signalling to enhance excitatory drive for extended periods of time. NA utilizes the presynaptic terminal to prime glutamate release by inactivating mGluRs and to trigger MVR by recruiting calcium release from internal stores. The two remaining elements of the tripartite synapse are utilized concurrently to elicit AMPA receptor insertion postsynaptically and thus a long-lasting change in synaptic strength. NA acts on glial cells to trigger the release of ATP which subsequently acts on postsynaptic P2X channels to induce activity-independent changes in synapse function. Finally, glia-derived d-serine acts as an essential co-transmitter with synaptically released glutamate to activate postsynaptic NMDA receptors in the induction of classical activity-dependent plasticity.
While elucidated in a very specific set of synapses, the types of aforementioned plasticity or derivatives of them may be utilized in other autonomic centres or in other regions of the brain. At excitatory synapses in the caudal nucleus tractus solitarius (cNTS), where visceral afferent information enters the CNS, ATP has been shown to elicit large-amplitude mEPSCs by acting on presynaptic ATP-gated P2X channels (Shigetomi & Kato, 2004). Ionotropic P2X channels allow for calcium influx, a process that here, is speculated to trigger the synchronization of multiple vesicles. The similarity of this effect to the NA effect described at glutamate synapses on MNCs is interesting because there have been many documented synergistic and/or collaborative actions between NA and ATP (Burnstock, 2004). For example, in the hypothalamus, experiments have demonstrated that hormone release from an explant preparation is potentiated when ATP and NA are co-applied (Kapoor & Sladek, 2000). Furthermore, ATP plays a pivotal role in relaying signals from A1 noradrenergic cell groups to MNCs (Day et al. 1993; Buller et al. 1996). In light of this information, the observation of NA-mediated MVR in the PVN and ATP-mediated MVR in the cNTS begs for an examination of potential cooperative or synergistic effects between these two molecules.
Since synaptic glutamatergic input drives the activity of MNCs (Nissen et al. 1995; Jourdain et al. 1998), which in turn is coupled tightly to hormone release from the posterior pituitary, understanding the mechanisms that regulate the strength of these synapses promotes a more comprehensive understanding of the processes that regulate the output of MNCs. These findings also begin to shine the spotlight on synaptic mechanisms that may contribute to learning and memory in autonomic circuits.
Acknowledgments
This work was made possible by a grant from the Canadian Institutes for Health Research (CIHR). JSB is supported by the Alberta Heritage Foundation for Medical Research (AHFMR). GRJG is supported by AHFMR, Heart and Stroke Foundation of Canada and Natural Sciences and Engineering Council of Canada (NSERC). We thank Dr. Quentin Pittman and members of the lab for comments on an earlier draft of the manuscript.
References
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Gallagher MJ, Sladek CD. Noradrenergic stimulation of supraoptic neuronal activity and vasopressin release in vitro: mediation by an alpha 1-receptor. Brain Res. 1986;365:192–197. doi: 10.1016/0006-8993(86)90739-0. [DOI] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Linn DM, Halmos KC, Tasker JG. Increased tonic activation of presynaptic metabotropic glutamate receptors in the rat supraoptic nucleus following chronic dehydration. J Physiol. 2003;551:815–823. doi: 10.1113/jphysiol.2003.042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Kirkpatrick K, Jarvis CR. Extrinsic modulation of spike afterpotentials in rat hypothalamoneurohypophysial neurons. Cell Mol Neurobiol. 1998;18:3–12. doi: 10.1023/A:1022566924921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller KM, Khanna S, Sibbald JR, Day TA. Central noradrenergic neurons signal via ATP to elicit vasopressin responses to haemorrhage. Neuroscience. 1996;73:637–642. doi: 10.1016/0306-4522(96)00156-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Day TA, Ferguson AV, Renaud LP. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol. 1984;355:237–249. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA, Sibbald JR, Khanna S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993;607:341–344. doi: 10.1016/0006-8993(93)91528-z. [DOI] [PubMed] [Google Scholar]
