Abstract
The hippocampus, a key structure for learning and memory processes, receives an important cholinergic innervation and is densely packed with a variety of nicotinic acetylcholine receptors (nAChRs) localized on principal cells and interneurons. Activation of these receptors by nicotine or endogenously released acetylcholine enhances activity-dependent synaptic plasticity processes. Deficits in the cholinergic system produce impairment of cognitive functions that are particularly relevant during senescence and in age-related neurodegenerative pathologies. In particular, Alzheimer's disease (AD) is characterized by a selective loss of cholinergic neurons in the basal forebrain and nAChRs in particular regions controlling memory processes such as the cortex and the hippocampus. Field excitatory postsynaptic potentials were recorded in order to examine whether nicotine was able to regulate induction of long-term potentiation at CA3–CA1 synapses in hippocampal slices from adult anti-NGF transgenic mice (AD 11), a comprehensive animal model of AD, in which cholinergic deficits due to nerve growth factor depletion are accompanied by progressive Alzheimer-like neurodegeneration. Both AD 11 and wild-type (WT) mice exhibited short- and long-lasting synaptic plasticity processes that were boosted by nicotine. The effects of nicotine on WT and AD 11 mice were mediated by both α7- and β2-containing nAChRs. In the presence of GABAA receptor antagonists, nicotine failed to boost synaptic plasticity in AD 11 but not in WT mice, indicating that in anti-NGF transgenic mice GABAergic interneurons are able to compensate for the deficit in cholinergic modulation of glutamatergic transmission. This compensation may occur at different levels and may involve the reorganization of the GABAergic circuit. However, patch-clamp whole-cell recordings from principal cells failed to reveal any change in spontaneous release of GABA following pressure application of nicotine to nearby GABAergic interneurons. Together, these experiments indicate that in AD 11 mice a rearrangement of the GABAergic circuit can ‘rescue’ nicotine-induced potentiation of synaptic plasticity. This may be relevant for developing proper therapeutic tools useful for the treatment of AD.
Most synapses in the CNS, including the cortex and the hippocampus, undergo bidirectional dynamic regulations of their efficacy following activity-dependent synaptic plasticity processes such as long-term potentiation (LTP) or depression (LTD). These processes, which provide the most compelling cellular models for learning and memory (Bliss & Collingridge, 1993; Bear & Malenka, 1994; Bear, 1999), are modulated by the cholinergic system. Acetylcholine, released from cholinergic fibres, acts on muscarinic and nicotinic acetylcholine receptors (nAChRs) to produce a variety of different effects on target neurons.
The hippocampus, a key structure for learning and memory processes, receives an important cholinergic innervation and is densely packed with a variety of nAChRs localized on principal cells and interneurons. These include fast desensitizing α7 receptors and slow desensitizing non-α7 receptor types (Zoli et al. 1998; Fabian-Fine et al. 2001; Kawai et al. 2002; Khiroug et al. 2003; Alkondon & Albuquerque, 2004). Activation of these receptors by nicotine or endogenously released acetylcholine enhances activity-dependent synaptic plasticity events. Thus, in hippocampal slices from young rats systemic administration of nicotine was found to potentiate or depress synaptic strength according to the initial state of the synapses (Maggi et al. 2003, 2004). Bath exposure to nicotine was also found to lower the threshold for LTP induction in such a way that stimuli that would normally elicit only short-term potentiation (STP), in the presence of nicotine would produce LTP (Fujii et al. 1999; Mann & Greenfield, 2003). Recently, evidence was provided that properly timed nicotinic activity on pyramidal neurons and interneurons by local application of acetylcholine in the presence of muscarinic receptor antagonists would determine the direction of synaptic modulation. Whereas local application of acetylcholine on the dendrites of principal cells boosted the induction of LTP via presynaptic and postsynaptic pathways (Ji et al. 2001; McGehee, 2002), nicotinic activity on interneurons inhibited nearby pyramidal cells and thereby prevented or diminished the induction of synaptic potentiation (Ji et al. 2001). At Schaffer collateral synapses, LTP was also induced when a mild presynaptic stimulation coincided with or preceded by few seconds nAChR-induced action potentials, suggesting that LTP induction crucially depends on the timing of the postsynaptic response relative to presynaptic glutamate release (Ge & Dani, 2005).
Deficits in the cholinergic system produce impairment of cognitive functions that are particularly relevant during senescence and in age-related neurodegenerative pathologies, which are increasingly considered as synaptic-failure pathologies (Selkoe, 2002). In particular, Alzheimer's disease (AD) is characterized by a selective loss of cholinergic neurons in the basal forebrain (Bartus et al. 1982) and nAChRs in particular regions controlling memory processes such as the cortex and the hippocampus (Paterson & Nordberg, 2000; Perry et al. 2000). Basal forebrain cholinergic neurons (BFCNs) crucially depend for their survival and differentiation on nerve growth factor (NGF) (Hefti, 1986; Li et al. 1995; Molnar et al. 1998; Cattaneo et al. 1999; Debeir et al. 1999). This neurotrophin has been proposed as a potential therapeutic agent to prevent degeneration of BFCNs and age-related neurodegenerative disorders on the basis of animal model studies (Capsoni et al. 2002a; De Rosa et al. 2005) and clinical studies in patients (Tuszynski et al. 2005). It is interesting that chronic deprivation of NGF in anti-NGF mice expressing recombinant neutralizing antibodies (Ruberti et al. 2000) leads to important deficits of the cholinergic function paralleled with an age-dependent progressive neurodegenerative pathology closely resembling that found in AD (Capsoni et al. 2000). Adult AD 11 mice display a neurodegenerative phenotype characterized by impairment in retention and transfer of spatial memory tasks associated with cholinergic atrophy, neuronal loss, hyperphosphorylation and insolubility of the protein too abnormalities of the neuronal cytoskeleton reminiscent of tangles (Capsoni et al. 2000), β-amyloid (Aβ) plaques (Capsoni et al. 2002b) and deficit in cortical synaptic plasticity (Pesavento et al. 2002).
In this study we examined whether, at the network level, nicotine was still able to regulate LTP induction at Schaffer collateral–CA1 synapses in hippocampal slices from adult anti-NGF transgenic mice, which start expressing intracellular Aβ and Aβ aggregates in the hippocampus and cortex at 6 months of age (Pesavento et al. 2002). We found that in AD 11 mice nicotine was able to convert STP into LTP in the same way as in age-matched wild-type (WT) mice. However, after blocking GABAA-mediated synaptic transmission, with GABAA receptor antagonists, nicotine failed to boost synaptic plasticity in AD 11 but not in WT mice, indicating that in anti-NGF transgenic mice GABAergic neurotransmission can ‘rescue’ nicotine-induced modulation of synaptic plasticity. Thus, the mechanisms of nicotinic response and of nicotine-induced modulation of synaptic plasticity are modified in 6-month-old anti-NGF mice.
Methods
Animals
Generation of AD 11 transgenic mice has been previously described (Ruberti et al. 2000). Briefly, AD 11 mice express a recombinant version of the monoclonal antibody αD11 that specifically recognizes and neutralizes NGF (Cattaneo et al. 1988; Molnar et al. 1998). Transgenic mice were obtained by digesting the plasmids encoding for the light and the heavy chain of the monoclonal antibody in order to isolate the transcriptional units. The fragments were individually microinjected into the pronucleus of single-cell fertilized C57BL/6 × SJLF2 hybrid mouse eggs and the injected eggs were reintroduced into foster pseudopregnant females (Ruberti et al. 2000). The mice expressing the light chain of the recombinant antibody were crossed with those expressing the heavy chain to obtain AD 11 mice. From this crossing, a percentage of animals did not express the two chains of the antibody and from these non-transgenic mice derives the line of mice used as controls. Female and male AD 11 and non-transgenic control mice aged 6 months were used for this study. Mice were housed in a group with no more than five animals per cage and kept in a colony room at 22°C on a 12 h light–12 h dark cycle (lights on at 07.00 h). Food and water were available ad libitum.
