Abstract
The aim of this study was to further understand the central processing of inputs arising from unmyelinated and myelinated nociceptors by (i) determining the response characteristics of Class 2 dorsal horn neurones to preferential activation of C- and A-fibre heat nociceptors, and (ii) investigating the control exerted by the dorsolateral/lateral region of the midbrain periaqueductal grey (DL/L-PAG) on C- and A-fibre-evoked responses of these neurones. The use of different rates of skin heating to preferentially activate unmyelinated (C-fibre; 2.5°C s−1) versus myelinated (A-fibre; 7.5°C s−1) heat nociceptors revealed that, in response to C-nociceptor activation, Class 2 neurones encode well only over the first 5°C above threshold, and that at higher temperatures responses decline. In contrast, responses to A-nociceptor activation are linear and encode skin temperature over more than 10°C, and almost certainly into the tissue-damaging range. PAG stimulation raised thresholds and decreased significantly the magnitude of responses to A- and C-nociceptor activation. However, differences were revealed in the effects of descending control on the relationships between skin temperature and neuronal firing rate; the linear relationship that occurred over the first 5°C of slow rates of skin heating was no longer evident, whereas that to fast rates of skin heating was maintained over the entire range, albeit shifted to the right. These data indicate that the sensori-discriminative information conveyed in A-fibre nociceptors is maintained and that the information from C-nociceptors is lost in the presence of descending control from the DL/L-PAG. The data are discussed in relation to the role of the DL/L-PAG in mediating active coping strategies.
Information about noxious events in the periphery is conveyed to the spinal cord in myelinated and unmyelinated nociceptors. These two classes of peripheral nociceptor convey different qualities of the nociceptive message, first and second pain, respectively (Raja et al. 1999), and have different roles in animal models of chronic pain (Fuchs et al. 2000). They have distinct pharmacological and physiological properties, such as their conduction velocities and their ability to encode the intensity of suprathreshold stimuli (Slugg et al. 2000; Cain et al. 2001), and recent evidence from this laboratory indicates that they activate distinct central pathways linking the hypothalamus and midbrain (Lumb et al. 2002).
A major determinant of the pain experience is descending control, which originates from a number of supraspinal sites and acts to modulate nociceptive transmission at early stages in pain pathways in the dorsal horn of the spinal cord (Willis, 1988; Willis & Westlund, 1997; Millan, 2002). Recently we reported that descending control from the dorsolateral/lateral sector of the midbrain periaqueductal grey (DL/L-PAG) has differential effects on nociceptive reflexes evoked by activity in unmyelinated (C-fibre) versus myelinated (A-fibre) heat nociceptors: C-fibre-evoked withdrawal responses to noxious heating were strongly inhibited, whereas those to A-fibre activation were relatively unaffected (McMullan & Lumb, 2006). This has important implications as neurones in the DL/L-PAG co-ordinate active coping strategies, of which modulation of nociceptive transmission is one component, in response to stressors and in emergency situations (Lovick & Bandler, 2005). One interpretation of this finding is that the PAG has the capacity to selectively filter out potentially distracting information conveyed in C-nociceptors while preserving the rapidly conducted well-localized aspects of first pain; this combination of effects would aid survival in emergency situations.
Given the different roles of unmyelinated and myelinated nociceptors in normal and pathological pain states, together with our recent evidence of a separation in the central pathways that they activate, and a selectivity in their descending control, it is of considerable importance to gain a better understanding of the spinal processing of input from these two classes of nociceptor.
To this end, the aims of the current study were twofold: (i) to determine the response characteristics of dorsal horn neurones to stimulation of C- and A-fibre heat nociceptors, and (ii) to investigate the control exerted by the DL/L-PAG region of the midbrain on C- and A-fibre-evoked responses of dorsal horn neurones.
Methods
Animal preparation
Experiments were conducted on 18 adult male Wistar rats (275–300 g). All experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986, and associated guidelines. Anaesthesia was induced with halothane (2–3% in O2), and the trachea, carotid artery and external jugular vein were cannulated, and subsequent anaesthesia was maintained by continuous intravenous infusion of alphaxalone/alphadolone (Saffan, 14–27 mg kg−1 h−1; Schering-Plough Animal Health, UK). Arterial blood pressure and rectal temperature were monitored and maintained within physiological limits. During preparatory surgery, anaesthesia was maintained at a level at which firm pinch of the hindpaw did not evoke withdrawal or blood pressure changes.