- Di S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Tasker JG, Wuarin JP. Intrinsic and synaptic mechanisms of hypothalamic neurons studied with slice and explant preparations. J Neurosci Meth. 1989;28:59–69. doi: 10.1016/0165-0270(89)90010-1. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8:1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Priming of excitatory synapses by α1 adrenoceptor-mediated inhibition of group III metabotropic glutamate receptors. J Neurosci. 2003;23:6223–6231. doi: 10.1523/JNEUROSCI.23-15-06223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Bains JS. Noradrenaline triggers multivesicular release at glutamatergic synapses in the hypothalamus. J Neurosci. 2005;25:11385–11395. doi: 10.1523/JNEUROSCI.2378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Tweedle CD. Magnocellular neuropeptidergic neurons in hypothalamus: increases in membrane apposition and number of specialized synapses from pregnancy to lactation. Brain Res Bull. 1982;8:197–204. doi: 10.1016/0361-9230(82)90046-6. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Israel JM, Dupouy B, Oliet SH, Allard M, Vitiello S, Theodosis DT, Poulain DA. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J Neurosci. 1998;18:6641–6649. doi: 10.1523/JNEUROSCI.18-17-06641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci. 2000;20:8868–8875. doi: 10.1523/JNEUROSCI.20-23-08868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Chen X, Pittman QJ. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. J Neurophysiol. 2000;83:2542–2553. doi: 10.1152/jn.2000.83.5.2542. [DOI] [PubMed] [Google Scholar]
- Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Lilly MP, Engeland WC, Gann DS. Responses of cortisol secretion to repeated hemorrhage in the anesthetized dog. Endocrinology. 1983;112:681–688. doi: 10.1210/endo-112-2-681. [DOI] [PubMed] [Google Scholar]
- Lilly MP, Engeland WC, Gann DS. Pituitary-adrenal responses to repeated small hemorrhage in conscious dogs. Am J Physiol. 1986;251:R1200–R1207. doi: 10.1152/ajpregu.1986.251.6.R1200. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Onaka T, Yagi K. Vasopressin regulation of noradrenaline release within the supraoptic nucleus. J Neuroendocrinol. 2000;12:477–479. doi: 10.1046/j.1365-2826.2000.00516.x. [DOI] [PubMed] [Google Scholar]
- Macek TA, Schaffhauser H, Conn PJ. Protein kinase C and A3 adenosine receptor activation inhibit presynaptic metabotropic glutamate receptor (mGluR) function and uncouple mGluRs from GTP-binding proteins. J Neurosci. 1998;18:6138–6146. doi: 10.1523/JNEUROSCI.18-16-06138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Leng G, Selvarajah JR, Bicknell RJ, Russell JA. Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience. 2000;101:1013–1021. doi: 10.1016/s0306-4522(00)00300-6. [DOI] [PubMed] [Google Scholar]
- Michaloudi HC, El MM, Poulain DA, Papadopoulos GC, Theodosis DT. The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. J Neuroendocrinol. 1997;9:17–23. doi: 10.1046/j.1365-2826.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- Moos F, Gouzenes L, Brown D, Dayanithi G, Sabatier N, Boissin L, Rabie A, Richard P. New aspects of firing pattern autocontrol in oxytocin and vasopressin neurones. Adv Exp Med Biol. 1998;449:153–162. doi: 10.1007/978-1-4615-4871-3_18. [DOI] [PubMed] [Google Scholar]
- Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol. 1995;484:415–424. doi: 10.1113/jphysiol.1995.sp020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Oliet SH. Functional consequences of morphological neuroglial changes in the magnocellular nuclei of the hypothalamus. J Neuroendocrinol. 2002;14:241–246. doi: 10.1046/j.0007-1331.2001.00766.x. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Panatier A, Gentles SJ, Bourque CW, Oliet SH. Activity-dependent synaptic plasticity in the supraoptic nucleus of the rat hypothalamus. J Physiol. 2006a;573:711–721. doi: 10.1113/jphysiol.2006.109447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Poulain DA, Oliet SH. Regulation of transmitter release by high-affinity group III mGluRs in the supraoptic nucleus of the rat hypothalamus. Neuropharmacology. 2004;47:333–341. doi: 10.1016/j.neuropharm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell. 2006b;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Parker SL, Crowley WR. Stimulation of oxytocin release in the lactating rat by a central interaction of alpha 1-adrenergic and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-sensitive excitatory amino acid mechanisms. Endocrinology. 1993;133:2855–2860. doi: 10.1210/endo.133.6.7694847. [DOI] [PubMed] [Google Scholar]
- Peavy RD, Sorensen SD, Conn PJ. Differential regulation of metabotropic glutamate receptor 5-mediated phosphoinositide hydrolysis and extracellular signal regulated kinase responses by protein kinase C in cultured astrocytes. J Neurochem. 2002;83:110–118. doi: 10.1046/j.1471-4159.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci. 2004;24:3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SD, Macek TA, Cai Z, Saugstad JA, Conn PJ. Dissociation of protein kinase-mediated regulation of metabotropic glutamate receptor 7 (mGluR7) interactions with calmodulin and regulation of mGluR7 function. Mol Pharmacol. 2002;61:1303–1312. doi: 10.1124/mol.61.6.1303. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Su Y, Plotsky PM. Patterns of Fos-immunoreactivity in the CNS induced by repeated hemorrhage in conscious rats: correlations with pituitary-adrenal axis activity. Stress. 1997;2:145–158. doi: 10.3109/10253899709014745. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Ultrastructural changes in rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber-Purkinje cell synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]