Hippocampal slice preparation
Male or female WT and AD 11 mice (6 months old) were decapitated under urethane anaesthesia (i.p. injection, 2 g kg−1). All experiments were carried out in accordance with the European Community Council Directive of 24 November 1986 (86/609EEC) and were approved by the local authority veterinary service. The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): sucrose 215, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, CaCl2 2, MgCl2 1.3, glucose 25 and ascorbic acid 4 10−4, saturated with 95% O2–5% CO2 (pH 7.3–7.4). Transverse hippocampal slices (thickness, 400 μm) were prepared using a vibratome (vibratome 1000 Plus, CE) and were maintained at room temperature (20–22°C)in ACSF for recording containing 130 mm NaCl instead of sucrose that was gassed with 95% O2–5% CO2. After incubation for at least 1 h, an individual slice was transferred to a submerged recording chamber and continuously superfused at 33°C with oxygenated ACSF at a rate of 4–5 ml min−1. No gender differences in the ability of nicotine to induce changes in short- and long-term synaptic plasticity processes were observed. Therefore data obtained from male and female mice were pooled together.
Electrophysiological recordings and data analysis
Field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of CA1 in response to stimulation of the Schaffer collateral pathway. They were mediated by AMPA receptors as they were readily abolished by 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μm). In some experiments to reveal the NMDA component, field potentials were recorded using an extracellular solution containing 0.5 mm MgCl2 instead of 1.3 mm and DNQX (20 μm) to block AMPA/kainite receptor subtypes. Electrical stimuli (100-μs square pulses at 0.1 Hz) were delivered through a bipolar twisted NiCr-insulated electrode controlled via a stimulus isolation unit (Digitimer, Welwyn Garden City, UK), with the stimulus strength adjusted to evoke a half-maximal response (in the range of 4–8 V). The recording micropipettes (resistance, < 3 MΩ) were pulled from thick-walled borosilicate glass capillary (Hilgenberg, Malsfeld, Germany) and filled with ACSF. fEPSPs were recorded using a DAM-800 differential amplifier (World Precision Instruments, Sarasota, FL, USA), low-pass filtered at 1 kHz, digitized at 10 kHz (DigiData 1200, Axon Instruments, Union City, CA, USA) acquired with LTP114J software (courtesy of W.W. Anderson, Bristol University, UK) and analysed off-line using Clampfit 9.0 software (Axon Instruments).
The signal magnitude was measured considering the peak amplitude of fEPSPs and the values were averaged over 1 min (six responses). After establishing a stable baseline, STP was induced by tetanic stimulation (50 pulses at 100 Hz). If there was no clear STP lasting more than 10 min, the slice was rejected. Otherwise, the responses were allowed to stabilize (> 20 min), and the effect of nicotine treatment (during 4 min) was assessed on a second identical STP tetanus given immediately after drug application. In pharmacological experiments, STP tetanus was initially applied in the presence of the drugs (AChR antagonists and bicuculline). In the presence of bicuculline, a cut was made between the CA1 and the CA3 region to prevent the propagation of epileptiform activity.
All the fEPSP amplitudes were normalized to baseline values. The amount of nicotine-induced potentiation was calculated as the ratio between fEPSP amplitude after the STP2 and that obtained after STP1 (STP2/STP1, see Results). In order to estimate the saturating level of the system, an LTP was induced at the end of the experiment by high-frequency stimulation (2 trains of 100 pulses at 100 Hz, delivered 20 s apart). The paired-pulse facilitation (PPF) was induced by a double-pulse (50 ms apart) stimulation protocol and it was evaluated by calculating the paired-pulse ratio (PPR) between the second (fEPSP2) and the first (fEPSP1) responses). To generate the input–output curves, slices were stimulated every 10 s (averaged over 1 min) with stimuli from the threshold to the maximal amplitude evoked responses (0.5–1 V increments) at the beginning of the experiment.
For statistical comparison between treatment groups, fEPSP values were averaged over the last 5 min (30 responses) preceding the next experimental condition. Data are presented as the mean ±s.e.m. Comparisons for differences in the means were assessed by ANOVA followed by multiple comparison Tukey's and Dunnett's post hoc tests or paired and unpaired Wilcoxon tests, as appropriate. For input–output curves, comparison Kolmogorov-Smirnov test was used. The differences were considered significant for P < 0.05.
In one set of experiments, the patch-clamp technique was used to record miniature GABAA-mediated inhibitory post synaptic currents (mIPSCs) from CA1 principal cells in slices obtained from both AD 11 and WT mice. Whole-cell GABAergic currents were recorded at 33°C and at the holding potential of −60 mV, in the presence of DNQX (20 μm), d-(−)-2-amino-5-phosphonopentanoic acid (d-APV) (50 μm) and tetrodotoxin (TTX, 1 μm). Patch pipettes were obtained from thin borosilicate glass capillaries (Hilgenberg, Malsfeld, Germany) and were filled with a solution containing (mm): CsCl 140, Hepes 10, MgATP 2 and BAPTA 1; pH was adjusted to 7.3 with CsOH. When filled with the intracellular solution they had a resistance of 4–6 MΩ. Data were acquired at 20 kHz, filtered with a cut-off frequency of 1 kHz with an Axopatch 1D amplifier (Axon Instruments) and stored on computer in order to perform an off-line analysis using Clampfit 9.0 software. The stability of the patch was checked by repetitively monitoring the input and series resistance during the experiments. Cells exhibiting more than 20% changes were excluded from the analysis. Membrane potentials were corrected for the liquid junction potential of 10 mV.
In another set of experiments, the effects of nicotine were tested on action potential-dependent and independent spontaneous GABAA-mediated IPSCs recorded from CA1 principal cells in the presence of DNQX and d-APV. Nicotine (concentration into the pipette 1 mm) was applied by pressure (5 psi; duration, 1–2 s) with the PicoPump PV 820 (World Precision Instruments) from a glass pipette localized close to a nearby interneuron in stratum radiatum (to avoid a direct effect of nicotine on principal cells the pipette was aligned parallel to the pyramidal cell layer 100–200 μm lateral and > 100 μm vertical to the patch-clamped pyramidal neuron). Data are presented as the mean ±s.e.m. Comparisons for differences in the means were assessed by paired and unpaired t tests, as appropriate.
Drugs used
Nicotine, dihydro-β-eritroidine (DHβE), bicuculline, α-bungarotoxin (α-BTX) and mecamylamine were purchased from Sigma (Poole, UK). SR95531 hydrobromide (gabazine), methyllycaconitine citrate (MLA), d-APV and DNQX were purchased from Tocris Cookson (Bristol, UK). Tetrodotoxin (TTX) was purchased from Latoxan (Valence, France). All drug stock solutions were made in distilled water and then divided into aliquots and frozen at −20°C.
α-BTX was incubated with the slices, for at least 1 h, during the recovering time. All other drugs were diluted in oxygenated ACSF immediately prior to use, and were applied via the perfusion system.