Preferential activation of myelinated and unmyelinated heat nociceptors
A heating apparatus that delivered ‘slow’ or ‘fast’ rates of skin heating to the dorsal hindpaw was used to preferentially activate unmyelinated (capsaicin-sensitive) or myelinated (capsaicin-insensitive) heat nociceptors, respectively (McMullan et al. 2004). The apparatus was a modified version of that described by Yeomans and colleagues (Yeomans & Proudfit, 1994, 1996; Yeomans et al. 1996), in which slow and fast rates of heating activated nociceptive primary afferents with conduction velocities < 2.3 m s−1 (i.e. C-fibres) and > 3 m s−1 (i.e. A-fibres), respectively. In brief, heat from a sputter-coated projector bulb was focused onto a thin disk of blackened copper (4 mm in diameter), which was placed in firm even contact with the dorsal surface of the skin of the hindpaw. A custom-made t-type thermocouple (0.02 mm in diameter) was fixed in position between the surface of the copper disk and the skin, and used to record skin temperature, which was digitized and captured at 50 samples s−1 on a PC running Spike2 version 3.21 (Cambridge Electronic Design, Cambridge, UK). Using a constant bulb voltage, fast rates of heating (7.5 ± 1°C s−1, measured over 2 s from the start of heating) were used to preferentially activate myelinated heat nociceptors, whereas slow rates of heating (2.5 ± 1°C s−1, measured over 4 s from the start of heating) were used to preferentially activate unmyelinated nociceptors. Data resulting from heating ramps with slopes that fell outside of ±1°C s−1 of 7.5°C s−1 and 2.5°C s−1 for fast and slow ramps, respectively, were rejected. In order to prevent tissue damage, skin heating was terminated by a feedback-controlled cut-off device fitted to the power source. Cut-offs to fast and slow rates of heating were set at 57 and 55°C, respectively; data from heating trials in which no response occurred were assigned the appropriate cut-off value and included in the analysis.
Single-unit dorsal horn recordings
Extracellular single-unit recordings were made from Class 2 (Menetrey et al. 1977) deep dorsal horn neurones, as previously described (Waters & Lumb, 1997). In brief, the vertebrae at each end of a laminectomy between T13 and L2 were clamped, and a 4% agar pool was formed over the exposed tissue. To allow access to the spinal cord, a small window was cut in the agar and filled with warm mineral oil. The dura were removed, and extracellular recordings of single unit activity were made using homemade borosilicate glass microelectrodes mounted on an active-probe headstage (Intra 767b; WPI, UK). Electrodes were filled with 4 m NaCl and had a resistance of 4–10 MΩ. The filtered (Neurolog 125; low cut-off, 500–700 Hz; high cut-off, 5 kHz) and amplified (Neurolog 105; ×1000) signal was monitored on an oscilloscope, captured by a PC running Spike2 version 3.21, and played over a loudspeaker. Action potentials were also captured as Spike2 ‘Wavemarks’, permitting online arithmetic plotting of instantaneous firing frequency on a separate data channel. In some cases multiunit recordings were made. Wavemarks were used to compare spike shape and amplitude; spike templates were constructed (sampled at 20 kHz) and compared using K-means cluster analysis. Individual units were sorted and filtered post hoc. Receptive fields of recorded neurones typically extended over an area equivalent to one quarter of the dorsal surface of the hindpaw, and were responsive to both low- (e.g. brush) and high-intensity (e.g. noxious pinch, heating) stimuli. Only cells that had excitatory receptive fields that were both accessible to the heater and larger than the copper disk that formed the heating contact with the skin were included in the study.
Neuronal activation in the PAG
The head was fixed in a flat skull position in a stereotaxic instrument and a small window, extending approximately 3 mm lateral and 5 mm rostral to lambda, was removed from the temporal bone using a small drill fitted with a round dental burr.
Triple-barrelled glass micropipettes (overall tip diameter ∼40 μm) were driven vertically into the PAG at approximately 7.5 mm caudal to bregma, 0.8 mm lateral to the midline, and between 4.0 and 5.3 mm deep to the cortical surface. Micropipette barrels contained: 50 mm dl-homocysteic acid (DLH; Sigma, UK) solution in pH-buffered Ringer solution for neuronal stimulation, Ringer solution for control injections, and saturated pontamine sky blue (PSB) solution to mark injection sites. Drug solutions (6 nl), or larger volumes of dye solution (∼60 nl), were pressure-ejected under microscopic guidance. A calibrated graticule was used to measure meniscus movement, from which ejection volumes were estimated. Micropipettes were positioned in the dorsolateral/lateral PAG at sites at which injection of DLH evoked increases in arterial blood pressure.
Experimental protocol
In a control period, neuronal responses to fast or slow rates of heating were recorded at 8 min intervals. Following three control trials, a 6 nl injection of DLH was made into a previously identified pressor site within the PAG. Approximately 20 s after injection (at which point cardiovascular changes evoked by DLH injection were maximal), a fourth heating trial was delivered to the receptive field. Neuronal responses to three subsequent (post-test) heating trials were then recorded at 8 min intervals. If, following a complete series of heating trials, the condition of the animal and the quality of the recorded signal were still optimal, a second series of heating trials, using the other rate of heating, was conducted (i.e. if the first series used slow rates of heating, the second series used fast rates of heating).
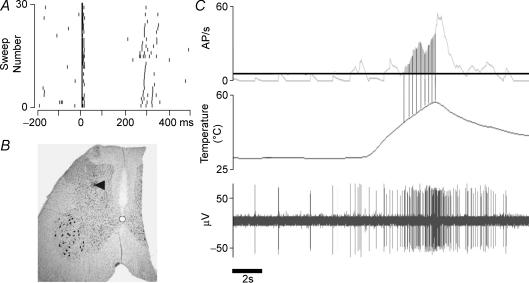
At the end of recording the composition of afferent input to the unit was assessed using electrical stimulation in the cutaneous receptive field. Bipolar needle electrodes were inserted into the centre of the receptive field and single rectangular pulses, 1–1.5 ms wide, were delivered. Pulse amplitude was gradually increased until single action potentials were reliably evoked. Stimulus intensity was then increased by a factor of 20 (up to a maximum of 100 V). Raster displays were constructed from repeated sweeps and units were classified as C+ve if a distinct late volley of action potentials was evoked with a latency of ∼200 ms. In 5/19 cells it was not possible to determine afferent composition. Raster displays from all other neurones indicated excitatory inputs from both myelinated and unmyelinated afferents (e.g. Fig. 1A). Data from cells in which it was not possible to characterize the composition of afferent input were not included in further analysis.
Figure 1. Data from an individual Class 2 dorsal horn neurone.
A, raster plot to illustrate the response of the neurone to supramaximal electrical stimulation in its cutaneous receptive field at time zero. B, photomicrograph to illustrate the recording site (pontamine sky blue (PSB) deposit) in the dorsal horn. C, response to a fast heating trial. Top trace, average discharge frequency per second. Middle trace, skin temperature. Lower trace, extracellular unit recording. Vertical lines, 1°C bins. Continuous horizontal line, background level of firing which is subtracted from the firing rate to calculate the magnitude of the response (shaded area).
In some experiments the electrode depth display on the stepping motor drive was calibrated by replacing recording electrodes with micropipettes containing saturated PSB in sodium acetate. PSB was ejected under iontophoresis (7 μA, 7–10 min) at the cord surface and at the depth of the recording site (e.g. Fig. 1B). Rats were killed with a lethal intravenous bolus of pentobarbitone at the end of experiments, and the midbrain and marked sections of spinal cord were removed and placed in 7% phosphate-buffered paraformaldehyde. Fixed tissue was sectioned at 50 μm and stained with neutral red. Sections were visualized under a microscope and camera lucida drawings made of injection and recordings sites. Cells included in this study were recorded at an average depth of 904 ± 101 μm from the surface of the spinal cord.
Data analysis
In order to determine thresholds of responses to slow and fast rates of heating, the rate of spontaneous activity occurring between 10 and 5 s before the start of heating was used as a reference period and response threshold was defined as the point at which heat-evoked activity exceeded and remained elevated relative to average spontaneous firing frequency during the reference period. If, after injection of DLH, heating did not evoke a neuronal response, a value equivalent to the lamp cut-off temperature was assigned. Two-way analysis of variance (Graphpad Prism 4.0) was used to determine any differences in the effects of DLH microinjection on neuronal responses evoked by slow and fast rates of heating. Bonferroni post-tests were used to detect significantly different pairings within responses evoked by slow or fast rates of heating.
Response magnitude was also quantified. Activity occurring between the response threshold and the heating cut-off was broken into 1°C bins (Fig. 1C), and the number of action potentials occurring in each bin was normalized with respect to bin width and expressed relative to the first bin of the response. Pre-trial spontaneous activity was measured and, after correcting for bin width, subtracted from each bin of the response. Data from repeated measures, i.e. data from trios of control and post-test trials, were averaged. All data points were normalized with respect to the first bins of control responses, which were assigned the value of 100%. Data points that were averaged from less than three experiments were excluded from analysis. Curve fitting and linear correlations were used to determine the relationships between skin temperature and neuronal firing frequency. ANOVA was used to compare slopes of stimulus–response functions (Graphpad Prism 4.0). Magnitudes of evoked responses were determined by calculating the normalized area under the temperature–response curve. Data are expressed as means ±s.e.m.
Results
Neuronal responses to slow and fast rates of heating
Recordings were made from 19 Class 2 neurons in the deep dorsal horn. Of these, five neurons were not included in analysis because their afferent composition could not be determined by electrical stimulation of the receptive field. Of the 14 neurons that received convergent input from both myelinated and unmyelinated afferent input, responses to fast rates of heating were examined in 12, and responses to slow rates were examined in 11 cells (i.e. it was possible to examine responses to both fast and slow rates of heating in nine units).
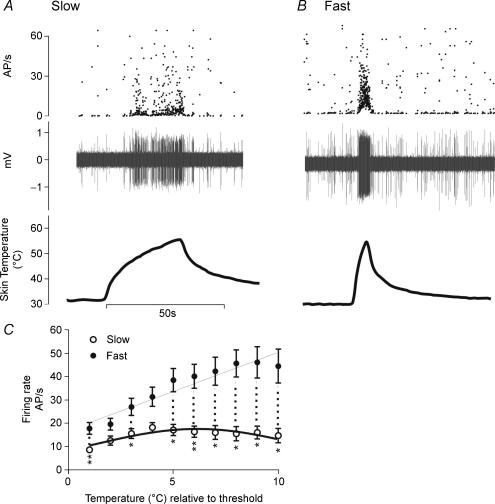
Responses of an individual neurone to slow and fast rates of skin heating are illustrated in Fig 2A and B, and the relationship between firing frequency and temperature relative to threshold for the entire population in Fig. 2C. Activity evoked by slow heating trials increased linearly with skin temperature over the first 5°C of a response, reached a plateau, and subsequently decreased. This pattern of response fitted a Gaussian response profile (r2= 0.63, Fig. 2C) rather than a straight line (r2= 0.24). In contrast, neuronal activity evoked by fast rates of skin heating exhibited a strong linear correlation with skin temperature (r2= 0.9; Fig. 2C) over the full range of skin heating (i.e. from average threshold of 46.1°C to lamp cut-off at 57°C). At any given temperature above threshold, fast rates of skin heating generally evoked significantly more activity than slow rates of heating (Fig. 2C). When expressed as area under the curve, activity evoked by fast rates of heating (322.1 AP s−1°C) was greater than that evoked by slow rates of heating (139.1 AP s−1°C; P < 0.01).