Results
Anti-NGF AD 11 mice exhibit short- and long-lasting activity-dependent synaptic plasticity processes similar to WT animals
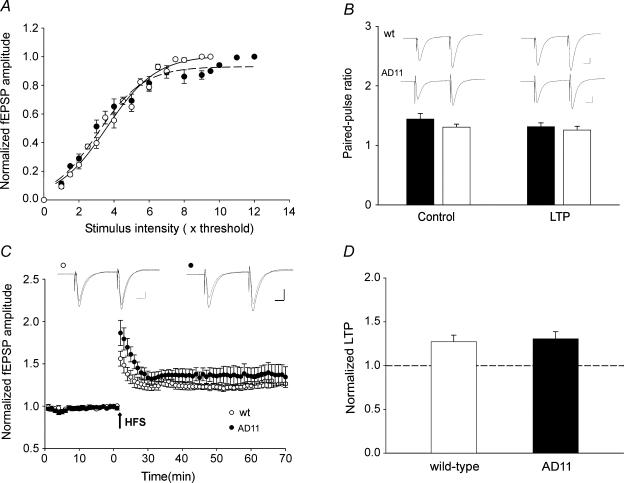
Previous studies with anti-NGF mice have demonstrated that the progressive pattern of neurodegeneration is associated at the cortical level with a reduction of the cholinergic innervation and a severe deficit of synaptic plasticity that was rescued by acute application of acetylcholine or galantamine indicating the involvement of the cholinergic system (Pesavento et al. 2002). Therefore the following experiments were undertaken to test whether in anti-NGF mice activation of nAChRs by nicotine is able to modulate synaptic plasticity in the hippocampus, a structure directly involved in learning and memory processes. For this purpose, extracellular fEPSPs evoked by Schaffer collateral stimulation were recorded from stratum radiatum of the CA1 hippocampal region in slices obtained from 6-month-old WT and AD 11 mice. These consist of a negative field potential preceded by a presynaptic fibre volley. In a first set of experiments, the strength of synaptic transmission was analysed by delivering single test stimuli of graded intensity to the Schaffer collaterals at 0.1 Hz. Figure 1A shows the input–output curve obtained from the hippocampus of WT (n = 9; ^) and AD 11 mice (n = 7; •). Overall, the shape and amplitude of fEPSPs were similar in both groups of animals. Although in both cases the input–output curves were nearly linear, their slopes slightly differed (Kolmogorov-Smirnov test, P < 0.05). In WT and in AD 11 mice the values of the slope were 1.41 and 1.31, respectively, indicating a modest reduction in network excitability in AD 11 mice. To test whether this small change in cell excitability reflects modification in transmitter release, in additional experiments we measured PPF, a short-term presynaptic phenomenon that at CA3–CA1 synapses is directly related to the probability of neurotransmitter release (Zucker, 1989). An increase or a decrease in PPF ratio indicates a reduction or an increase in neurotransmitter release, respectively (Zucker, 1989). In our experiments, the fEPSPs evoked by a second stimulus (50 ms apart) was always facilitated and the degree of facilitation (expressed as PPR) was virtually identical in AD 11 and WT mice in both controls (WT, 1.65 ± 0.08; AD 11, 1.56 ± 0.07) and/or after potentiation conditions (WT, 1.31 ± 0.06; AD 11, 1.33 ± 0.05; Fig. 1B). This suggests that in anti-NGF AD 11 mice the probability of glutamate release from the Schaffer collateral was not altered.
Figure 1. Chronic NGF deprivation in AD 11 mice does not alter activity-dependent synaptic plasticity processes in the hippocampus.
A, the amplitudes of field EPSPS (fEPSPs) evoked by Schaffer collateral stimulation in wild-type (WT) (^) and AD 11 mice (•), normalized to the maximal responses are plotted against stimulus intensities. Each point is the average of six responses obtained from WT (n = 9) and AD 11 (n = 7) animals. In this and in the following figures, bars represent s.e.m. Data points are fitted with sigmoid functions (WT, r2= 0.98; AD, 11 r2= 0.99). With respect to WT mice (continuous line), the curve obtained in AD 11 mice (dashed line) shows a slight but significant decrease in the slope (from 1.41 to 1.31; P < 0.05, Kolmogorov-Smirnov test). B, examples of paired responses (50-ms interval, average of six traces) obtained in WT (upper traces) and AD 11 (lower traces) mice in control conditions (left) and 45 min after LTP induction (right). The columns below the traces represent the mean values of the paired-pulse ratio obtained in WT (open columns, n = 6) and AD 11 mice (fiflled columns, n = 6) in control and after LTP. C, LTP induced by two HFS trains (1 s at 100 Hz, 20 s apart; arrow) delivered to the Schaffer collateral in WT (^; n = 6) and in AD 11 mice (•; n = 6). The amplitudes of the fEPSPs were normalized to those obtained before HFS. In the insets above the graph two superimposed average traces of fEPSPs obtained from WT (left) and AD 11 mice (right) before and 45 min after LTP induction. D, amount of potentiation measured 45 min after LTP induction in WT (open column, n = 6) and AD 11 mice (filled column, n = 6). Scale bars in B and C, 0.1 mV (10 ms)−1.
The next series of experiments were undertaken to test whether activity-dependent LTP could be induced in the hippocampus of 6-month-old anti-NGF transgenic mice. In WT mice, high-frequency stimulation (HFS) of the Schaffer collateral (two trains of 100 pulses at 100 Hz delivered at 20-s interval) induced an NMDA receptor (NMDAR)-dependent form of LTP. On average, the LTP was 56 ± 9% of the control value immediately after the HFS and 27 ± 8% of the control value 45 min later (n = 6; Fig. 1C and D). In AD 11 mice, HFS induced a LTP with similar time course and degree of potentiation. On average, LTP was 86 ± 15% and 30 ± 8% immediately and 45 min after HFS, respectively (n = 6; Fig. 1C and D). The steady-state degree of potentiation measured 45 min after HFS in both WT and AD 11mice (27 ± 8% and 30 ± 8%, respectively) was not significantly different between the two groups of animals (P = 0.87, unpaired Wilcoxon test). In both cases, LTP was associated with a reduction in PPF, indicating an activity-dependent increase in the probability of glutamate release (Fig. 1B). These results suggest that at CA3–CA1 synapses of 6-month-old AD 11 mice short- and long-term synaptic plasticity processes are not affected.
Nicotine facilitates the development of LTP in both anti-NGF AD 11 and WT mice
This set of experiments was undertaken to assess whether a sustained application of nicotine to hippocampal slices is able to change STP into LTP as recently demonstrated in the adult guinea-pig hippocampus (Mann & Greenfield, 2003). For this purpose, the amplitude of fEPSPs evoked by Schaffer collateral stimulation at 0.1 Hz was measured for 10 min to establish a baseline and then an STP protocol (50 pulses at 100 Hz) was applied in order to produce a robust STP (STP1). Immediately after the tetanus, the amplitude of the fEPSP reached a peak that was ∼60% of the baseline value and then declined exponentially to reach either the baseline value (WT, n = 3/7; AD 11, n = 1/7) or a value above control (WT, n = 4/7; AD 11, n = 6/7). In these cases fEPSP amplitude was 23 ± 5% and 19 ± 2% of control values for WT and AD 11 mice, respectively (Fig. 2A and B). After 30 min from STP1, nicotine (1 μm, horizontal bar in Fig. 2C and D) was applied in the bath for 4 min. This drug did not modify the amplitude or the shape of fEPSPs. Immediately after washing out nicotine, a second STP protocol (STP2), identical to the first, was applied. This procedure generated a significant increase of STP2 with respect to STP1 leading to a robust LTP. After STP2, the peak amplitude of the fEPSP was increased 105 ± 35% of the control baseline in WT and 106 ± 24% in AD 11 mice (Fig. 2C and D). This value was significantly different from that obtained after STP1 in both groups (P < 0.05 paired Wilcoxon test). At the end of the experiments, an HFS protocol (see Methods) was applied in order to assess the upper plasticity level of our system.
Figure 2. Nicotine boosts activity-dependent synaptic plasticity processes in both wild-type (WT) and AD 11 mice.
A and B, in the absence of nicotine, successive stimulation protocols to Schaffer collateral (i.e. STP1, STP2 (black arrows) and LTP (grey arrows)) produce a progressive increase in the degree of potentiation in both WT (^, n = 7) and AD 11 mice (•, n = 7). In the insets above the graphs three superimposed average traces of field EPSPs obtained in WT (left) and AD 11 mice (right) after the first, second and third stimulation protocol. C and D, bath application of nicotine (1 μm for 4 min, filled bars) boosts the amount of activity-induced synaptic plasticity in WT (^, n = 12) and in AD 11 transgenic mice (•, n = 10). Insets above the graphs as in A and B. Note that nicotine did not saturate the degree of potentiation in either in WT or in transgenic mice as a further potentiation could be obtained by two HFS trains (1 s at 100 Hz, 20 s apart, C and D). Scale bars, 0.1 mV (10 ms)−1.