Figure 2. Activation of class 2 dorsal horn neurons by slow and fast rates of skin heating.
A and B, firing frequencies (upper traces) and extracellular recordings (middle traces) of an individual neurone to illustrate the relationship between skin temperature (lower traces) and responses evoked by slow (A) and fast (B) rates of skin heating. C, temperature–response relationships of Class 2 neurones to slow (open symbols, n = 11) and fast (filled symbols, n = 12) rates of skin heating; data are means ±s.e.m.; responses to fast rates of heating follow a linear relationship (r2= 0.9), whereas responses to slow rates best fit a bell-shaped function (r2= 0.63); *P < 0.05, **P < 0.01 (two-way Student's t test), statistical significance between responses at any given temperature above threshold (hashed line).
When responses evoked by slow and fast rates of heating were normalized and expressed as a percentage of action potential frequency recorded over the first 1°C of control responses (as in Fig. 6), the values for goodness of fit (r2) of linear (fast) and Gaussian (slow) regressions were increased to 0.97 and 0.78, respectively.
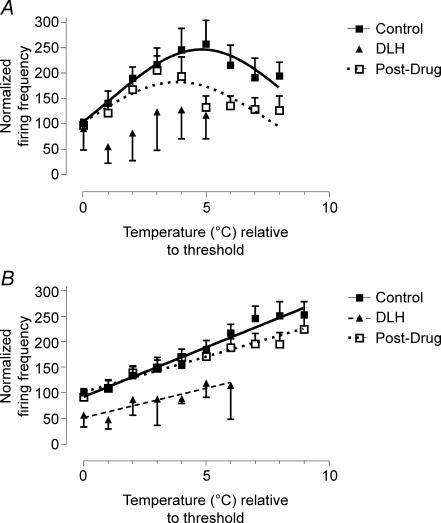
Figure 6. Effect of PAG stimulation on response profiles.
Effects of neuronal activation in the periaqueductal grey on the responses of Class 2 neurones to slow (A, n = 11) and fast (B, n = 12) rates of skin heating. Data (means ±s.e.m.) are normalized to threshold responses in the control period.
Effects of PAG stimulation
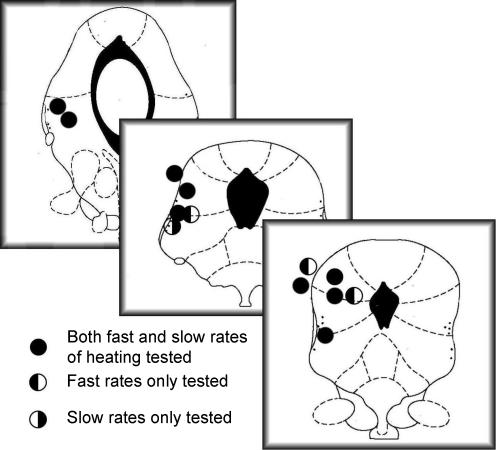
Microinjection of DLH into sites in the DL/L-PAG (Fig. 3) evoked pressor responses of 19.7 ± 1.9 mmHg. In 9/14 cells, spontaneous firing was reduced shortly after DLH injection (latency, 1–2 s). In five of these cells, the reduction in firing was preceded by a transient (∼0.5 s) but reproducible increase in action potential frequency. In these cases, the increase in firing frequency was almost simultaneous with DLH injection, and in several cases was at such short latency that the initial bursting had concluded before the completion of drug injection (data not shown).
Figure 3. Midbrain sites at which microinjection of 50 mm dl-homocysteic acid evoked pressor responses.
Sections shown are 7.5, 8.0 and 8.72 mm caudal to bregma (redrawn from Paxinos & Watson, 1986). NB, one pressor site that was not confirmed histologically is not included.
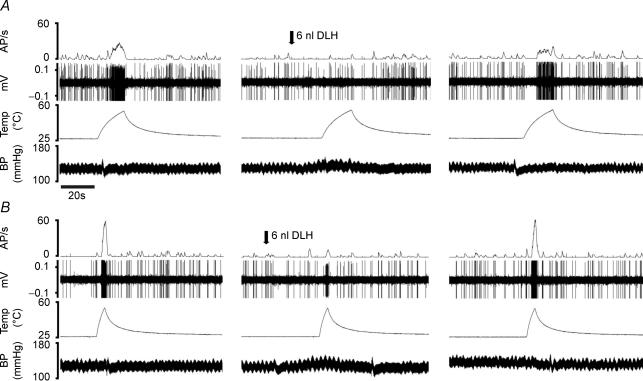
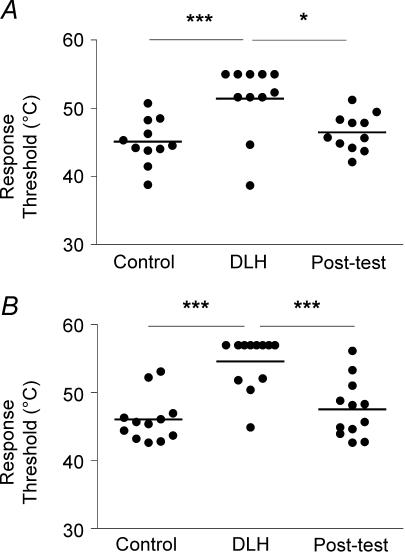
PAG activation evoked strong inhibition of neuronal responses to both slow and fast rates of skin heating (Fig. 4). On average, thresholds of responses to slow rates of heating were increased significantly from 45.1 ± 1 to 51.4 ± 1.6°C (n = 11, P < 0.001; Fig. 5A) and thresholds to fast rates of heating were increased from 46.1 ± 1 to 54.6 ± 1.1°C (n = 12, P < 0.001; Fig. 5B). In the 24 min period following PAG stimulation (post-test), thresholds of responses to both slow and fast rates of heating recovered to their control values. Two-way analysis of variance indicated that the degree to which the threshold of neuronal responses to heating was increased by DLH microinjection was independent of rate of skin heating (P > 0.05).
Figure 4. Effects of neuronal activation in the PAG on the responses of an individual neurone to slow (A) and fast (B) rates of skin heating.
Traces: top, action potential frequency; second, extracellular recording; third, skin temperature; bottom, arterial blood pressure. Arrows indicate the timing of microinjection of DLH (dl-homocysteic acid) in the periaqueductal grey.
Figure 5. Effect of PAG stimulation on response thresholds.
Scatterplots illustrating the effect of microinjection of DLH in the periaqueductal grey on thresholds of neuronal responses to slow (A, n = 11) and fast (B, n = 12) heating ramps. *P < 0.05, ***P < 0.001 (two-way ANOVA).
The magnitudes of responses evoked by noxious heating in control, test and post-test periods were calculated by measuring the area under the curve of normalized activity plotted against temperature relative to threshold (see Fig. 6). Note, however, that due to the increase in response threshold evoked by PAG stimulation, there was a reduction in the range of temperatures over which neuronal responses were evoked (i.e. the range between threshold and heating cut-off was reduced from 8 or 9°C to 5 or 6°C). In view of this, for consistency, area under the curve measurements were made only over the first 5 or 6°C of control responses to slow and fast rates of heating, respectively. During PAG activation, responses to slow and fast heating trials were reduced to 50.7 and 58% of control, respectively.
The profiles of responses to slow rates of skin heating immediately after PAG activation no longer fitted a Gaussian profile (r2= 0.37; Fig. 6A). In the 24 min period following PAG stimulation (post-test), profiles of responses evoked by slow heating returned to a Gaussian profile (r2= 0.5), and the area under the curve of the response had returned to 82.6% of control. Responses to fast rates of heating continued to increase linearly with temperature following PAG stimulation (r2= 0.86). However, responses were decreased in magnitude and the slope of the response profile was reduced from 19.4 ± 1.2 to 11.5 ± 2.1%°C−1 (P < 0.01). Responses to fast heating trials recovered in the post-test period: area under the curve was restored to 92% control, and action potential frequency continued to rise linearly with increasing temperature. However, the slope of the regression was still significantly reduced (13.4 ± 1%°C−1; P < 0.01).
Discussion
Stimulus–response functions of Class 2 neurones to slow and fast rates of skin heating
This study targeted a selective population of deep dorsal horn neurones (Class 2 or wide dynamic range), as recent studies (see Suzuki et al. 2004) indicate that neurones in the superficial dorsal horn, many of which are nociceptive specific, have different roles in nociceptive processing. The data reveal differences in the ability of Class 2 neurones to encode dynamic changes in suprathreshold temperatures signalled by unmyelinated C- and myelinated A-fibre heat nociceptors.
In response to slow rates of heating (to preferentially activate C-nociceptors), neurones encoded skin temperature well over the first 5°C above the mean threshold of 45 ± 1°C. However, at higher temperatures firing frequency progressively decreased until the cut-off temperature of 55°C was reached, resulting in a Gaussian stimulus–response curve. In contrast, fast rates of heating, which preferentially activate A-fibre nociceptors, evoked linear response functions that encoded skin temperature from a threshold of 46.1 ± 1°C up to the cut-off temperature of 57°C, i.e. over more than 10°C and almost certainly into the tissue damaging range. It should be borne in mind that this linear relationship could not continue indefinitely, as neuronal firing rate is finite, and responses must therefore level off at some point. It is unclear from the current data at what temperature such a plateau might occur.
This is the first study to compare dynamic temperature–response functions of dorsal horn neurones following differential activation of myelinated and unmyelinated afferents. Studies that have used ramp-and-hold heat stimuli report that the magnitude of heat-evoked responses generally increases at progressively higher temperatures (e.g. Gebhart et al. 1983; Carstens & Douglass, 1995; Pertovaara, 1999; Craig et al. 2001). However, data derived from ramp-and-hold protocols cannot be interpreted with respect to the class of heat nociceptor. There are two studies (Morgan, 1998, 1999) in which relatively slow rates (1.8°C s−1) of skin heating were used to evoke responses in dorsal horn neurones. Interestingly, these resulted in Gaussian response profiles in Class 2 neurones, presumably as a result of C-fibre activation.