It is interesting that when potentiation was calculated as the ratio between the amplitude of the fEPSP obtained after the STP2 and the fEPSP amplitude obtained after STP1 (STP2/STP1) a significant potentiation was observed when nicotine was applied prior to STP2 (STP2/STP1: WT, 1.49 ± 0.14, n = 12; AD 11, 1.47 ± 0.12, n = 10; Fig. 2C and D). These two values were not significantly different (P = 0.86 unpaired Wilcoxon test). No potentiation of STP2 was observed when 50 pulses at 100 Hz were delivered after STP1 in the absence of nicotine (STP2/STP1: WT, 1.05 ± 0.03, n = 7; AD 11, 1.08 ± 0.02, n = 7; Fig. 2A and B). These values differed significantly from those obtained with nicotine (P < 0.05, Tukey's test; see also Fig. 6C and D). These results indicate that a second stimulation protocol, which does not induce any additional potentiation per se can produce a strong facilitation of the fEPSP if delivered immediately after nicotine application and that the amount of nicotine-induced facilitation is the same in both WT and anti-NGF AD 11 mice. These experiments also showed that the STP2 protocol delivered after application of nicotine does not saturate LTP either in WT or in transgenic mice as a further potentiation could be obtained at the end of the experiments by HFS trains to the Schaffer collateral (Fig. 2C and D). The degree of LTP obtained in this way was maximal as additional trains did not produce any further potentiation (n = 4; data not shown).
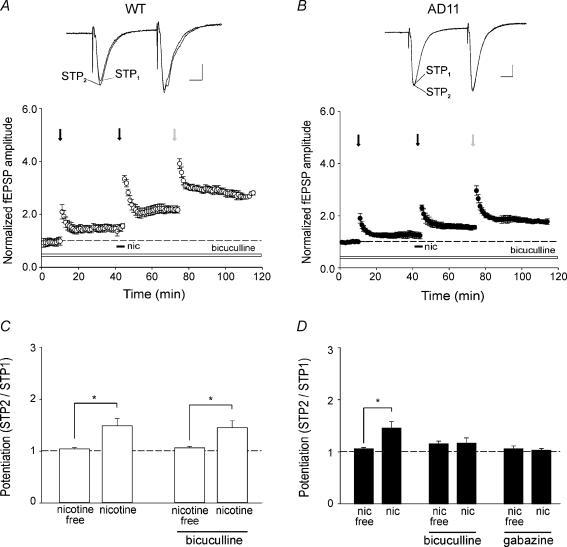
Figure 6. Activation of GABAA receptors is required for nicotine-induced potentiation of AMPA-mediated field EPSP in AD 11 mice.
A and B, activity-induced potentiation evoked by stimulation protocols (arrows as in Fig. 2) before and/or after nicotine application (filled bars) in the presence of bicuculline (10 μm, open bars) in wild-type (WT) (^, n = 7) and in AD 11 mice (•, n = 7). In the insets above the graphs two superimposed average traces obtained after the first (STP1) and the second (STP2) simulation protocol. C and D, summary data showing average values of STP2/STP1 obtained in the presence or absence of nicotine and in the presence or absence of nicotine plus bicuculline in WT (open columns) and AD 11 mice (filled columns). Note the total lack of nicotine effect in AD 11 mice when bicuculline was present in the bathing solution. *P < 0.05, Tukey's test.
Nicotine-induced potentiation requires NMDA receptor activation
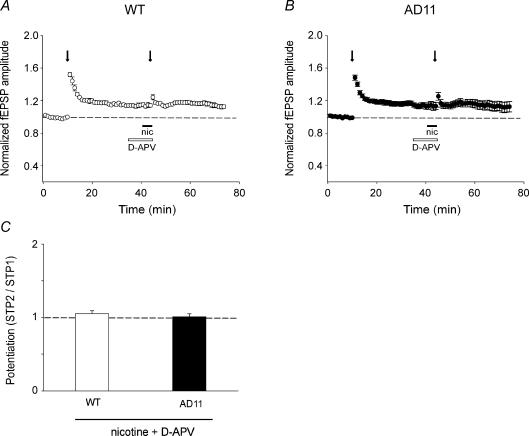
The induction of LTP in the hippocampal CA1 region depends upon NMDAR activation (Bliss & Collingridge, 1993; Nicoll & Malenka, 1995). Thus, one possible mechanism underlying the effect of nicotine could be nicotine-induced modulation of NMDARs. In order to evaluate the role of these receptors in nicotine potentiation, d-APV was bath applied 6 min before and for 4 min during nicotine application and fEPSP amplitude was analysed in this experimental condition. We found that in both experimental groups, d-APV (50 μm) was able to fully prevent the facilitatory effect of nicotine (Fig. 3A and B) without modifying the amplitude or the shape of the fEPSPs. The STP2/STP1 ratio was 1.05 ± 0.04 in WT and 1.01 ± 0.04, in AD 11 mice (n = 4 for both; Fig. 3C). Moreover, this pharmacological treatment prevented the induction of LTP after STP2 stimulation. This set of experiments demonstrates that in our experimental conditions nicotine-induced modulation of hippocampal plasticity processes requires the activation of NMDARs, although the mechanisms underlying this action are still unclear.
Figure 3. Nicotine-induced potentiaton of field EPSPS (fEPSPs) is mediated by NMDA receptors.
A and B, nicotine (filled bars) applied in the presence of d-APV (50 μm, open bars) fails to produce any additional potentiation of fEPSPs evoked by STP stimulation (arrows) in both wild-type (WT) and AD 11 mice. C, each column represents the mean STP2/STP1 value obtained in WT (n = 4) and AD 11 (n = 4) mice in the presence of nicotine plus d-APV.
Nicotine as gain setter for neuronal plasticity
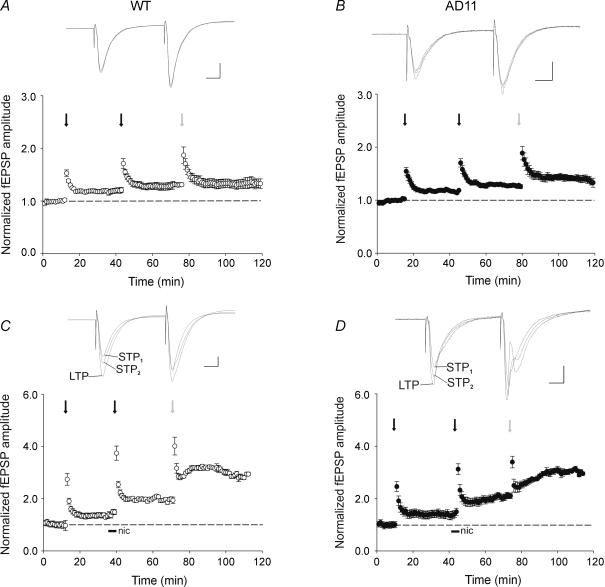
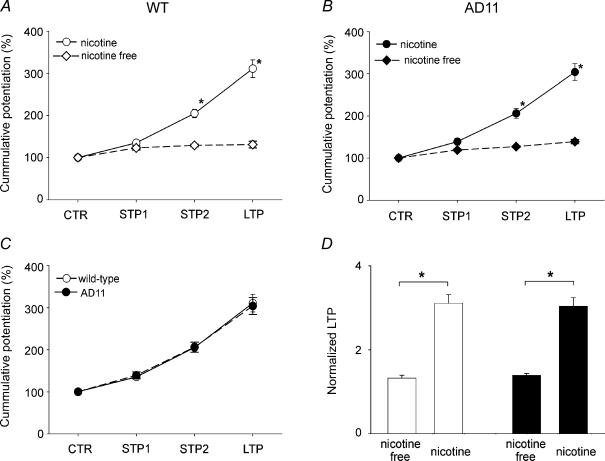
Considering the effects of nicotine on STP2, we next addressed the question of whether the alkaloid was able to modify the saturating level of LTP produced by an HFS protocol to the Schaffer collateral. To this aim, the degree of potentiation induced by different stimulation protocols in the presence or absence of nicotine was compared. As shown in the plots of Fig. 4A and B from WT and anti-NGF AD 11 mice, nicotine not only significantly enhanced the level of the potentiation induced by the STP2 protocol but also produced a three-fold increase of the saturating level of LTP in both control and transgenic mice (P < 0.05 unpaired Wilcoxon test). The effects of nicotine on potentiation followed the same progression in both anti-NGF AD 11 and WT mice (Fig. 4C). Moreover, in both WT and transgenic mice, the degree of LTP boosted by nicotine was almost the same (WT, 311 ± 21%; AD 11, 304 ± 20% of the baseline; Fig. 4D).
Figure 4. Nicotine acts as a ‘gain setter’ of neuronal plasticity.
A and B, cumulative potentiation of field EPSPs obtained in different experimental conditions (as indicated) in wild-type (WT) (open symbols, n = 19) and in AD 11 mice (filled symbols, n = 17) in the absence (diamonds) or in the presence (circles) of nicotine, expressed as percentage of the normalized potentiation. C, the amount of potentiation induced by different experimental protocols in both WT and AD 11 is very similar. D, each column represents the mean LTP value obtained in the absence or in the presence of nicotine in WT (open columns) and AD 11 mice (filled columns). *P < 0.05, unpaired Wilcoxon test.
Altogether, these results indicate that, in both strains of mice, a brief exposure to nicotine is able to induce a gain of function at hippocampal CA3–CA1 synapses.
Nicotine effects are mediated by both α7- and β2-containing nAChRs in AD 11 and WT mice
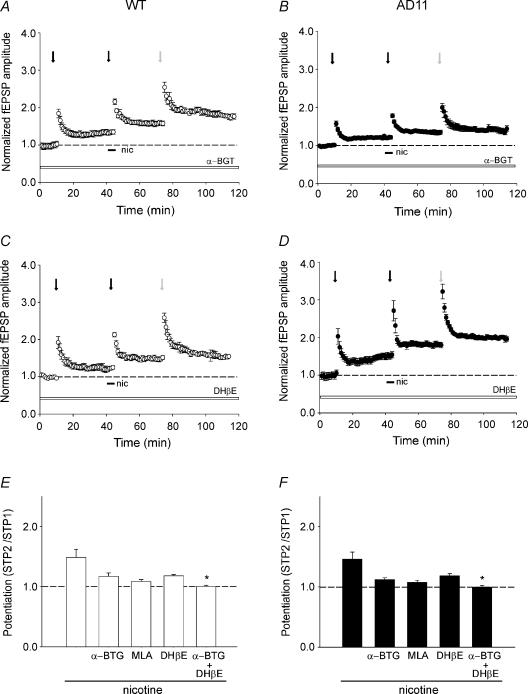
In order to investigate which types of nAChRs were involved in the facilitatory effect of nicotine on synaptic plasticity, a set of pharmacological experiments was performed. We used α-BGT and MLA to selectively antagonize homomeric α7-nAChRs and DHβE which at 1 μm, selectively blocks β2-containing nAChRs (Bertrand et al. 1992). In the case of α-BGT, because of its irreversible effect, slices were incubated in the presence of the toxin for at least 1 h before recording and then perfused with a drug-free solution. These antagonists did not affect short- and/or long-term synaptic plasticity processes (Fig. 5A–D) but partially prevented the potentiating effect of nicotine. In particular, in WT mice α-BGT (100 nm) and MLA (10 nm) reduced the mean STP2/STP1 ratio from 1.49 ± 0.14 to 1.18 ± 0.06 (P = 0.18, Dunnett's test; n = 9) and 1.1 ± 0.04 (P = 0.07, Dunnett's test; n = 6), respectively, (Fig. 5A and E). Similarly, DHβE (1 μm) partially antagonized the action of nicotine (mean STP2/STP1 ratio, 1.19 ± 0.02; P = 0.11, Dunnett's test; n = 8; Fig. 5C and E). The combined treatment with α-BGT and DHβE completely blocked nicotine effects (mean STP2/STP1 ratio, 1.01 ± 0.02, P < 0.05, Dunnett's test; n = 4; Fig. 5E). As in WT, α-BGT and MLA partially prevented the action of nicotine in AD 11 mice. The toxin reduced the mean STP2/STP1 ratio from 1.47 ± 0.12 to 1.12 ± 0.03 (P = 0.09, Dunnett's test; n = 9) whereas MLA caused a decrease from 1.47 ± 0.12 to 1.09 ± 0.04 (P = 0.06, Dunnett's test; n = 6; Fig. 5B and F). A partial block was also produced by DHβE (STP2/STP1, 1.19 ± 0.03, n = 8; P = 0.3, Dunnett's test; Fig. 5D and F). Also in AD 11 mice, the combined treatment of α-BGT and DHβE completely blocked the effects of nicotine (mean STP2/STP1 ratio, 1.00 ± 0.03, n = 4; P < 0.05, Dunnett's test; Fig. 5F).
Figure 5. Effects of nicotine in wild-type (WT) and AD 11 mice are mediated by different nAChR subtypes.
A and B, activity-induced potentiation evoked by different stimulation protocols (arrows as in Fig. 2) before and/or after nicotine application (horizontal bars) in the presence of α-bungarotoxin (α-BGT; 100 nm, open bars) in WT (^, n = 7) and in AD 11 mice (•, n = 8). Each circle is the average of six traces. C and D, the same experimental procedure as used in A and B, but in the presence of dihydro-β-eritroidine (DHβE; 1 μm, open bars) for WT (^, n = 6) and in AD 11 mice (•, n = 7). E and F, summary data of nicotine-induced potentiation (STP2/STP1) obtained from WT (open columns) and AD 11 mice (filled columns) in different experimental conditions (as indicated). Note that in AD 11 animals, preincubation with α-BGT but not DHβE significantly prevents nicotine-induced potentiation. *P < 0.05, Dunnett's test.
These experiments indicate that in both AD 11 and WT mice the effect of nicotine on synaptic plasticity is mediated by both homomeric α7 and heteromeric β2-containing nAChRs. It is interesting that in both experimental groups the degree of potentiation of fEPSPs induced by the first stimulation protocol (STP1) in the presence of nAChR antagonists was similar to controls (obtained in the absence of the drugs), indicating that nicotine-induced potentiation following STP2 was mainly due to the activation but not to the desensitization of nAChRs.
In AD 11 mice but not WT mice, GABAergic interneurons are required for nicotine-induced potentiation of AMPA-mediated fEPSP
Principal cells receive two types of inhibition: recurrent and feed-forward (Freund & Buzsaki, 1996) which control cell excitability and plasticity processes. To elucidate the role of GABAA-mediated inhibition in nicotine-induced facilitation of activity-dependent synaptic plasticity processes, slices obtained from both WT and anti NGF AD 11 mice were superfused with an extracellular solution containing bicuculline (10 μm, n = 7), or gabazine (5 μm, n = 4) in order to block GABAA-mediated synaptic transmission. However, this led to the development of interictal discharges, which precluded any further analysis of fEPSPs. Epileptiform bursts observed in the absence of GABAA-mediated inhibition may be triggered at the level of recurrent collaterals between CA3 principal cells and propagate to the CA1 region via Schaffer collaterals (Miles & Wong, 1987). Therefore, in order to isolate the CA1 from the CA3 hippocampal region, a cut was produced in the slice. In these conditions, interictal discharges did not propagate to the CA1 region and fEPSPs could be recorded without the interference of epileptiform bursts. Blocking GABAA receptors produced diverse effects in WT and AD 11 mice. Whereas in WT mice nicotine was still able to enhance synaptic plasticity, it failed to modify synaptic responses in anti-NGF AD 11 mice (Fig. 6). However, as shown in time course plots of Fig. 6A and B, treatment with bicuculline did not interfere with basic short- and long-term synaptic plasticity processes in both experimental groups.