Similarly, it is difficult to draw comparisons between the response properties of primary afferents and those of dorsal horn neurones reported here, as very few investigations have used dynamic heating ramps. Numerous studies have used ramp and hold protocols of skin heating, and describe monotonic encoding of skin temperature by nociceptive myelinated (Treede et al. 1998; Cain et al. 2001) and unmyelinated (LaMotte & Campbell, 1978; Treede et al. 1990; Andrew & Greenspan, 1999; Cain et al. 2001) primary afferent nociceptors.
The study that is most directly comparable to the current one is that of Yeomans & Proudfit (1996), in which similar rates of skin heating were used to preferentially activate myelinated and unmyelinated afferents in the rat. Those authors reported that activity in myelinated nociceptors encoded fast rates of skin heating (6.5°C s−1) linearly over a wide temperature range (to a maximum of 59°C). In contrast, although unmyelinated afferents encoded temperature well from a mean threshold of approximately 43°C to around 18 s after the start of heating (at which point skin temperature would have been approximately 4°C above threshold), at higher temperatures firing progressively decreased. In other words, the stimulus–response functions of Class 2 neurones in the deep dorsal horn to slow and fast rates of skin heating bear marked similarities to those of C-fibre and A-fibre nociceptors, respectively. Similar differences in the encoding properties of C- and A-fibre high-threshold mechanoceptors (HTMs) have also been described (Slugg et al. 2000) and Gaussian stimulus–response functions have been described in human C-fibres following graded heating of the skin (Handwerker et al. 1991).
Taken together, the available data suggest that the stimulus–response functions of Class 2 dorsal horn neurones to different rates of skin heating are a direct result of the properties of C- versus A-fibre nociceptors, rather than, for example, differences in the segmental circuitry conveying input from myelinated and unmyelinated afferents to the deep dorsal horn, which could conceivably evoke different rates of depolarization in second order neurones.
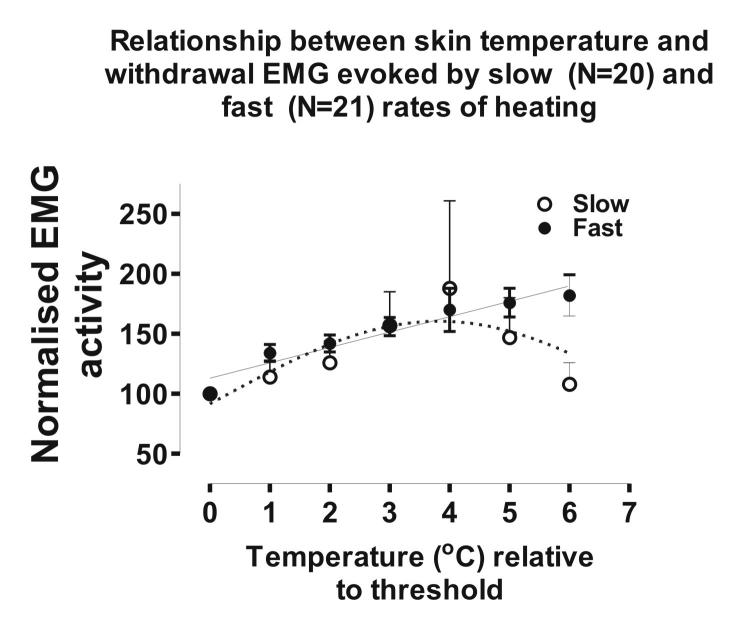
In a recent study, we reported that reflex withdrawal EMG evoked by slow and fast rates of noxious skin heating encoded temperature linearly over the first 4°C above threshold (McMullan & Lumb, 2006). In light of the current findings, we have now plotted the magnitude of reflex EMGs in response to slow and fast rates of skin heating up to 6°C above threshold. This analysis reveals that at temperatures higher than 4°C above threshold, responses to slow rates of skin heating decline, resulting in a bell-shaped function (see online Fig. 1 in Supplemental material). In contrast, EMG responses to fast rates of skin heating continue to encode in a linear fashion. The similarities in the stimulus–response relationships to slow and fast rates of skin heating observed in withdrawal EMG (McMullan & Lumb, 2006), Class 2 neurones (present study) and primary afferent nociceptors (Yeomans & Proudfit, 1996) suggest that Class 2 neurones may form part of the reflex withdrawal pathway (see Morgan, 1998).
Effects of neuronal activation in the PAG on stimulus–response functions of dorsal horn neurones
Stimulation in the PAG raised the thresholds and decreased the magnitude of responses to slow and fast rates of skin heating. Increased thresholds of responses of dorsal horn neurones to heat stimuli following activation in the PAG have been reported by others (Gebhart et al. 1983; Sandkuhler et al. 1991; Carstens & Douglass, 1995; Waters & Lumb, 1997; Budai & Fields, 1998). However, none of these studies differentiated between responses evoked by C- and A-fibre nociceptors.
The current study investigated the responsiveness of deep Class 2 neurons to myelinated and unmyelinated noxious inputs in order to compare response characteristics with those obtained previously for withdrawal reflexes. Class 2 deep dorsal horn neurons are thought to be more likely than Class 3 neurons (i.e. nociceptive specific, NS) to contribute to the withdrawal reflex (Schouenborg et al. 1995; Morgan, 1998), and their activity and modulation by descending and segmental spinal mechanisms correlate closely with motor activity evoked by noxious stimuli (Schouenborg & Dickenson, 1985; Carstens & Campell, 1992). Similarly, superficial dorsal horn neurons, the majority of which are NS, have properties inconsistent with a role in nociceptive withdrawal reflexes. For example, the augmentation of both motor (Wall & Woolf, 1984) and deep dorsal horn responses (Dickenson & Sullivan, 1990) seen following repeated high-intensity electrical stimulation (‘wind up’) is not present in the superficial dorsal horn (Seagrove et al. 2004).
The effects of PAG stimulation on thresholds for neuronal activation could contribute to the increased thresholds of reflex responses to slow rates of skin heating we reported previously (McMullan & Lumb, 2006). However, the data suggest that additional populations of neurones are likely to have a role in setting the threshold of reflex responses to fast rates of skin heating, as these were unchanged in our previous report. Candidate neurones include: (i) the 10% of Class 2 neurones in the dorsal horn whose nociceptive inputs are mediated exclusively by A-nociceptors (C–ve neurones), as suggested by Waters & Lumb (1997); and/or (ii) nociceptive neurones in the superficial laminae of the dorsal horn, many of which are NS. In on-going studies we are testing the latter hypothesis, by determining the effects of stimulation in the PAG on the numbers and distributions of neurones that express Fos protein in the superficial dorsal horn following fast or slow rates of skin heating.
The effects of PAG stimulation on encoding properties of dorsal horn neurones to slow rates of skin heating were similar to those we observed previously on EMG responses, namely that the initial component of the response no longer increased linearly with temperature (McMullan & Lumb, 2006). This finding adds further support to the notion that C+ve Class 2 neurones have a role in driving withdrawal reflexes to C-fibre stimulation. In contrast, and as we reported on EMG responses to fast rates of skin heating (McMullan & Lumb, 2006) the linear relationship between neuronal firing and skin temperature during fast rates of skin heating was maintained, albeit shifted to the right, following activation in the PAG. This observation suggests that, at suprathreshold intensities, C+ve Class 2 neurones may contribute to the A-fibre drive to withdrawal reflexes. However, as discussed above, it is unlikely that their properties underlie the threshold to withdrawal.
Although the effects of PAG stimulation on normalized responses to slow and fast rates of heating reported here are equivalent, the discussion of the data thus far does not take into account differences in the magnitude or patterns of neuronal responses evoked by the two rates of heating: fast rates of heating evoked 2.3 times as much neuronal activity compared with slow rates of heating (see Fig. 2C). Consequently during an equivalent attenuation of responses by PAG stimulation (∼50%), a considerably larger volume of A-fibre input would be available to activate nociceptive pathways. That being the case, if the Class 2 neurones investigated here are part of the withdrawal reflex pathway, then the reduction in C-fibre-evoked firing from the PAG may be sufficient to attenuate reflex responses to slow rates of skin heating, whereas the more robust firing in response to A-fibre activation in the presence of descending control may continue to drive withdrawal reflexes, albeit at a higher threshold. This could account, at least in part, for the differential effects of the PAG on reflex responses to slow and fast rates of skin heating (McMullan & Lumb, 2006).
Functional significance
The results reported here demonstrate that rapidly conducted A-fibre-mediated information about potentially damaging events in the periphery is encoded very precisely in Class 2 neurones which, it is suggested, drive protective withdrawal responses. In emergency situations, during which descending controls from the DL-PAG become active, these neurones would continue to encode the stimulus into the tissue-damaging range (to at least 57°C) and may therefore retain their protective function. In contrast, even in the absence of descending control, Class 2 neurones only encode C-fibre-mediated information at temperatures less than 49°C and, in the presence of descending control, these encoding properties are no longer evident. As such, we suggest that the slowly conducted C-fibre-mediated inputs do not drive protective reflexes in acute emergency situations.
Supplementary Material
Acknowledgments
The authors would like to thank Simon Lishman and Dilys Parry for their assistance. Work was supported by the Wellcome Trust. S. McM. was a University of Bristol Scholar.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.117754 http://jp.physoc.org/cgi/content/full/jphysiol.2006.117754/DC1 and contains supplemental material consisting of a figure and legend entitled: Supplementary Fig. 1, Biceps femoris EMG evoked by slow and fast rates of skin heating (adapted from McMullan & Lumb, 2006).