Summary data from disinhibited slices obtained from WT and transgenic mice are represented in Fig. 6C and D, respectively. It is clear from Fig. 6 that after nicotine application, the STP2 protocol produced in WT but not in AD 11 mice an increase in the peak amplitude of the fEPSP. In WT mice, the STP2/STP1 ratio observed in the presence of bicuculline was 1.45 ± 0.1 (n = 7, P < 0.05, Tukey's test) whereas in anti-NGF AD 11 mice it was 1.18 ± 0.1 (n = 7, P = 0.85, Tukey's test). As it has been reported that bicuculline significantly reduces currents mediated by heteromeric and homomeric nAChRs expressed in Xenopus oocytes (Demuro et al. 2001), we tested another more selective GABAA receptor antagonist, gabazine. As with bicuculline, gabazine (5 μm) prevented the effect of nicotine on STP2/STP1 ratio (the value of this ratio was 1.04 ± 0.03; n = 4, P = 0.6, Tukey's test) indicating that in AD 11 mice the action of nicotine was indeed mediated via GABAA receptors.
Nicotine-induced potentiation also involves the NMDA-mediated component of synaptic responses
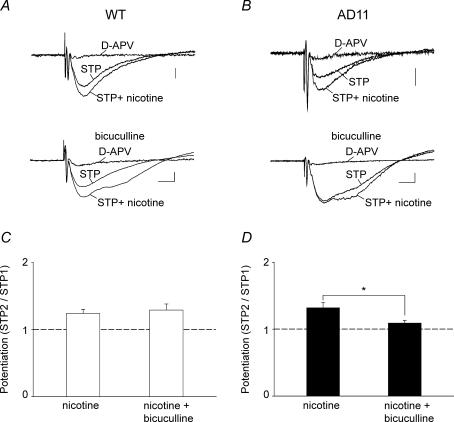
In a previous study with 192-IgG-saporin-treated rats, it was found that the loss of cholinergic innervation in the hippocampus was associated with an increased threshold for LTP induction an effect that could be reversed by nicotine (Yamazaki et al. 2002). According to these authors, the rescue was dependent on the activation of nAChRs localized on feed-forward GABAergic interneurons leading to principal cell disinhibition. In particular, it was found that the NMDA component of synaptic responses was significantly increased. To see whether this occurred also in our experiments, hippocampal slices were incubated for at least 30 min in the presence of an extracellular solution containing a low concentration (0.5 mm) of magnesium plus DNQX (20 μm). In these conditions, stimulation of the afferent pathway evoked field potentials which were reversibly blocked by d-APV (50 μm) indicating that they were mediated by glutamate acting on NMDA receptor types (Fig. 7A and B). The NMDA-mediated fEPSPs evoked in WT and AD 11 mice were very similar in amplitude (WT, 0.09 ± 0.007 mV; AD 11, 0.09 ± 0.01 mV) and decay time (WT, 28.3 ± 2.2 ms; AD 11, 27.8 ± 2.4 ms). They also exhibited similar STP when a tetanus (50 pulses 100 Hz) was delivered to the Schaffer collateral (WT, 1.43 ± 0.1; AD 11, 1.39 ± 0.1) suggesting that NGF deprivation does not affect the NMDA component of synaptic activity. Application of nicotine (1 μm for 4 min) induced a similar increase in amplitude and duration of the fEPSP in both WT and transgenic mice (on average the area underlying the fEPSP increased from 2.59 ± 0.19 mV ms to 3.49 ± 0.2 mV ms in WT and from 2.58 ± 0.17 mV ms to 3.46 ± 0.25 mV ms in AD 11 mice). It is interesting that, as for AMPA-mediated synaptic responses, bath application of bicuculline (10 μm) prevented the potentiating effects of nicotine in AD 11 but not in WT mice, suggesting that in transgenic mice, the effects of nicotine occur via nAChRs localized on GABAergic interneurons. In WT mice, STP2/STP1 was 1.24 ± 0.06 (n = 6) and 1.30 ± 0.09 (n = 4) whereas in AD 11 mice it was 1.32 ± 0.08 (n = 6) and 1.09 ± 0.04, (n = 4) in control and in the presence of bicuculline, respectively (P < 0.05, unpaired Wilcoxon test). It should be stressed that, as for AMPA-mediated responses, for NMDA-mediated fEPSPs the CA1 region was isolated from the CA3 with a knife cut to prevent the propagation of interictal bursts from the CA3 to the CA1 area.
Figure 7. Activation of GABAA receptors is required for nicotine-induced potentiation of the NMDA components of the field EPSPS (EPSPs) in AD 11 mice.
A and B, upper traces, examples of NMDA-mediated fEPSPs recorded from wild-type (WT) (left) and AD 11 mice (right), in the presence of an extracellular solution containing 0.5 mm magnesium and 20 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX; each trace is the average of six responses). Nicotine induces a potentiation of the NMDA component of the fEPSPs, which were blocked by d-(−)-2-amino-5-phosphonopentanoic acid (d-APV) (50 μm). Lower traces, in AD 11 (right) but not WT (left) mice, nicotine-induced potentiation of STP is prevented by bicuculline (10 μm). Scale bars, 50 μV (10 ms)−1. C and D, summary data from WT (open columns; nicotine, n = 6; nicotine + bicuculline, n = 4) and AD 11 mice (filled columns; nicotine, n = 6; nicotine + bicuculline, n = 4). *P < 0.05, unpaired Wilcoxon test.
Direct application of nicotine to GABAergic interneurons increases the frequency of spontaneous GABA-mediated IPSCs in principal cells of both WT and AD 11 mice
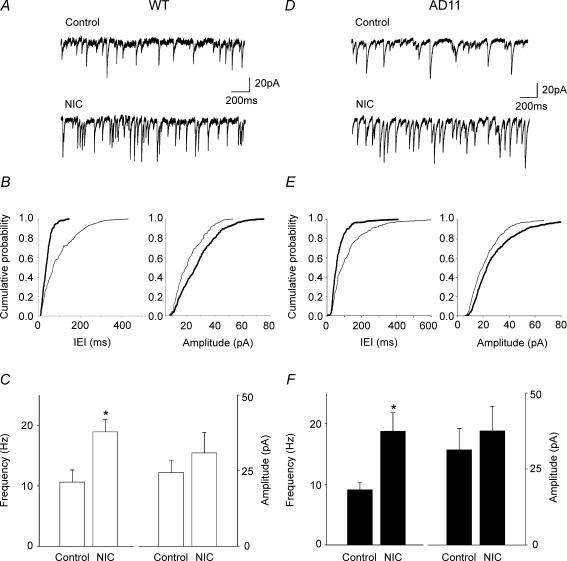
In order to elucidate whether in AD 11 mice nicotine-induced potentiation of the fEPSPs was due to disinhibition of principal cells, in a set of experiments whole-cell recordings in voltage-clamp conditions were obtained from principal cells in the presence of DNQX (20 μm) and d-APV (50 μm) to block AMPA/kainite- and NMDA-mediated synaptic currents. In these conditions, spontaneous synaptic currents were completely blocked by gabazine (5 μm) indicating that they were mediated by GABA acting on GABAA receptors (data not shown). Nicotine was directly applied to nearby interneurons localized in stratum radiatum and identified under infrared differential interference contrast microscopy. In both strains of mice, pressure application of nicotine to GABAergic interneurons induced a sudden increase of GABAA-mediated synaptic currents in principal cells that lasted for a few seconds (21 ± 4 s and 20 ± 3 s in WT and AD 11 mice, respectively; Fig. 8). On average in seven cells from WT mice, nicotine produced a significant increase in frequency (from 11 ± 2 Hz to 19 ± 2 Hz; P < 0.05, paired t test) but not in amplitude (from 24 ± 4 pA to 31 ± 7 pA; P > 0.05, paired t test) of spontaneous synaptic currents. A similar effect was observed in AD 11 mice (n = 7). In AD 11 mice, nicotine increased the frequency of IPSCs from 9 ± 1 Hz to 19 ± 3 Hz (P < 0.05, paired t test) whereas the amplitude was not significantly altered (31 ± 7 pA and 38 ± 8 pA, before and after nicotine application, respectively). In both WT and AD 11 mice, the potentiating effect of nicotine was prevented by the broad spectrum nAChR antagonist mecamylamine (20 μm; data not shown). These results suggest that in AD 11 mice, nicotine-induced potentiation of the field EPSPs is not due to disinhibition of principal cells.