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Budai D, Fields HL. Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol. 1998;79:677–687. doi: 10.1152/jn.1998.79.2.677. [DOI] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- Carstens E, Campell IG. Responses of motor units during the hind limb flexion withdrawal reflex evoked by noxious skin heating: phasic and prolonged suppression by midbrain stimulation and comparison with simultaneously recorded dorsal horn units. Pain. 1992;48:215–226. doi: 10.1016/0304-3959(92)90061-F. [DOI] [PubMed] [Google Scholar]
- Carstens E, Douglass DK. Midbrain suppression of limb withdrawal and tail flick reflexes in the rat: correlates with descending inhibition of sacral spinal neurons. J Neurophysiol. 1995;73:2179–2194. doi: 10.1152/jn.1995.73.6.2179. [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol. 2001;86:1459–1480. doi: 10.1152/jn.2001.86.3.1459. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Differential effects of excitatory amino acid antagonists on dorsal horn nociceptive neurones in the rat. Brain Res. 1990;506:31–39. doi: 10.1016/0006-8993(90)91195-m. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Sandkuhler J, Thalhammer JG, Zimmermann M. Quantitative comparison of inhibition in spinal cord of nociceptive information by stimulation in periaqueductal gray or nucleus raphe magnus of the cat. J Neurophysiol. 1983;50:1433–1445. doi: 10.1152/jn.1983.50.6.1433. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Bandler R. The organization of the midbrain periaqueductal grey and the integration of pain behaviours. In: Hunt SP, Koltzenburg M, editors. The Neurobiology of Pain, Molecular and Cellular Neurobiology. Oxford: Oxford University Press; 2005. pp. 267–287. [Google Scholar]
- Lumb BM, Parry DM, Semenenko FM, McMullan S, Simpson DA. C-nociceptor activation of hypothalamic neurones and the columnar organisation of their projections to the periaqueductal grey in the rat. Exp Physiol. 2002;87:123–128. doi: 10.1113/eph8702348. [DOI] [PubMed] [Google Scholar]
- McMullan S, Lumb BM. Midbrain control of spinal nociception discriminates between responses evoked by myelinated and unmyelinated heat nociceptors in the rat. Pain. 2006;124:59–68. doi: 10.1016/j.pain.2006.03.015. [DOI] [PubMed] [Google Scholar]
- McMullan S, Simpson DA, Lumb BM. A reliable method for the preferential activation of C- or A-fibre heat nociceptors. J Neurosci Methods. 2004;138:133–139. doi: 10.1016/j.jneumeth.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Menetrey D, Giesler GJ, Jr, Besson JM. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977;27:15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM. Direct comparison of heat-evoked activity of nociceptive neurons in the dorsal horn with the hindpaw withdrawal reflex in the rat. J Neurophysiol. 1998;79:174–180. doi: 10.1152/jn.1998.79.1.174. [DOI] [PubMed] [Google Scholar]
- Morgan MM. Paradoxical inhibition of nociceptive neurons in the dorsal horn of the rat spinal cord during a nociceptive hindlimb reflex. Neuroscience. 1999;88:489–498. doi: 10.1016/s0306-4522(98)00238-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. The influence of stimulus temperature rise rate, adapting temperature, and stimulus duration on suprathreshold responses evoked by noxious heat in the glabrous skin of the limb. Comparison of neuronal discharge in the rat spinal dorsal horn with human sensations. Expbrain Res. 1999;126:482–494. doi: 10.1007/s002210050756. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN, Wall PD, Melzack R. Textbook of Pain. Edinburgh: Churchill-Livingston; 1999. Peripheral neural mechanisms of nociception; p. 11. [Google Scholar]
- Sandkuhler J, Willmann E, Fu QG. Characteristics of midbrain control of spinal nociceptive neurons and nonsomatosensory parameters in the pentobarbital-anesthetized rat. J Neurophysiol. 1991;65:33–48. doi: 10.1152/jn.1991.65.1.33. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Dickenson A. Effects of a distant noxious stimulation on A and C fibre-evoked flexion reflexes and neuronal activity in the dorsal horn of the rat. Brain Res. 1985;328:23–32. doi: 10.1016/0006-8993(85)91318-6. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Weng HR, Kalliomaki J, Holmberg H. A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Exp Brain Res. 1995;106:19–27. doi: 10.1007/BF00241353. [DOI] [PubMed] [Google Scholar]
- Seagrove LC, Suzuki R, Dickenson AH. Electrophysiological characterisations of rat lamina I dorsal horn neurones and the involvement of excitatory amino acid receptors. Pain. 2004;108:76–87. doi: 10.1016/j.pain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. J Neurophysiol. 2000;83:2179–2191. doi: 10.1152/jn.2000.83.4.2179. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Comparison of heat and mechanical receptive fields of cutaneous C-fiber nociceptors in monkey. J Neurophysiol. 1990;64:1502–1513. doi: 10.1152/jn.1990.64.5.1502. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat–response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- Wall PD, Woolf CJ. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Lumb BM. Inhibitory effects evoked from both the lateral and ventrolateral periaqueductal grey are selective for the nociceptive responses of rat dorsal horn neurones. Brain Res. 1997;752:239–249. doi: 10.1016/s0006-8993(96)01462-x. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr Anatomy and physiology of descending control of nociceptive responses of dorsal horn neurons: comprehensive review. Prog Brain Res. 1988;77:1–29. doi: 10.1016/s0079-6123(08)62776-4. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioural evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–151. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DOI: 10.1113/jphysiol.2006.117754 http://jp.physoc.org/cgi/content/full/jphysiol.2006.117754/DC1 and contains supplemental material consisting of a figure and legend entitled: Supplementary Fig. 1, Biceps femoris EMG evoked by slow and fast rates of skin heating (adapted from McMullan & Lumb, 2006).