Figure 8. Pressure application of nicotine to GABAergic interneurons enhances GABA release in principal cells of both wild-type (WT) and AD 11 mice.
A, individual tracings obtained from the hippocampus of WT mice in control and after pressure application (5 psi, 1 s) of nicotine (NIC, 1 mm) from a pipette localized near a GABAergic interneuron in stratum radiatum. B, cumulative distributions of inter event intervals (IEI) and amplitude of EPSCs shown in A. C, each column represents the mean frequency and amplitude of IPSCs recorded in control and after nicotine (NIC) application (n = 7). D–F, as in A–C, but for AD 11 mice (n = 7). *P < 0.05, paired t test.
In order to see whether differences in action potential-independent release of GABA exist between the two strains of mice, mIPSCs were recorded in the presence of TTX (1 μm) from principal cells held at −60 mV in the hippocampus obtained from WT (n = 9) and AD 11 mice (n = 8). No significant differences in frequency (WT, 10 ± 2 Hz; AD 11, 10 ± 1 Hz, P > 0.05, unpaired t test) or in amplitude (WT, 18 ± 2 pA; AD 11, 15 ± 3 pA, P > 0.05, unpaired t test) of mIPSCs were detected, indicating that in AD 11 mice action potential-independent release of GABA is not altered (data not shown).
Discussion
The present experiments clearly show that in the hippocampus of WT and anti-NGF AD 11 mice, a sustained activation of nAChRs by nicotine was able to lower the threshold for LTP induction and to convert STP or weak LTP into robust LTP. These data also demonstrate that nicotine was able to boost synaptic plasticity processes thus acting as a positive modulator of neuronal gain. However, whereas in WT mice nicotine-induced modulation of synaptic plasticity persisted in the presence of GABAA receptor antagonists, in AD 11 mice it was abolished, indicating the involvement of the GABAergic network. It should be stressed that chronic NGF deprivation in AD 11 mice did not affect AMPA- or NMDA-mediated field EPSPs that were similar in amplitude and shape to those observed in WT mice. We failed also to demonstrate any modification in short- (paired pulse facilitation, STP) or long-term (LTP) synaptic plasticity processes in AD 11 mice with respect to controls suggesting that at least at 6 months of age, in spite of the initial accumulation of Aβ in the hippocampus, the mechanisms controlling these processes are not altered. Recent studies from our group have shown a severe impairment of LTP in the visual cortex of aged-matched anti-NGF mice (Pesavento et al. 2002). Several hypotheses can be put forward to explain these different results including variations in the cholinergic innervation between the hippocampus and the visual cortex and/or different sensitivity to NGF deprivation of basal forebrain cholinergic projecting neurons to the hippocampus or to the visual cortex. However, the observation that, as in the present case, disruption of cholinergic fibres projecting to the hippocampus did not affect LTP expression (Jouvenceau et al. 1996) suggests that in the hippocampus LTP is not necessarily dependent on cholinergic innervation.
Similar to the present findings, nicotine-induced conversion of STP into LTP in cholinergic deprived animals required the activation of GABAA receptors (Yamazaki et al. 2002, 2005). The powerful priming effect of nicotine on STP has previously been described in rats (Fujii et al. 1999) and guinea-pigs (Mann & Greenfield, 2003). As in guinea-pigs, the effects of nicotine were mediated via NMDA receptors in the present experiments as they were prevented by d-APV (Schulz & Fitzgibbons, 1997). However, in contrast with the results obtained by Mann & Greenfield (2003) on guinea-pigs, in both WT and AD 11 mice we failed to see any slowly developing nicotine-induced synaptic potentiation in the presence of d-APV. Although the mechanisms underlying the reported novel form of potentiation have not been elucidated, the lack of this form of nicotine-induced synaptic plasticity in WT and AD 11 mice can be attributed to differences in nAChRs subtypes, localization and signalling pathways in the hippocampal network of these two animal species.
In our case, the observation that both the AMPA and the NMDA component of the field EPSPs were similarly potentiated suggests that the main effect of nicotine was to enhance glutamate release. Although we cannot completely exclude a postsynaptic change in the expression of AMPA and/or NMDA receptors this is expected to be associated with different modifications in the respective components of the fEPSPs.
In AD 11 mice, the fact that nicotine-induced conversion of STP into LTP was prevented when GABAA receptors were blocked strongly indicates that GABAergic interneurons are able to compensate for the deficit in the cholinergic modulation of glutamatergic transmission. This compensation may occur at different levels and may involve a major reorganization of the GABAergic circuitry and/or a redistribution of nAChRs within the interneuronal network. However, GABAergic inputs to pyramidal cells were unaltered as demonstrated by the observation that the action potential-independent release of GABA was similar in both AD 11 and WT mice. In addition, the present data enable us to exclude disinhibition of principal cells as the main cause for nicotine-induced potentiation, as in both strains of mice, activation of nAChRs localized on stratum radiatum interneurons by local application of nicotine, enhanced GABA release into principal cells in a similar way (see Ji & Dani, 2000). Reorganization of the GABAergic network in AD 11 mice may include other GABAergic interneurons not directly projecting to principal cells or their excitatory or inhibitory interconnections. Changes in the functional properties of the axons of septal GABAergic cells may also contribute to the observed effects, as it is known that these terminals selectively inhibit hippocampal inhibitory cells thus exerting a powerful disinhibitory action on principal cells (Toth et al. 1997). Furthermore, we cannot exclude the possibility that, in AD 11 mice, nicotine-induced increase in GABA release in interneurons may spillover to activate presynaptic GABAA receptors present on Schaffer collaterals leading to an increase in glutamate release. Whereas activation of GABAA receptors present on mossy fibre terminals is known to reduce principal cell excitability (Ruiz et al. 2003), at CA3–CA1 synapses activation of presynaptic GABAA receptors has been shown to facilitate glutamate release (Jang et al. 2005). Finally, as a matter of speculation we can hypothesize that chronic NGF deprivation alters chloride homeostasis leading to high [Cl−]i and to the shift of GABA from the hyperpolarizing to the depolarizing direction (Cherubini et al. 1991; Rivera et al. 1999; Payne et al. 2003). GABA-induced depolarization of principal cells would remove the magnesium block from NMDA channels thus exerting a positive excitatory feedback on pyramidal cells (Ben-Ari, 2002).
In WT mice, the persistent potentiating effect of nicotine in the presence of bicuculline or gabazine could be attributed to the strong facilitatory action of nicotine on glutamate release which would counterbalance the lack of GABA-mediated disinhibition. Nicotine not only would facilitate glutamate release from presynaptic nerve endings (Gray et al. 1996) but would also depolarize principal cells. Depolarization paired with presynaptic stimulation has been shown to convert STP into LTP (Ji et al. 2001). However, at the network level, the direction of synaptic plasticity can be modified depending on the distribution and timing of nAChR activity in principal cells and interneurons. Another possibility is that in WT mice, the spatial and temporal differences in nAChR activity on both excitatory and inhibitory neurons produces a net shift towards excitation as demonstrated in the mesolimbic reward system (Mansvelder et al. 2002).
In conclusion, it appears from the present study that in chronically NGF-deprived AD 11 mice, which can be considered an animal model for sporadic AD (Capsoni et al. 2000), GABAergic interneurons are the ideal targets for nicotine action on LTP. It is interesting that these interneurons are relatively resistant to loss in AD (Cotman & Anderson, 1995). As nicotine has been shown to reverse cognitive impairment caused by lesions of the cholinergic system (McGurk et al. 1991; Decker et al. 1992; Levin et al. 1993) and to improve cognitive function in AD patients (Jones et al. 1992; Sahakian et al. 1994), the present model may be relevant for developing properly targeted therapeutic tools useful for the treatment of AD.
Acknowledgments
We are grateful to Elisabetta Sola for her useful comments during the preparation of this manuscript. This work was supported by Telethon Grant GGP 030416 to E.C. and A.C.
References
- Alkondon M, Albuquerque EX. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog Brain Res. 2004;145:109–120. doi: 10.1016/S0079-6123(03)45007-3. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bear MF. Homosynaptic long-term depression: a mechanism for memory? Proc Natl Acad Sci U S A. 1999;96:9457–9458. doi: 10.1073/pnas.96.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric alpha 7 receptor. Neurosci Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, Cattaneo A. Nerve growth factor and galantamine ameliorate early signs of neurodegeneration in anti-nerve growth factor mice. Proc Natl Acad Sci U S A. 2002a;99:12432–12437. doi: 10.1073/pnas.192442999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Giannotta S, Cattaneo A. Beta-amyloid plaques in a model for sporadic Alzheimer's disease based on transgenic anti-nerve growth factor antibodies. Mol Cell Neurosci. 2002b;21:15–28. doi: 10.1006/mcne.2002.1163. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc Natl Acad Sci U S A. 2000;97:6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Capsoni S, Margotti E, Righi M, Kontsekova E, Pavlik P, Filipcik P, Novak M. Functional blockade of tyrosine kinase A in the rat basal forebrain by a novel antagonistic anti-receptor monoclonal antibody. J Neurosci. 1999;19:9687–9697. doi: 10.1523/JNEUROSCI.19-22-09687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Rapposelli B, Calissano P. Three distinct types of monoclonal antibodies after long-term immunization of rats with mouse nerve growth factor. J Neurochem. 1988;50:1003–1010. doi: 10.1111/j.1471-4159.1988.tb10565.x. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Anderson AJ. A potential role for apoptosis in neurodegeneration and Alzheimer's disease. Mol Neurobiol. 1995;10:19–45. doi: 10.1007/BF02740836. [DOI] [PubMed] [Google Scholar]
- De Rosa R, Garcia AA, Braschi C, Capsoni S, Maffei L, Berardi N, Cattaneo A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD 11 anti-NGF transgenic mice. Proc Natl Acad Sci U S A. 2005;102:3811–3816. doi: 10.1073/pnas.0500195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeir T, Saragovi HU, Cuello AC. A nerve growth factor mimetic TrkA antagonist causes withdrawal of cortical cholinergic boutons in the adult rat. Proc Natl Acad Sci U S A. 1999;96:4067–4072. doi: 10.1073/pnas.96.7.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ, Anderson DJ. Effects of nicotine on spatial memory deficits in rats with septal lesions. Brain Res. 1992;572:281–285. doi: 10.1016/0006-8993(92)90485-r. [DOI] [PubMed] [Google Scholar]
- Demuro A, Palma E, Eusebi F, Miledi R. Inhibition of nicotinic acetylcholine receptors by bicuculline. Neuropharmacology. 2001;41:854–861. doi: 10.1016/s0028-3908(01)00137-x. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, Davies HA, Sher E, Stewart MG, Fine A. Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986;6:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Ito Y, Akaike N. Feed-forward facilitation of glutamate release by presynaptic GABA(A) receptors. Neuroscience. 2005;135:737–748. doi: 10.1016/j.neuroscience.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jones GM, Sahakian BJ, Levy R, Warburton DM, Gray JA. Effects of acute subcutaneous nicotine on attention, information processing and short-term memory in Alzheimer's disease. Psychopharmacology (Berl) 1992;108:485–494. doi: 10.1007/BF02247426. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Billard JM, Lamour Y, Dutar P. Persistence of CA1 hippocampal LTP after selective cholinergic denervation. Neuroreport. 1996;7:948–952. doi: 10.1097/00001756-199603220-00024. [DOI] [PubMed] [Google Scholar]
- Kawai H, Zago W, Berg DK. Nicotinic alpha 7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci. 2002;22:7903–7912. doi: 10.1523/JNEUROSCI.22-18-07903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug L, Giniatullin R, Klein RC, Fayuk D, Yakel JL. Functional mapping and Ca2+ regulation of nicotinic acetylcholine receptor channels in rat hippocampal CA1 neurons. J Neurosci. 2003;23:9024–9031. doi: 10.1523/JNEUROSCI.23-27-09024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, Briggs SJ, Rose JE. Chronic nicotine reverses working memory deficits caused by lesions of the fimbria or medial basalocortical projection. Brain Res Cogn Brain Res. 1993;1:137–143. doi: 10.1016/0926-6410(93)90021-v. [DOI] [PubMed] [Google Scholar]
- Li Y, Holtzman DM, Kromer LF, Kaplan DR, Chua-Couzens J, Clary DO, Knusel B, Mobley WC. Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J Neurosci. 1995;15:2888–2905. doi: 10.1523/JNEUROSCI.15-04-02888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS. Nicotinic receptors and hippocampal synaptic plasticity … it's all in the timing. Trends Neurosci. 2002;25:171–172. doi: 10.1016/s0166-2236(00)02127-5. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Levin ED, Butcher LL. Impairment of radial-arm maze performance in rats following lesions involving the cholinergic medial pathway: reversal by arecoline and differential effects of muscarinic and nicotinic antagonists. Neuroscience. 1991;44:137–147. doi: 10.1016/0306-4522(91)90256-n. [DOI] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature ‘silent’ connections in the developing hippocampus. Proc Natl Acad Sci U S A. 2003;100:2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. J Physiol. 2004;559:863–874. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar M, Tongiorgi E, Avignone E, Gonfloni S, Ruberti F, Domenici L, Cattaneo A. The effects of anti-nerve growth factor monoclonal antibodies on developing basal forebrain neurons are transient and reversible. Eur J Neurosci. 1998;10:3127–3140. doi: 10.1046/j.1460-9568.1998.00314.x. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Perry E, Martin-Ruiz C, Lee M, Griffiths M, Johnson M, Piggott M, et al. Nicotinic receptor subtypes in human brain ageing, Alzheimer and Lewy body diseases. Eur J Pharmacol. 2000;393:215–222. doi: 10.1016/s0014-2999(00)00064-9. [DOI] [PubMed] [Google Scholar]
- Pesavento E, Capsoni S, Domenici L, Cattaneo A. Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex of anti-nerve-growth-factor mice. Eur J Neurosci. 2002;15:1030–1036. doi: 10.1046/j.1460-9568.2002.01937.x. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, Gonfloni S, Rossi G, Berardi N, Cattaneo A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J Neurosci. 2000;20:2589–2601. doi: 10.1523/JNEUROSCI.20-07-02589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Coull JJ, Hodges JR. Selective enhancement of executive function by idazoxan in a patient with dementia of the frontal lobe type. J Neurol Neurosurg Psychiatr. 1994;57:120–121. doi: 10.1136/jnnp.57.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Fitzgibbons JC. Differing mechanisms of expression for short- and long-term potentiation. J Neurophysiol. 1997;78:321–334. doi: 10.1152/jn.1997.78.1.321. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DPU HS, Bakay R, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res. 2002;946:148–152. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. Eur J Neurosci. 2005;22:845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]